Changes in bone marrow (BM) architecture and its remodeling after hematopoietic stem cell transplantation (HSCT) following a reduced-intensity conditioning regimen for treating malignant hematologic diseases are poorly documented. We assessed these changes and their correlation with the clinical course to investigate the dynamics of this process.
Material and methodsHematology patients who received a reduced intensity conditioning (RIC) were followed after successful allogeneic or autologous HSCT. Patients had at least 100 days after hematopoietic grafting. Post-transplant BM architecture was assessed by expert examination of a trephine biopsy using age-adjusted scores and histopathology results were contrasted with clinical post-transplant evolution to determine meaningful associations.
ResultsTwenty-seven HSCT recipients, 19 allogeneic and 8 autologous, were studied at a median 155 (100–721) days post-transplant. Eleven (40.7%) had hypocellular biopsies with around 50% of the expected cell composition for a normal individual at that age. Patients with increased fibrosis had lower peripheral white blood cell counts compared to those with normal reticulin distribution (p=0.015). A decrease in overall survival (OS) was documented in the group with more severe myelofibrosis at a median 813 days of follow-up. Infectious complications were more frequent in patients receiving an allogeneic (n=6) compared with the recipients of an autologous transplant (n=0).
ConclusionsDespite normal peripheral blood count and composition, significant post-transplant hypocellularity, revealing incomplete bone marrow reconstitution, was documented after HSCT in patients conditioned with a RIC regimen. Severe BM fibrosis was associated to decrease OS.
The bone marrow (BM) is one of the largest organs in the body,1 it accounts for approximately 5% of the weight in adults, its analysis by means of an aspirate and/or biopsy is essential in the diagnosis of hematologic and non-hematological diseases, to detect metastatic spread, storage disorders, in the staging of solid tumors, in performing microbiological culture in patients with unexplained fever, HIV infection, and in the follow-up of patients after chemotherapy or transplantation.2
The success of hematopoietic stem cell transplantation for the treatment of hematologic malignancies is dependent on efficient eradication of the malignant clone and the successful reconstitution of the host's hematopoiesis and immune system. Reduced Intensity Conditioning (RIC) is a non-myeloablative regimen with the same survival as with myeloablative regimens3 in addition there is an earlier immune reconstitution and decreased morbidities. The principal mechanism of this conditioning is the graft-versus-tumor response, suggested by the mixed chimerism observed in the host cellularity.
We have carried out hematopoietic stem cell transplants at our center for the past 25 years; since the year 2000 we accomplish hematopoietic grafting employing exclusively a RIC protocol.4,5
There are few clinical studies assessing histological reconstitution of the bone marrow in patients with malignant hematologic diseases grafted after RIC regimens.6–8 We documented this process, its relationship with peripheral blood count characteristics, and relevant clinical events in our patients.
Material and methodsPatientsTwenty-seven clinically asymptomatic patients attending the Hematology Department at the Dr. José E. Gonzalez University Hospital of the School of Medicine of the Universidad Autónoma de Nuevo León, in Monterrey, México, who had passed day +100 after receiving an allogeneic or autologous HSCT, from January 2013 to December 2014, for diverse malignant or pre-malignant hematologic diseases fitting with the guidelines of the American Society for Blood and Marrow Transplantation,9 and accepted to participate in the study and give a bone marrow biopsy specimen for analysis were included. Informed consent was obtained from each patient. The Institutional Review Board and the Ethics Committee approved the protocol of the study. Clinical follow-up was conducted until August 2016.
Conditioning regimen, transplant procedure and follow-upThe administered RIC conditioning consists of a scheme with dosage based on ideal weight, which includes busulphan 4mg/kg p.o. on days −6 and −5; cyclophosphamide (CyA) 350mg/m2 once daily i.v. on days −4, −3 and −2; fludarabine 30mg/m2 i.v. once daily on days −4, −3 and −2; ib 5mg/kg p.o. starting on day −1; and methotrexate 5mg/m2 i.v. on days +1, +3, +5 and +11. CyA is continued through day 180, with adjustments to obtain serum CyA levels of 150–275ng/ml, and then tapered over 30–60 day.5 In patients with aplastic anemia, busulphan was not used and the cyclophosphamide dose was doubled from days −4 to −1. If graft-versus-host disease (GvHD) developed, CyA was tapered over longer periods. Ondansetron 1mg i.v. every hour over 4h after chemotherapy, an oral quinolone and an azole were used in all patients until granulocytes were >0.5×109/L.
