To determine the effectiveness of three different combinations for the “loading phase” in the treatment of diabetic macular edema (DME), using bevacizumab (BVZ), triamcinolone (TCL) and subthreshold macular photocoagulation (SMPC).
MethodsExperimental, longitudinal, prospective, comparative and blind. Patients were randomly assigned to three treatment branches: Group 1: BVZ+SMPC (12 eyes), Group 2: SMPC+BVZ+TCL (7 eyes), Group 3: BVZ+TCL (11 eyes). Treatment with BVZ and TCL was given every 4 weeks for 3 months, SMPC was applied once at the beginning of treatment. Initial and final measurements of best corrected visual acuity (BCVA), central macular thickness (CMT) and intraocular pressure (IOP) were tested.
ResultsThe improvement in BCVA and the reduction in CMT was statistically superior in group of BVZ+SMPCwhen compared to the other groups. There were no differences in IOP.
ConclusionsCombined therapies in the “loading phase”are a good option when treating DME. Although the group with BVZ+SMPC obtained the best results, further studies with longer follow-up and a higher number of participants to establish this combined therapy as the first treatment option are required.
Diabetic macular edema (DME) is the main cause of visual loss in patients with diabetic retinopathy (DR). It is considered to be the number one cause of blindness at a productive age in developed countries.1–6 DME is the result of alterations of the inner and outer blood-retinal barriers (BRB) due to the imbalance between the inflammatory and angiogenic factors of the retinal pigment epithelium (PE) and the vitreoretinal interface. Among these, there is the vascular endothelial growth factor (VEGF), the hepatocyte growth factor and the interleukin 1B. The reduction in the pigment epithelium-derived anti-angiogenic factor, a potent anti-inflammatory, antioxidant and anti-angiogenic which regulates, among other things, VEGF levels, also plays an important role in DME pathophysiology.7–9 The treatment focuses on reestablishing BRB, modulating inflammatory and angiogenic factors. Among the current options to accomplish said effect, there are the thermal laser and intravitreal drug therapies (corticosteroids and anti-angiogenics).
The laser stimulates the PE, acting as a substance modulator for PEDF and VEGF. Moreover, the thermal destruction of the outer layers of the retina reduces the metabolic demand and oxygen expenditure with the consequent VEGF reduction.10–18 Triamcinolone is the main intraocular corticosteroid in the treatment of RD, DME and other neovascular and inflammatory diseases because it inhibits overregulation of inflammatory molecules and VEGF. Part of this regulation was completed through the reduction of vascular permeability in the retina by reducing the liberation of arachidonic acid derivatives, such as prostaglandins.19–22 Bevacizumab is a recombinant humanized monoclonal antibody (lgG1) which unifies all isoforms of VEGF-A. It was approved by the FDA in 2004 for metastatic colon cancer treatment. Since then, it has been successfully used in an unofficial manner to treat different ocular neovascular illnesses, such as age-associated macular degeneration, proliferative DR, neovascular glaucoma, premature retinopathy, macular edema secondary to retinal venous obstruction and DME, among others. Even though, to this day, it has not been approved by the FDA nor the COFEPRIS for its ophthalmologic use, the injection of 1.25–2.5mg in the vitreous cavity has been performed in a safe and effective manner.23–29 Different regimes in DME treatment have been described. The laser is recommended for its application in a selective manner and on a single occasion, and, if necessary, reapply it in intervals of no less than 12 weeks apart.18,30,31 Intravitreal pharmacological therapy has been proposed for the different ocular neovascular pathologies, from having one dose and repeating treatment as deemed appropriate by the examiner pro re nata (PRN), up to a monthly dose for 24 months, without regard to visual and anatomic changes.32–41 This study showed that the maximum visual and anatomical effect occurs during the first three doses, and those following them only helped to maintain the inactivity of the pathology; thus, the decision in the selection of the scheme during this “loading stage” is fundamental. The “treat and observe” regime is currently being proposed. This is to apply three doses in a row with an interval of 4 weeks in between these “loading doses” until accomplishing the maximum visual and anatomic effect, repeating the same treatment PRN.42 Based on the possible synergy between the laser, the corticosteroids and the anti-angiogenics, the combination between these has been utilized with a dual intention; to accomplish a greater visual and anatomic effect, and to accomplish the minimum number of repetitions in long-term treatment of this chronic degenerative illness.35,36,43–49 In spite of all of this, the question about which combination may be the best option remains unanswered.
