Objective: To compare the efficacy and safety of 4 mg of ondansetron vs. 4 mg of nalbuphine for the treatment of neuraxial morphine-induced pruritus, in patients at the “Dr. José Eleuterio González” University Hospital from September 2012 to August 2013.
Material and methods: A controlled, prospective, randomized study of 28 patients (14 per group) receiving neuraxial morphine analgesia was conducted, which was registered and approved by the ethics Committee of the Institution and patients agreed to participate in the study under informed consent. The results were segmented and contrasted (according to drug) by hypothesis testing; the association was determined by X2 with a 95% confidence interval (CI).
Results: Pruritus was effectively resolved in both groups and no significant difference was found in the rest of the variables. An increase in the visual analogue scale (EVA) was observed at 6 and 12 hours for the ondansetron group, which was statistically significant (p≤0.05), however both groups had an EVA of less than 3.
Conclusions: When comparing the efficacy and safety of ondansetron 4 mg vs. nalbuphine 4 mg for the treatment of neuraxial morphine induced pruritus, the only significant difference found was the mean EVA at 6 and 12 hours, favoring the ondansetron group. However, both groups scored less than 3 on the EVA. Therefore, we consider that both treatments are effective and safe in the treatment of pruritus caused by neuraxial morphine.
Introduction
Neuraxial administration of opioids provides excellent postoperative analgesia, however its use is associated with a high incidence of side effects such as pruritus (itching), nausea, vomiting, urinary retention and respiratory depression.1-4
The term “pruritus” or “prurire” is an unpleasant sensation which causes the desire to scratch and varies in intensity. It is one of the most common side effects in epidural and/or intrathecal administration of opiates with an incidence of approximately 60%, compared to 2%-10% of the patients treated systematically; thus, its incidence depends on the route of administration. This symptom is common in postpartum women. The most affected areas are those innervated by the trigeminal, probably due to their higher number of opiate receptors in the spinal nucleus of the trigeminal nerve, causing patients to scratch their nose and the upper part of the face. There are several chemical mediators responsible for pruritus (histamine, serotonin, cytokines, growth factors, prostaglandins, etc.).1-6,7
The cause of pruritus induced by the administration of neuraxial opiates is uncertain; nevertheless there are 3 theories that may explain its origin. The 1st theory is associated with the release of peripheral histamine caused by the administration of morphine; however, this theory has not been proven because the antihistamines were ineffective in the treatment of pruritus caused by intrathecal morphine. The 2nd theory involves μ-opioid receptors responsible for pain modulation and some side effects, especially pruritus, nausea and vomiting, in the central nervous system activated by morphine. This explains the antipruritic effects of naloxone and nalbuphine, both μ-antagonists.2,3,8 A 3rd theory is that pruritus induced by the administration of neuraxial opiates could be related to the excitatory effects of opioids over nociceptive and non-nociceptive neurons in the anterior and posterior horns. It has been reported that morphine can activate serotonin receptors due to an independent mechanism of the opioid receptors. Therefore, the direct irritation of the serotonin type-3 receptors in the spinal cord, dorsal horn and bone marrow caused by the administration of morphine is possibly the mechanism of pruritus.8
In light of the above, a great variety of medications has been evaluated for the prevention and treatment of pruritus induced by opioids, including antihistamines, 5-hydroxytryptamine (5-HT3) antagonists, opioid receptor antagonists, non-steroidal anti-inflammatory drugs, propofol and droperidol. None of them have had satisfactory results.1-3,9,10 Naloxone and nalbuphine are opioid antagonist drugs used for this purpose and proven to be effective by different authors as a therapeutic agent for the reversion of pruritus induced by the neuraxial administration of opioids. Nalbuphine is an agonist-antagonist opioid and its analgesic effect and probable antipruritic effect are mediated by its action in mu and kappa receptors.4,10,11 These medications could play a role in the treatment of pruritus, with a disadvantage, however, because its preventive administration reduces its analgesic effictiveness.3
Other medications used for the treatment of pruritus are the 5-HT3 receptor antagonists. Ondansetron, for example, which has been successfully used in the treatment of neuraxial morphine-induced pruritus, because of its antipruritic effect and its role in the nociception, contrary to the use of opioid receptor antagonists.
The objective of our study was to compare the efficiency and safety of 4 mg of nalbuphine vs. 4 mg of ondansetron, for the treatment of neuraxial morphine-induced pruritus as post-operative analgesic in patients programed for elective surgery.