Donors were stimulated with 10μg/kg of G-CSF subcutaneously for five days prior to CD34+ cell automated collection CD 34+ cells at a dose ≥2.5×106/kg of body weight were infused on day 0. Afterwards, patients were closely followed to document time to neutrophil and platelet recovery to ≥500/μl and ≥20,000/μl, respectively. Engraftment was also assessed by chimerism analysis by flow cytometry and clinical follow-up was carried out on an outpatient basis. Transplants in our center are performed as an outpatient procedure, in order to make this modality of therapy financially viable and to reduce the risk of hospital acquired post-transplant infections.4,5
HistopathologyBone marrow biopsies were obtained following standard procedure from the posterior iliac crest in all the cases through a Jamshidi needle. The BM tissue was fixed in 10% formaldehyde for at least 12h. Subsequently they were placed in decalcification solution for 2–3h. The tissue was processed for inclusion in paraffin and subsequently cut seven levels at 5 microns and stained with routine hematoxylin and eosin technique. All the biopsies were examined by an experimented hematopathologist with cellularity scores assigned following previously published guidelines.10 Pathologist was blinded with regard to transplant type and primary diagnosis. The parameters evaluated were percentage cellularity, histological features of trabecular and stromal bone; special stains, including Gordon stain in search of abnormal reticulin distribution, were performed. Bone marrow fibrosis (MF) was graded fitting the European Consensus. (MF-0 Scattered linear reticulin with no intersections corresponding to normal bone marrow; MF-1 Loose network of reticulin with many intersections, especially in perivascular areas; MF-2 Diffuse and dense increase in reticulin with extensive intersections, occasionally with only focal bundles of collagen and/or focal osteosclerosis; MF-3 Diffuse and dense increase in reticulin with extensive intersections with coarse bundles of collagen, often associated with significant osteosclerosis).11 In each cell line number of components and its maturation sequence was evaluated, and any abnormal morphological data was registered. Iron staining was performed in cases of anemia documented in the complete blood count and if histological changes suggestive of increased iron deposition were present.
Statistical analysisFor data analysis, SPSS v. 22.0 statistical package (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.) was used. The normality distribution was evaluated with the Kolmogorov–Smirnov test. Descriptive analysis was performed, obtaining means and standard deviation, medians and ranges according to the variables distribution calculated with chi square test. The differences between groups were analyzed with the Student's t-test for parametric variables and Mann–Whitney U test for nonparametric distribution. A two-sided p-value of 0.05 was considered statistically significant. Overall survival was determined with the Kaplan–Meier and method, calculating time, status, cumulative survival, and standard error with a 95% confidence interval. Equality of data distribution was estimated with the log-rank test.
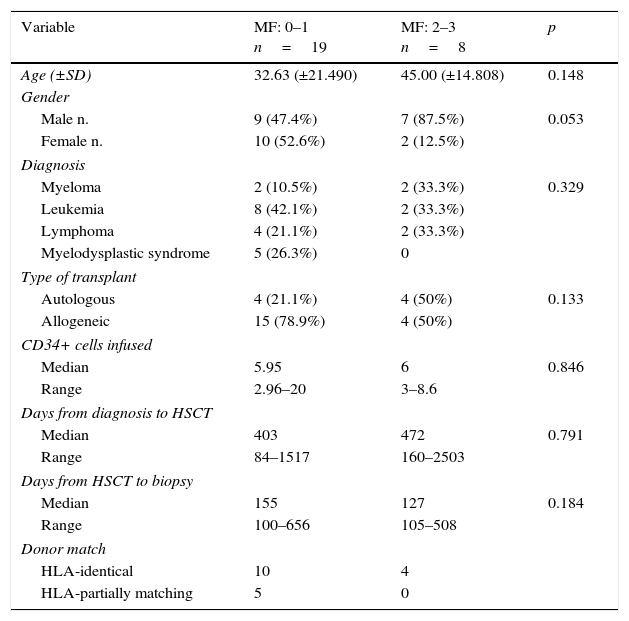
ResultsDemographic and clinical characteristics for the two groups are described in Table 1. Nineteen (70.4%) patients presented none or scarce myelofibrosis, whereas eight (29.6%) moderate to severe fibrosis. There were not differences in clinical characteristics between groups. A non-significant predominance of MF ≥2 on male patients, with 87.5%, was documented vs. 12.5% in females (p=0.53).