ObjectiveTo evaluate effectiveness with three different treatment combinations in the “loading phase” of diabetic macular edema (DME); using bevacizumab (BVZ), triamcinolone (TCL) and subthreshold macular photocoagulation (SMPC).
Method and materialsControlled clinical, experimental, prospective, longitudinal, comparative and blind essay, including those patients from the Department of Ophthalmology at the “Dr. José Eleuterio González” University Hospital using the following inclusion criteria: male and female with diabetes (type I or II), 18 years of age or older, with a clinical and tomographic DME diagnosis, best corrected visual acuity (BCVA) higher than 20/400. Patients who did not present any of the exclusion criteria; presence of significant cataract (according to the researcher's criteria), diagnosis of glaucoma, vitreous hemorrhage, previous intraocular surgery, macular laser treatment and/or intravitreal drug therapy in the three months previous to the study. Patients who for any reason did not complete treatment or developed complications during treatment were eliminated. The protocol was evaluated and approved by our institution's Ethics Committee and registered under the code OF11-010. The study was conducted following the guidelines established in the Helsinki Declaration and the International Conference on Harmonization Guidelines for Good Clinical Practices. All patients signed an informed consent form respecting the Official Mexican Standards on the patients’ right to know everything about their illness and its possible treatment options.
Clinical diagnosis was made through fundoscopy, using a magnifying glass of 90 diopters and a Goldman contact lens and DME was considered as the central thickening of at least a diameter of 1500 microns, situating the center of this circle in the umbo foveolar. Tomographic diagnosis was performed whenever there was a central macular thickness (CMT) greater than 230 microns using the “Macular Thickness Map” scanning modality of the optical coherence tomography (OCT) using Stratus OCT™ by Carl Zeiss.
Baseline BCVA measurements were taken by means of distant subjective refraction with a Snellen primer. IOP was taken by means of an applanation tonometry from Goldmann and clinical and OCT findings were recorded.
Later, the randomized selection of the study groups was made, using the six-sided die technique: numbers 1 or 4 to group 1 (BVZ+SMPC), numbers 2 or 5 to group 2 (BVZ+TCL+SMPC), and 3 or 6 to group 3 (BVZ+TCL). In this study, the principal investigator, who evaluated the study at the beginning and finalized the treatment regimen during the “loading phase”, did not know which group each patient belonged to.
The laser was only applied at the beginning of treatment (week 0), with the aim of avoiding possible complications from the laser threshold. The shots were made on subthreshold (invisible) mode,50 using VISULAS™ 532s laser equipment (Carl Zeiss Meditec AG. Jena, Germany).
The pharmacological treatment was performed on week 0, repeating at weeks 4 and 8. A dose of 1.25mg in 0.05ml of BVZ, commercial name Avastin™ (Genentech Inc., South San Francisco, CA, USA/Roche Mexico) was applied each session. The TCL utilized was ATLC™ (conservative-free), distributed by GRIN laboratories, Mexico, at a rate of 2mg in 0.05ml every injection. The procedure was performed in the “cure room,” with prior asepsis and antisepsis of the eyelids and ocular surface with a solution of povidone-iodine at 5% (BetadineMR Alcon Laboratories Inc., Fort Worth, TX) for 3min and posterior irrigation with a balanced saline solution. The topical anesthesia applied was tetracaine (Ponti™ Laboratorios Sophia, S.A. de C.V., Guadalajara, Mexico). The injection was made via pars plana in the superotemporal quadrant (4mm from the corneal limbo in phakic eyes and 3.5mm in pseudo-phakic eyes). A drop of moxifloxacin (Vigamoxi™, Alcon Laboratories Inc., Fort Worth, TX) was applied as a wide-spectrum antibiotic at the end of the procedure, which was used prophylactically at a rate of one drop every 6h for three consecutive days.