Material and methods
After the approval of the ethics Committee and the research Committee of the School of Medicine at the “Dr. José Eleuterio González” University Hospital of Universidad Autónoma de Nuevo León (UANL, by its Spanish acronym), we conducted a study from September 2012 to August 2013, a controlled, prospective, comparative and randomized clinical trial with 28 patients programed for surgery under neuraxial anesthesia and receiving epidural or subarachnoid morphine analgesia. Patients who agreed to participate in the trial had to sign an informed consent and meet the following criteria: ASA I and II patients, undergoing surgery with neuraxial anesthesia and presenting pruritus seconda- ry to the administration of epidural or subarachnoid morphine, either male and female between 18 and 50 years old, a signed consent form, neurologically intact and able to assess pain using the visual analogue scale (EVA). We excluded those patients with neurological alterations of the state of consciousness, patients with a history of allergy to nalbuphine and ondansetron and local anesthetics, patients under 18 and older than 50 years of age, patients who did not accept this type of administration of analgesia and patients with a dermatological condition.
All patients were administered intravenous 10 mg of metoclopramide and 50 mg of ranitidine intravenously as pre-anesthetic medications. We monitored all patients with a continuous electrocardiogram, pulse oximetry and noninvasive blood pressure every 5 minutes during the whole procedure; they received oxygen at 5 L per minute through a face mask. Subsequently, we applied a neuraxal block (epidural, subarachnoid or both), once applied we administered local anesthetic and 100 mcg of morphine (subarachnoid or epidural) using the following formula (-0.01 * age + 1.85 mg). We assessed the presence of pruritus at 30 minutes and 2, 6, 12 and 24 hours after morphine administration; the patients who presented pruritus were divided into 2 groups: group “O” (ondansetron) and group “N” (nalbuphine). We administered 4 mg of the corresponding drug to each group and we registered the presence of pruritus, nausea, vomiting, ramsay sedation scale, blood pressure, respiratory frequency and pain using the EVA scale from 0 to 10 (0 = no pain and 10 = intense pain) and the use of rescue medication.
The obtained results were gathered in a database developed using Microsoft excel® and analyzed using IBM SPSS® Statistics v. 20.0. We obtained traditional descriptive statistics, as well as observed frequencies in accordance with the administered medication (ondansetron and nalbuphine) through median and proportion hypothesis-testing, as the case may be for each type of variable (quantitative and qualitative respectively) with a confidence interval (CI) of 95%. Statistical association was determined using X2 with a 95% CI.
Results
A total of 28 patients were studied, randomly distributed into 2 groups of 14 patients each. The median age in the O group was 26.9 and 28.7 for the N group. All of the patients were female, programmed for a C-section. We performed 21 epidural blocks, 6 subarachnoid blocks and one combi- ned subarachnoid-epidural technique.
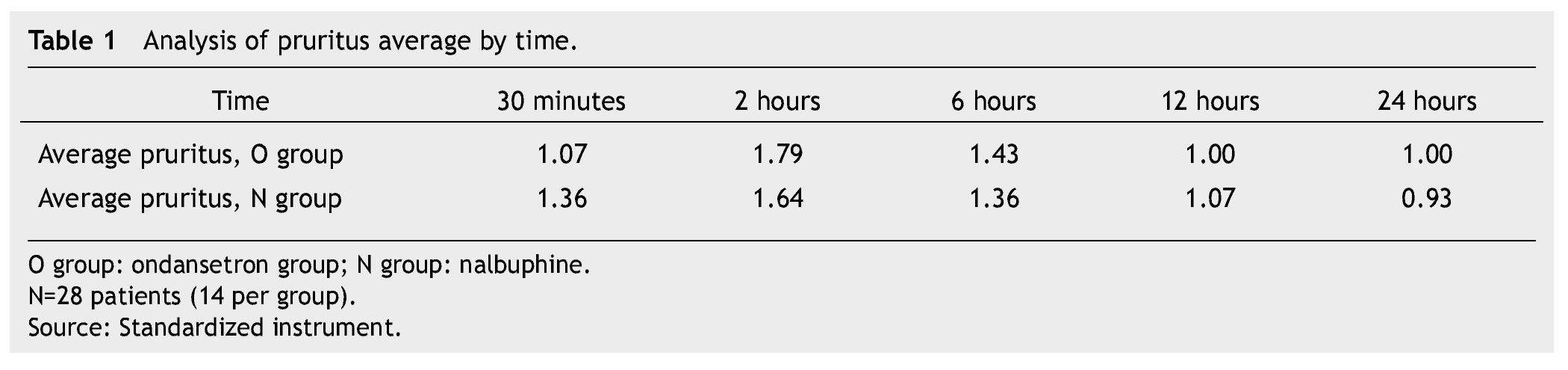
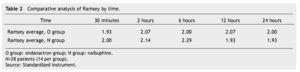
In both groups mild pruritus (itching) occurred in 15 patients during the first 12 hours, moderate pruritus in 13 patients between the 2nd and 6th hour subsequent to the administration of morphine; this was resolved in all patients. We did not find a significant difference between groups (Table 1).