Clinical characteristics of hematology patients who received an hematopoietic transplant after reduced intensity conditioning (RIC) according to the grade of myelofibrosis.
| Variable | MF: 0–1 n=19 | MF: 2–3 n=8 | p |
|---|---|---|---|
| Age (±SD) | 32.63 (±21.490) | 45.00 (±14.808) | 0.148 |
| Gender | |||
| Male n. | 9 (47.4%) | 7 (87.5%) | 0.053 |
| Female n. | 10 (52.6%) | 2 (12.5%) | |
| Diagnosis | |||
| Myeloma | 2 (10.5%) | 2 (33.3%) | 0.329 |
| Leukemia | 8 (42.1%) | 2 (33.3%) | |
| Lymphoma | 4 (21.1%) | 2 (33.3%) | |
| Myelodysplastic syndrome | 5 (26.3%) | 0 | |
| Type of transplant | |||
| Autologous | 4 (21.1%) | 4 (50%) | 0.133 |
| Allogeneic | 15 (78.9%) | 4 (50%) | |
| CD34+ cells infused | |||
| Median | 5.95 | 6 | 0.846 |
| Range | 2.96–20 | 3–8.6 | |
| Days from diagnosis to HSCT | |||
| Median | 403 | 472 | 0.791 |
| Range | 84–1517 | 160–2503 | |
| Days from HSCT to biopsy | |||
| Median | 155 | 127 | 0.184 |
| Range | 100–656 | 105–508 | |
| Donor match | |||
| HLA-identical | 10 | 4 | |
| HLA-partially matching | 5 | 0 | |
MF, myelofibrosis; HSCT, hematopoietic stem cell transplant; SD, standard deviation; HLA, human leukocyte antigen.
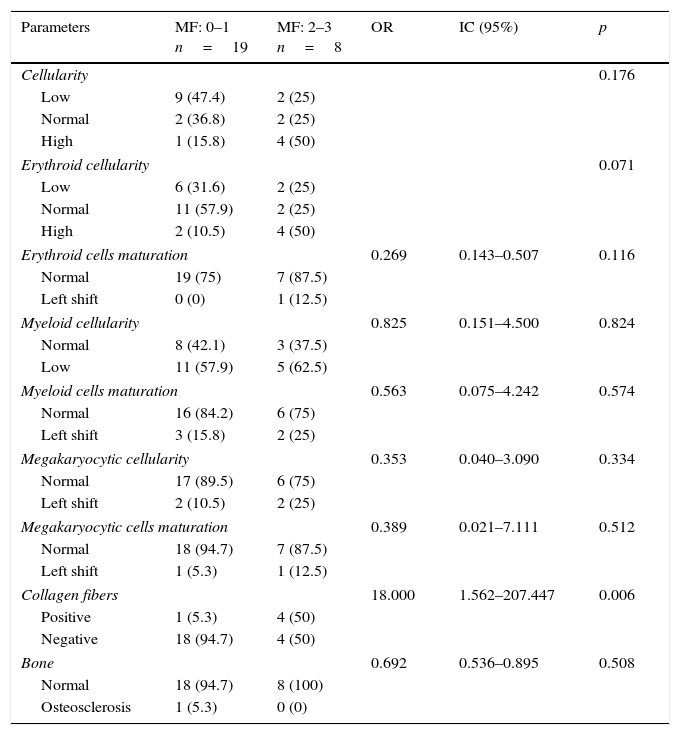
Histopathology evaluation showed hypocellularity in the bone marrow biopsy of 11 (40.75%) HSCT recipients, 9 (47.4%) in 0–1 MF and 2 (25%) in the group with 2–3 MF. Findings of diminished cellularity overlapped, as decreased erythroid series was found in 5 (62.5%). Myeloid series was decreased in 11 (57.9%) in the group with non or scarce MF and 5 (62.5%) patients with higher MF. In the whole cohort, an overall normally ordered maturation sequence was documented for the three hematopoietic cell series. Detailed results of all evaluated parameters are shown in Table 2.
