Background
Primary biliary cirrhosis (PBC) is characterized by nonsuppurative cholangitis with granulomatous destruction of the interlobular bile ducts, which may lead to advanced fibrosis, cirrhosis and the complications of portal hypertension.1 The worldwide prevalence of PBC varies is from 1 in 2500 to 1 in 100 000 and a female predominance exists with an estimated ratio of 9 to 1 women to men.2,3 In Canada, up to 10% of the patients who are listed for liver transplantation have a diagnosis of PBC.4
PBC is considered an autoimmune disease because 95% of patients develop anti-mitochondrial antibodies (AMA), which are highly specific for this disease. Moreover, up to 60% of patients make anti-nuclear antibodies (ANA) that have reactivity with several proteins found in the nucleus that include rim proteins, such as gp210 and centromere proteins.
AMA binds the lipoylated domains of the oxo-acid dehydrogenase proteins, and these antibodies are mainly directed towards the pyruvate dehydrogenase-E2 (PDCE2) and PDC-E3 binding protein complex.3 It is thought that patients make AMA because a protein resembling PDC-E2 is found aberrantly expressed and in large amounts on the cell surface of biliary epithelium and in lymphoid tissue.5-7 However, the role that AMA play in the development of bile duct damage is poorly understood. For example, we know that PBC patients who make AMA have a similar clinical course than AMA-negative patients.5 In addition, immunosuppression treatment has showed limited efficacy, and drug related side effects has limited the use of these agents in PBC patients.8
Management of Primary Biliary Cirrhosis
At present, there are limited options for the management of PBC. The progressive loss of bile ducts leads to accumulation of bile within the liver resulting in fibrosis. The only licensed therapy is ursodeoxycholic acid (UDCA), a water-soluble bile salt that acts as a choleretic to help excrete bile from the liver.2,3 With UDCA treatment, one third of patients normalize their liver function tests and a similar proportion develops sufficient benefit to avoid progression to liver failure. It is estimated that the survival rates for PBC with UDCA treatment have risen to more than 80% at 10 years,3 whereas those that develop end stage liver disease clearly benefit from liver transplantation.
Of interest, after liver transplantation PBC may reoccur in the allograft of up to a third of patients, suggestive of an infectious disease process. For example, 70% of recipients have AMA in serum as well as the disease specific "mitochondrial" phenotype in biliary epithelium and histological evidence for recurrent PBC could be observed in up to 40% of patients with granulomatous destruction of bile ducts after transplantation.2,4,9-11
It has also been reported from many transplant programs that more potent immunosuppressive regimens using the calcineurin inhibitor tacrolimus, accelerates the onset and severity of recurrent disease; whereas the use of weaker calcineurin inhibitor, such as cyclosporine A, reduces the incidence of recurrent disease.4,9,10 In an study performed in our center, 58% of patients on tacrolimus had recurrent PBC at 10 years, compared to 13% of patients on cyclosporine A.4 It is also notable that cyclosporine inhibits replication of many viral agents by binding cyclophilins and also has demonstrable antiviral activity against betaretroviruses (vide infra).12
Genetic factors associated with Primary Biliary Cirrhosis
An increased frequency of PBC has been reported among family members of patients with PBC (1% to 7%), and this disease has higher prevalence in monozygotic, compared to dizygotic twins.13 Moreover, some allelic variations have been found to be associated with higher risk for PBC, including the human leucocyte antigen (HLA) genes class II region [DR, DQ]13-16 in the adaptive immune system [IL-12/interferon g cytokine axis]15,17 and the cytotoxic T-lymphocyte associated antigen 4.18,19 These findings suggest that PBC patients may be genetically predisposed to a general disturbance in host response to environmental agents.
Environmental factors Associated with Primary Biliary Cirrhosis
There are other data on and above observations from recurrent disease in liver transplant patients that suggest an infectious etiology of PBC.20,21 For example, unrelated family members and care providers are at higher risk for developing PBC, suggesting the role of environmental factors in the pathogenesis of this disease.22,23 Also, PBC clusters in geographical regions23,24 and migration studies report that children develop a similar incidence of PBC observed in their adopted host country rather than their land of birth.25 Also, an increased risk and clustering of PBC cases has been found near toxic waste disposal sites, adding strength to the notion that environmental factors may play a key role in the development of the disease.26,27 Several environmental factors, such as Chlamydia pneumonia,28,29 other bacteria30 and xenobiotics have been implicated in the etiology of PBC, but there is no direct evidence that links these agents with PBC.28,31-33
Betaretrovirus related to mouse mammary tumor virus in Primary Biliary Cirrhosis
Preliminary studies reported viral antibody reactivity, viral sequences in liver tissue, and virus-like particles in biliary epithelium from patients with PBC.5,20,21,34 Then, in 2003, a human betaretrovirus (HBRV) was characterized in patients with PBC.7 The HBRV was found to have 97% homology with the "human mammary tumor virus" sequences found in human breast cancer sample35 and phylogenetic studies have showed that is genetically indistinguishable from the "mouse mammary tumor virus" (MMTV).36
Serological studies in patients with PBC have demonstrated that 70% have serum reactivity to betaretrovirus infection.37 This virus has been found predominantly in lymphoid tissue, rather than the liver, which is consistent with findings of MMTV infection in mice.38 Using reverse transcriptase (RT)-PCR and immunochemistry in frozen tissue samples, 75% of lymph node samples of patients with PBC were positive for viral protein and nucleic acid determination;7 whereas, liver samples showed no evidence of viral proteins and only 29% of PBC patients were positive for HBRV RNA.