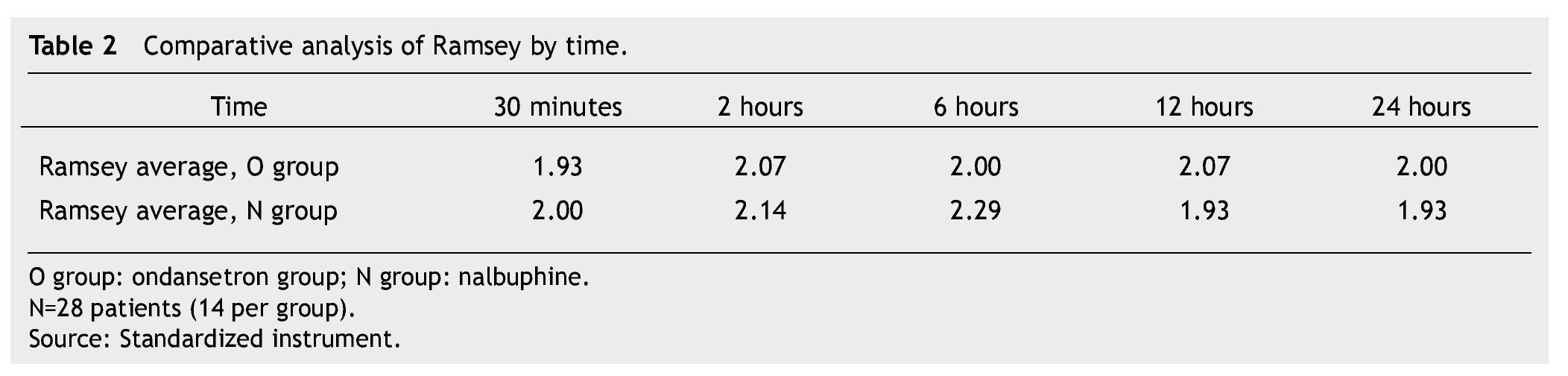
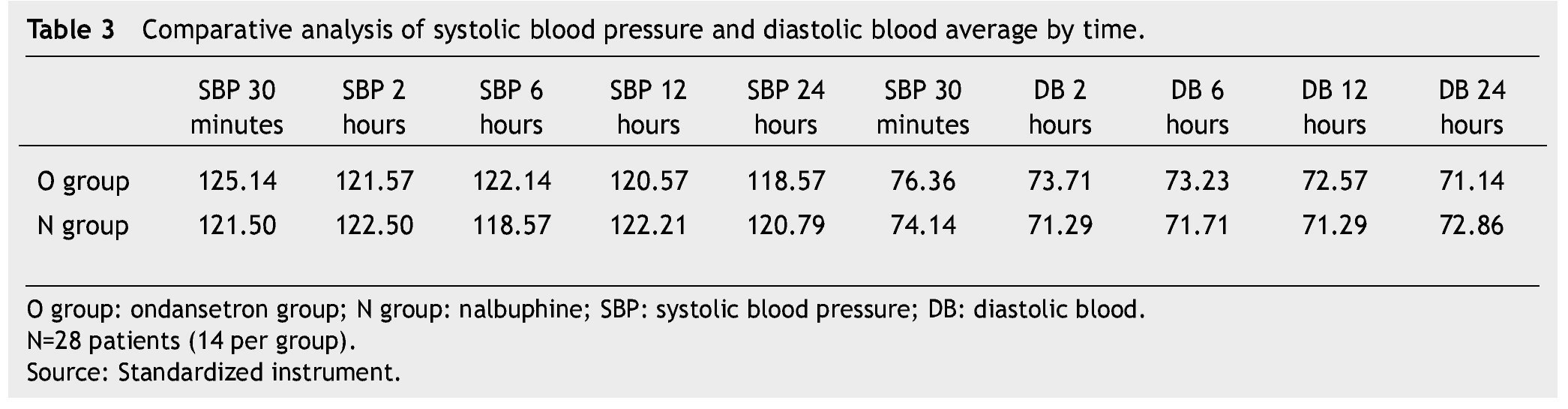
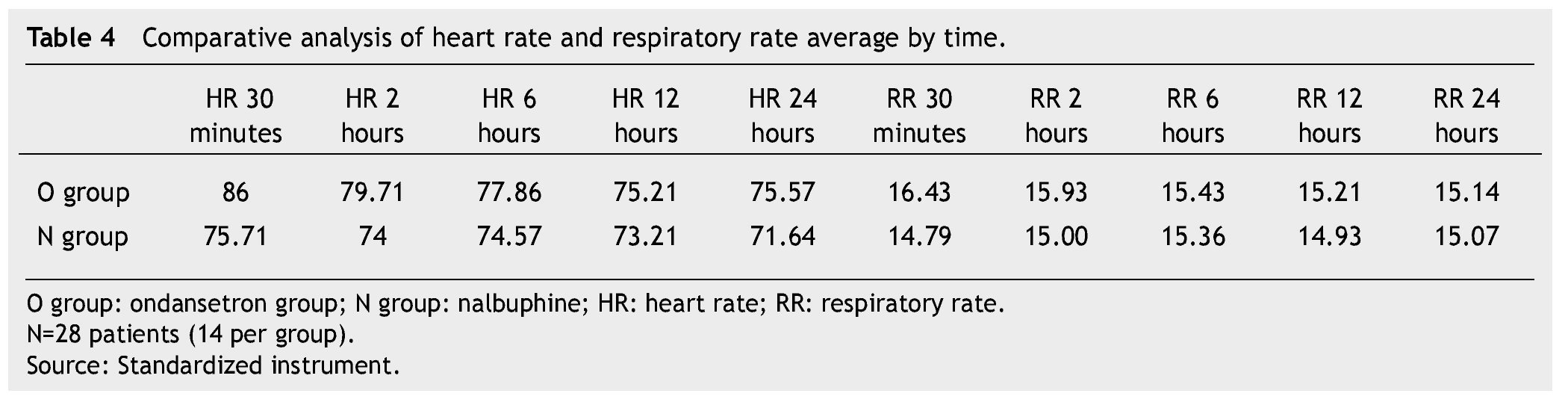
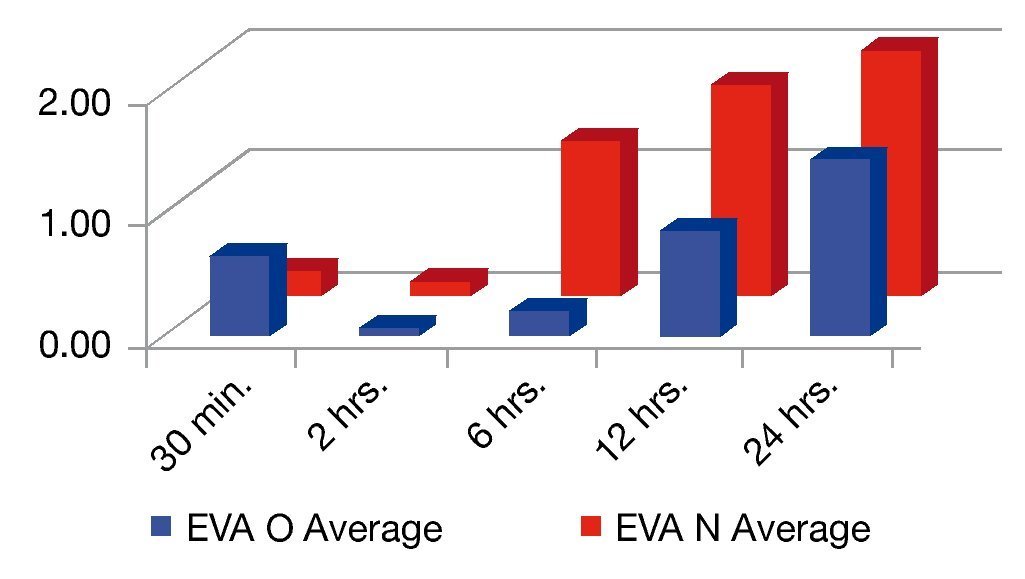
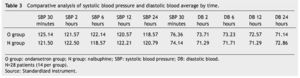
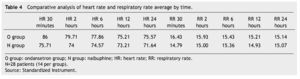
The presence of pain was assessed according to the EVA, a significant difference was found (p≤0.05) at 6 and 12 hours in favor of the O group. The average score in the EVA pain scale was less than 3, hence we did not administer any rescue medications (Fig. 1). When we analyzed the variables (blood pressure, ramsay, respiratory frequency, nausea and vomiting), we did not find a statistically significant difference (p>0.05) (Tables 2, 3 and 4).