Comparison of bone marrow characteristics and composition in patients with different grade of myelofibrosis after autologous hematopoietic stem cell transplant (HSCT) in 27 hematology patients conditioned with a reduced intensity scheme.
| Parameters | MF: 0–1 n=19 | MF: 2–3 n=8 | OR | IC (95%) | p |
|---|---|---|---|---|---|
| Cellularity | 0.176 | ||||
| Low | 9 (47.4) | 2 (25) | |||
| Normal | 2 (36.8) | 2 (25) | |||
| High | 1 (15.8) | 4 (50) | |||
| Erythroid cellularity | 0.071 | ||||
| Low | 6 (31.6) | 2 (25) | |||
| Normal | 11 (57.9) | 2 (25) | |||
| High | 2 (10.5) | 4 (50) | |||
| Erythroid cells maturation | 0.269 | 0.143–0.507 | 0.116 | ||
| Normal | 19 (75) | 7 (87.5) | |||
| Left shift | 0 (0) | 1 (12.5) | |||
| Myeloid cellularity | 0.825 | 0.151–4.500 | 0.824 | ||
| Normal | 8 (42.1) | 3 (37.5) | |||
| Low | 11 (57.9) | 5 (62.5) | |||
| Myeloid cells maturation | 0.563 | 0.075–4.242 | 0.574 | ||
| Normal | 16 (84.2) | 6 (75) | |||
| Left shift | 3 (15.8) | 2 (25) | |||
| Megakaryocytic cellularity | 0.353 | 0.040–3.090 | 0.334 | ||
| Normal | 17 (89.5) | 6 (75) | |||
| Left shift | 2 (10.5) | 2 (25) | |||
| Megakaryocytic cells maturation | 0.389 | 0.021–7.111 | 0.512 | ||
| Normal | 18 (94.7) | 7 (87.5) | |||
| Left shift | 1 (5.3) | 1 (12.5) | |||
| Collagen fibers | 18.000 | 1.562–207.447 | 0.006 | ||
| Positive | 1 (5.3) | 4 (50) | |||
| Negative | 18 (94.7) | 4 (50) | |||
| Bone | 0.692 | 0.536–0.895 | 0.508 | ||
| Normal | 18 (94.7) | 8 (100) | |||
| Osteosclerosis | 1 (5.3) | 0 (0) | |||
MF, myelofibrosis.
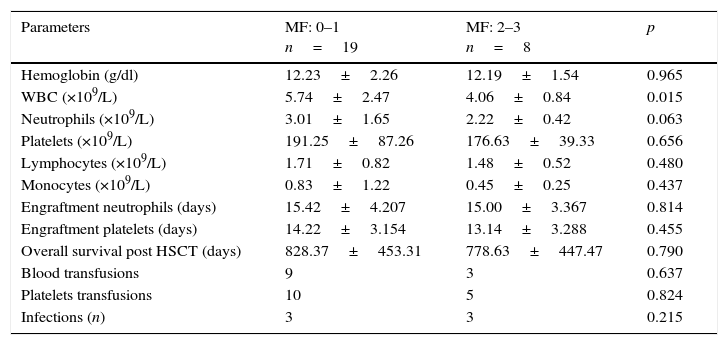
In Table 3 we compared the complete blood cells after the transplant and the clinical characteristics between the groups. Patients with marrow fibrosis MF 0–1 had a higher WBC count with median of 5.74×109/L, whereas the group with MF 2–3, had a median of 4.06×109/L (p=0.015).
Comparison of peripheral complete blood count (CBC) values according to bone marrow's myelofibrosis (MF) in histological examination. Twenty-seven recipients of a hematopoietic graft with hematologic disease were included.
| Parameters | MF: 0–1 n=19 | MF: 2–3 n=8 | p |
|---|---|---|---|
| Hemoglobin (g/dl) | 12.23±2.26 | 12.19±1.54 | 0.965 |
| WBC (×109/L) | 5.74±2.47 | 4.06±0.84 | 0.015 |
| Neutrophils (×109/L) | 3.01±1.65 | 2.22±0.42 | 0.063 |
| Platelets (×109/L) | 191.25±87.26 | 176.63±39.33 | 0.656 |
| Lymphocytes (×109/L) | 1.71±0.82 | 1.48±0.52 | 0.480 |
| Monocytes (×109/L) | 0.83±1.22 | 0.45±0.25 | 0.437 |
| Engraftment neutrophils (days) | 15.42±4.207 | 15.00±3.367 | 0.814 |
| Engraftment platelets (days) | 14.22±3.154 | 13.14±3.288 | 0.455 |
| Overall survival post HSCT (days) | 828.37±453.31 | 778.63±447.47 | 0.790 |
| Blood transfusions | 9 | 3 | 0.637 |
| Platelets transfusions | 10 | 5 | 0.824 |
| Infections (n) | 3 | 3 | 0.215 |
MF, myelofibrosis; WBC, white blood cells; HSCT, hematopoietic stem cell transplant.