However, other groups have not been able to confirm an association of betaretrovirus infection with PBC. For example, one laboratory could not detect viral DNA in liver's samples using a single round of PCR,39 and a different group found the virus in only 5% of patients with PBC using nested PCR of hepatic DNA.40 These results suggest that the HBRV DNA is rarely detected in the liver (~5%) using a nested-PCR technique; however, the detection rate in PBC patients is as high as 70% when viral RNA is assessed in lymphoid tissue7 (Figure 1). Accordingly, more studies are required to confirm the true prevalence of viral infection in patients with PBC.
Figure 1. Prevalence studies conducted in patients with PBC showing that the use of more sensitive techniques for identifying HBRV nucleic acid leads to a higher detection rate in separate tissue compartments.
Betaretrovirus and the mitochondrial phenotype of PBC of aberrant PDC-E2 expression
Both, HBRV and MMTV are associated with the PBC mitochondrial phenotype in mouse models,41 and in humans.6,37 Using antibodies reactive with the MMTV capsid (p27CA) protein, HBRV has been detected in cells with aberrant PDC-E2 expression in lymph nodes of PBC patients and not in controls. To date, lymph nodes are the major reservoir of HBRV identified in humans and co-cultures of PBC lymph nodes have been used to isolate HBRV.42 Similarly, homogenates of lymph nodes in co-culture with normal biliary epithelial cells (BEC) have been used to establish the PBC in vitro model.6 In these studies, normal BEC developed cell surface PDC-E2 expression in co-culture with PBC lymph node homogenate; whereas, control lymph nodes had no such effect. The development of the PDC-E2 expression in BEC was accompanied by evidence of HBRV infection with anti-p27CA reactivity as well as RT-PCR evidence of infection with passage of HBRV or MMTV in the supernatants.7 These findings provide a possible conceptual model for viral induction of autoimmunity in PBC.7,30
Viral infection triggers the cell surface expression of PDC-E2, and then incorporates host proteins in either the budding particle or as part of exosomal multi-vesicular bodies, as hypothesized in other viral infections.7,30
Mouse models of Primary Biliary Cirrhosis
Traditionally, mouse models of PBC that make AMA have been difficult to create and previous studies have demonstrated that is difficult to produce AMA or any liver disease process by immunizing different strains of mice with PDC-E2.43-45 It is notable, therefore, that spontaneous AMA production has been found in many mouse models with immune deficiency46 as AMA are highly specific for patients with PBC, although these antibodies may occasionally be found in other autoimmune diseases, such as systemic lupus erythematosus or autoimmune hepatitis.2,3,47 In contrast, many immune deficient mouse models spontaneously produce AMA. These models include the NOD.c3c4, that develops granulomatous cholangitis, as well as the T cell TGF-b receptor II dominant-negative (dnTGFBRII), IL-2Ra-/-, and the scurfy mouse lacking T regulatory cells.48 While the NOD.c3c4 mouse has the essential features of PBC with progressive biliary disease and dies from liver failure, the other models develop diffuse extra-hepatic inflammatory disease and usually die from multi-organ failure.48 Of interest, most of these mouse models have evidence of MMTV infection in the same cells that have the mitochondrial phenotype of PBC with aberrant PDC-E2 expression, suggesting that MMTV is linked with the autoantigen expression and loss of tolerance to mitochondrial proteins.48 Indeed, the NOD. c3c4 mouse has evidence of MMTV in inflamed bile ducts, suggesting the possibility that MMTV triggers cholangitis in this model.20,21
Antiviral studies in NOD.c3c4 mouse model of PBC.