The IOP, BCVA, clinical findings and CMT were documented on week 12 after the beginning of treatment, and these results were compared with the baseline measurements. A statistical analysis was made by the IBM SPSS Statistics software, with a descriptive analysis of the data, which was compared to the average through the Student's t-test for related samples. p<.05 was determined to be a statistically significant difference.
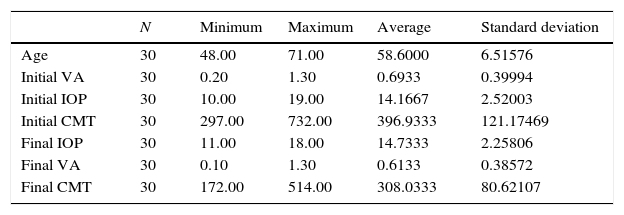
ResultsOur study included 30 eyes from 30 patients, with ages between 48 and 71 years old, an initial BCVA of .2 Logmar (20/30) to 1.3 Logmar (20/400), an initial CMT average of 396.93μm and an initial average IOP of 14.16mmHg (Table 1). The global BCVA change was from .6933 to .6133 Logmar (p=.24), a statistically insignificant difference. The global CMT change was from 396.93μm to 308.03μm (p=.01), a statistically significant difference. There were no significant differences between the initial and final IOP, which was from 14.16mmHg to 14.77mmHg (p=.176) (Table 1).
Description of the studied population.
| N | Minimum | Maximum | Average | Standard deviation | |
|---|---|---|---|---|---|
| Age | 30 | 48.00 | 71.00 | 58.6000 | 6.51576 |
| Initial VA | 30 | 0.20 | 1.30 | 0.6933 | 0.39994 |
| Initial IOP | 30 | 10.00 | 19.00 | 14.1667 | 2.52003 |
| Initial CMT | 30 | 297.00 | 732.00 | 396.9333 | 121.17469 |
| Final IOP | 30 | 11.00 | 18.00 | 14.7333 | 2.25806 |
| Final VA | 30 | 0.10 | 1.30 | 0.6133 | 0.38572 |
| Final CMT | 30 | 172.00 | 514.00 | 308.0333 | 80.62107 |
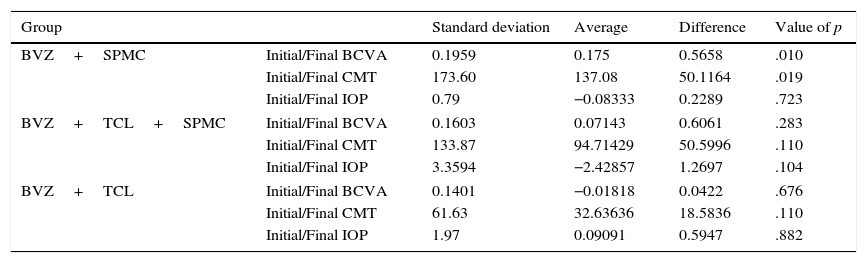
In the group analysis, we found that the average change in BCVA in group 1 (12 eyes, BVZ+SMPC) was from .64 to .46 Logmar (p=.01), a statistically significant difference compared to group 2 (7 eyes, SPMC+BVZ+TCL) which was from .74 to .67 (p=.28) and group 3 (11 eyes, BVZ+TCL) which was from .71 to .73 (p=.67).
In the same way, the decrease of CMT in group 1 was from 456.5μm to 319.41μm (p=.019), a statistically significant value in comparison to group 2, which showed a decrease of 385.28μm to 290.57μm (p=.110), and group 3, which showed a decrease of 339.36μm to 306.72μm (p=.110). There were no significant differences in IOP change in any of the 3 groups (Table 2). Two patients from group 3 (BVZ+TCL) and one from group 2 (SPMC+BVZ+TCL) showed cataract progression which required surgery more than IOL placement.