Figure 1 Analysis of visual analogue scale average by time.
Discussion
Pruritus incidence subsequent to neuraxial morphine administration is approximately 60%, compared to 2%-10% of the patients treated systematically. Thus, its incidence depends on the route of administration. This symptom is common in postpartum women. The most affected areas are those innervated by the trigeminal, probably due to their higher number of opiate receptors in the spinal nucleus of the trigeminal nerve, causing patients to scratch their nose and the upper part of the face. There are several chemical mediators responsible for pruritus (histamine, serotonin, cytokines, growth factors, prostaglandins, etc.).1-3,5-7
There is evidence that opioids and the serotonergic system interact closely in the central nervous system. One example is ondansetron, a 5-HT3 antagonist with an anti-pruritic effect. In 1995 Fan reported that morphine can activate serotonin type-3 receptors in the spinal cord dorsal horn and bone marrow and that could possibly be the mechanism causing pruritus.1-3,9,10,12,13
Since 1999 there have been studies comparing ondansetron’s effectiveness in the treatment of epidural morphine-induced pruritus, where its effectiveness has been demonstrated.8,12-15
In our study, pruritus occurred more frequently between 2 and 4 hours in both groups. These results are consistent with those reported in medical literature.16 Pruritus caused by opioids can be extremely difficult to manage and approximately 10%-15% of the patients showed no response to naloxone.17
In our study, pruritus was effectively treated in both groups and did not require the use of rescue medications.
We did not observe changes in the level of pain in the O group; however, there was an increment in the EVA scale at 6 and 12 hours in the N group, probably because of its agonist-antagonist effect of the mu, kappa and delta receptors. Nevertheless, both groups had an EVA score lower than 3. We did not find differences in sedation levels in any of the groups during the period of study.
The presence of nausea and vomiting is common after the administration of opioids.18 Nausea occurs within the first 4 hours subsequent to the administration and vomiting occurs right after this. High dosages of opioids can cause arteriolar and venous dilatation, a decrease in peripheral vascular resistance and baroreceptors reflex inhibition, which can cause postural hypotension. Opioids cause dose-dependent bradycardia, probably because of sympatholytic and parasympatholytic mechanisms. There were no statistically significant hemodynamic changes during the entire trial. Even though opioids cause dose-dependent respiratory depression, there were no alterations in respiratory frequency in the patients with nalbuphine.9
This study shows that nalbuphine was well-tolerated, only displaying changes in the level of analgesia (EVA 3), which did not require rescue medication, unlike ondansetron, which did not display changes in analgesia levels and no side effects were reported. However, both medications proved to be safe and efficient for the treatment of neuraxial morphine-induced pruritus.
Conclusions
When comparing the efficacy and safety of 4 mg of ondansetron vs. 4 mg of nalbuphine for the treatment of neuraxial morphine-induced pruritus, the only significant difference found was the mean EVA at 6 and 12 hours, favoring the ondansetron group. However, both groups had an EVA of less than 3. Based on the obtained results we reject the null hypothesis, which states that the administration of intravenous ondansetron at 4 mg is not as effective as the administration of nalbuphine at 4 mg in the treatment of neuraxial morphine-induced pruritus.
Therefore, we consider that both treatments are effective and safe in the treatment of pruritus caused by neuraxial morphine.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
No financial support was provided.
Received: March 2014;
Accepted: May 2014
* Corresponding author:
Anesthesiology Service,
“Dr. José Eleuterio González” University Hospital.
Francisco I. Madero and Gonzalitos Avenue,
Mitras Centro, Z.P. 64460, Monterrey, N.L., Mexico.
Telephone: (81) 1681 8404.
E-mail address: drferretti@hotmail.com (U. Cruz-Ferretti).