The NOD.c3c4 mice have subsequently been used to test whether MMTV may play a central role in the autoimmune biliary disease process by inhibiting viral infection with anti-retroviral therapy or neutralizing antibodies against MMTV (anti-MMTV).20,21 Following therapy, NOD.c3c4 mice were assessed for reduction of viral replication in the liver as well as improvements in hepatic biochemistry and histology.49 In these studies, mice were treated with combination reverse transcriptase inhibitors, such as CombivirTM, a formulation including Lamivudine (3TC) and Zidovudine (AZT) or TruvadaTM, a combination of Tenofovir (TFV) and Emtricitabine (FTC), as well as highly active anti-retroviral therapy (HAART) a regimen containing both reverse transcriptase inhibitors and protease inhibitors such as KaletraTM that includes Lopinavir and Ritonavir (LPVr). These combinations have been shown to inhibit the MMTV in vitro.12,50
We found that all antiviral combinations and anti-MMTV significantly improved histological disease in the NOD.c3c4 model.51 Also, mice treated with HAART or TFV/FTC (TruvadaTM) alone, developed significant reduction in hepatic biochemistry. However, mice treated with AZT/3TC (CombivirTM) alone did not experience significant biochemical benefit at the end of study despite improvements during therapy. Furthermore, these mice did not derive adequate suppression of MMTV levels within the liver49 and subsequent studies showed that these mice developed mutations in the MMTV pol gene suggestive of viral resistance to treatment on Combivir alone. These data support the hypothesis that biliary disease in the NOD.c3c4 mouse is triggered by MMTV infection. Furthermore, we believe that this mouse may provide a good in vivo model to test antiviral therapy for patients with PBC, as MMTV and HBRV are closely related viruses.
Anti-retroviral Treatment in Patients with Primary Biliary Cirrhosis
Translational studies have been conducted using combination anti-retroviral therapy to determine whether there is any clinical benefit from intervention.52 Preliminary studies using AZT/3TC (CombivirTM) therapy to treat PBC patients have showed significant histological benefit in bile duct injury, ductopenia and necroinflammatory score.52 One of the notable results from these studies was the return of bile ducts (Figure 2) as no other therapy has reversed ductopenia in patients with PBC.53 In a randomized controlled trial with AZT/3TC, the stated endpoints were not achieved,54 primarily because the endpoints, such as normalization of alkaline phosphatase, were too strict. A recent review summarizes more appropriate endpoints for randomized controlled trials for patients with PBC.55 It is important to note that some patients with PBC developed evidence of resistance to therapy with biochemical rebound,56 similar to the observations from NOD.c3c4 mice treated with AZT/3TC. However, it is also important to mention that AZT/3TC treatment was associated with significant improvement in clinical symptoms and liver biochemistry (Figure 3).54 Taken together with the data from the HAART studies in mice; it appears that patients with betaretrovirus infection may be better served with more antiviral potent regimens. Indeed, a recent report demonstrated that HAART completely normalized liver tests in a PBC patient with HIV and HBRV co-infection. This finding is encouraging, as most of the improvement occurred prior to initiation of standard therapy with ursodeoxycholic acid (UDCA).57
Figure 2. Serial liver biopsies assessed before and after (a) single and (b) combination antiviral treatment showing a significant reduction in necroinflammatory activity, bile duct injury and improvement in ductopenia for subjects receiving Combivir therapy.
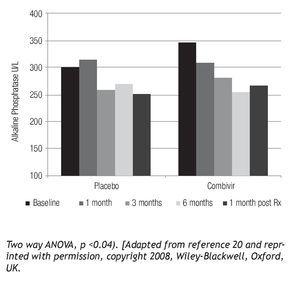
Figure 3. PBC patients treated with Combivir experienced a mean progressive drop in alkaline phosphatase (Pearson r = -0.9551, p < 0.05) with a rebound following discontinuation of therapy as well as a greater than 10% reduction compared to placebo.
In summary, the pathogenesis of PBC probably involves a combination of environmental and genetics factors. UDCA is the only approved treatment for PBC but many patients do not achieve a significant response. There is need for better treatments and there is a possibility that the studies of animal model as well as genetic and environmental factors that impact on PBC will provide novel insights for translational studies. Even though the pathogenesis of PBC remains poorly understood, preliminary data exist to support a link between the betaretrovirus and PBC. Accordingly, additional clinical trials using more potent antiviral therapies are warranted to authenticate the role of betaretrovirus infection in patients with PBC and to treat progressive disease in those unresponsive to UDCA.20,21
Correspondence author: Aldo J. Montano-Loza, M.D.
Zeidler Ledcor Centre, 130 University Campus, Edmonton, Alberta, Canada T6G 2X8.
Telephone: (780) 248 1892 fax (780) 248-1895.
E-mail:aldomontano-loza@med.ualberta.ca.
Recibido: Abril 2011.
Aceptado: Abril 2011