Results by groups.
| Group | Standard deviation | Average | Difference | Value of p | |
|---|---|---|---|---|---|
| BVZ+SPMC | Initial/Final BCVA | 0.1959 | 0.175 | 0.5658 | .010 |
| Initial/Final CMT | 173.60 | 137.08 | 50.1164 | .019 | |
| Initial/Final IOP | 0.79 | −0.08333 | 0.2289 | .723 | |
| BVZ+TCL+SPMC | Initial/Final BCVA | 0.1603 | 0.07143 | 0.6061 | .283 |
| Initial/Final CMT | 133.87 | 94.71429 | 50.5996 | .110 | |
| Initial/Final IOP | 3.3594 | −2.42857 | 1.2697 | .104 | |
| BVZ+TCL | Initial/Final BCVA | 0.1401 | −0.01818 | 0.0422 | .676 |
| Initial/Final CMT | 61.63 | 32.63636 | 18.5836 | .110 | |
| Initial/Final IOP | 1.97 | 0.09091 | 0.5947 | .882 | |
Since the first results of ETDRS were published in 1985, macular laser became, and still is, the “gold standard” for the treatment of DME.18 Despite this, laser offers suboptimal results, never mind the possible complications due to the burning of the external retina.In 2008, Faghihi et al.36 demonstrated that a single dose of BVZ or BVZ+TCL showed superiority in diminishing CMT with patients with DME in comparison to the laser alone. Regardless, the effect of BVZ on monotherapy was short, and the decrease of CMT with an improvement of BCVA only correlated the BVZ+TCL group.36 In 2010, Solaiman et al. compared laser and BVZ treatment against the monotherapy of either one, and his results indicated an improvement in the BCVA and CMT in the groups that were treated with BVZ in combination with laser as a starting therapy.48 In 2011, DRCR.net published results comparing combined therapy using macular lasers and BVZ or TCL. After receiving panphotocoagulation laser treatment, the visual improvement was greater in groups that received BVZ or TCL, although there were no differences in muscular thickness, suggesting that combined therapies utilizing laser+BVZ or laser+TCL were superior to laser monotherapy.51 The same year, Wang et al. demonstrated the beneficial effects of BVZ as a monotherapy or in combination with TCL in the treatment of DMC, without there being a difference between the two groups. In 2012, Soheilian et al.52 published results comparing BVZ monotherapy, BVZ+TCL and macular laser monotherapy. The group with the laser did not obtain a significant improvement on BCVA, but although the BCVA improvement in the BVZ group was significant during the first 6 months, there was no significant difference between the BVZ group and the combined treatment group (BVZ+TCL) at the end of the treatment. Although the CMT reduction was greater in the BVZ group, there were also no significant differences between the 3 groups.52 To our knowledge upon the printing of this publication, the only study to utilize a BVZ+TCL+macular laser combined triple therapy was published by Chan et al. in 2012,53 who compared the triple therapy to laser monotherapy. Regardless, unlike our study, TCL administration was subtenonian. They reported an important decrease and sustained CMT in the combined therapy group, when compared to laser monotherapy.53
The majority of the reports which include combined therapies in DME demonstrate the superiority of any one of them over laser monotherapy,25,33,36,40,41,43,47–49,52,53 and the small impact when using TCL.26,36,45–47,51–53 Our results indicate that the BVZ+laser combined therapy during the “loading phase” is significantly superior to therapies that included TCL, in achieving an BCVA improvement and a CMT decrease, and we suggest that this combination be repeated PRN in long-term follow-up.
One possible limitation, owing to the methodological design of our study (which did not include monotherapies) is that we could not conclude, as previous publications have, if the combination of anti-angiogenic+laser is equal to or superior than monotherapy with anti-angiogenics. Regardless, this was not the object of our study. Another of the limitations of our study is that we only evaluated the therapy during the “loading phase.” It would be interesting to follow-up on these patients in the long term, with the aim of determining if this therapeutic combination could additionally be effective in prolonging the intervals of the retreats in the long term.
ConclusionOur study shows that out of the combined therapies during the “loading phase” in DME treatment, the combination of BVZ and SMPC was the best option. However, further studies are necessary, with a longer follow-up period and a larger number of participants, to establish this alternative as a first treatment option.
Conflict of interestThe authors have no conflicts of interest to declare.
FundingNo financial support was provided.