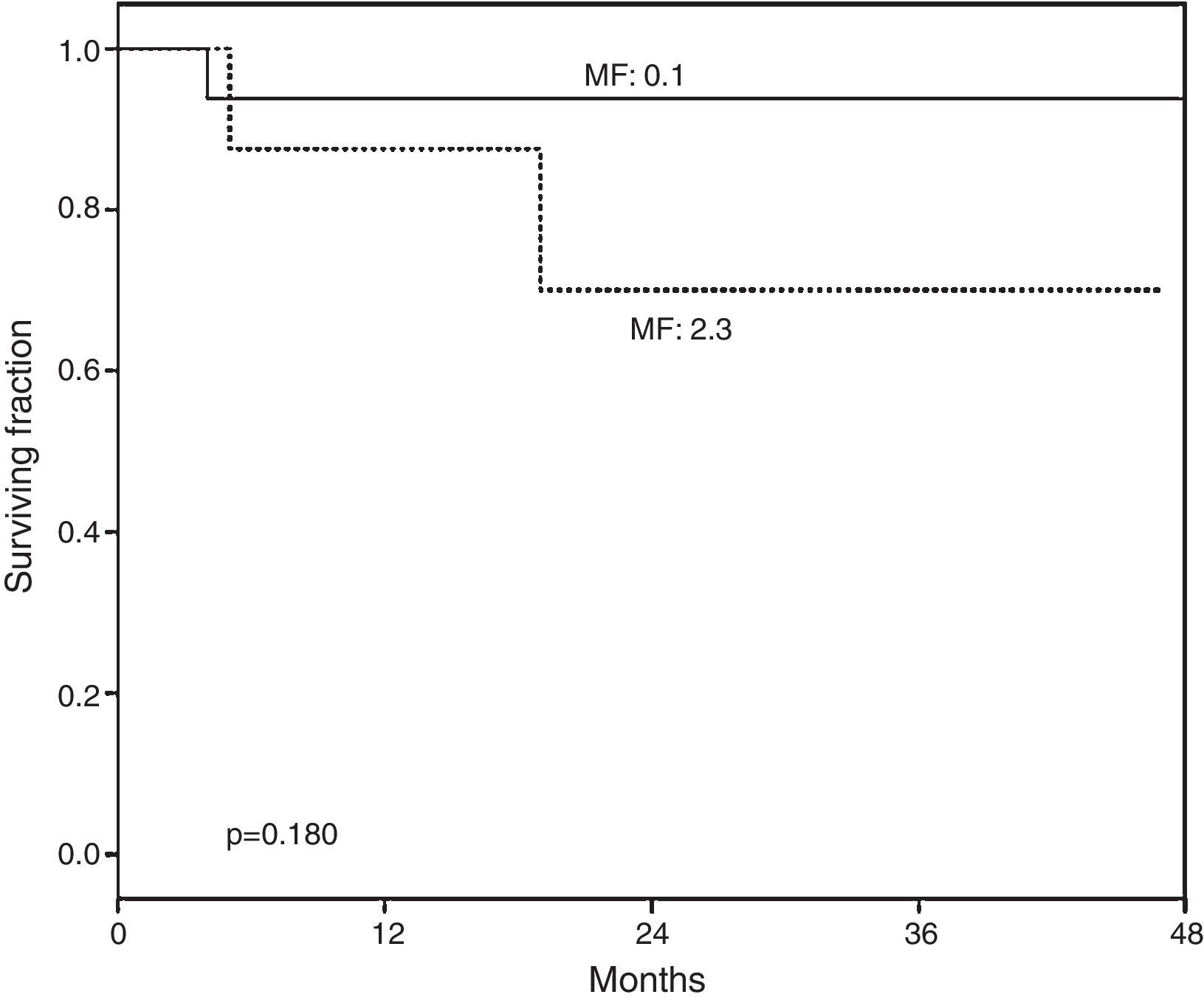
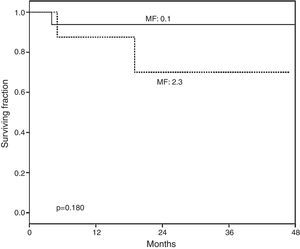
At a median post-HSCT follow-up of 813 days a non-significant higher overall survival was observed in the group of patients with none or mild vs. severe fibrosis (p=0.180) (Fig. 1).
Overall survival (OS) according to myelofibrosis after hematopoietic grafting. A non-significant tendency to lower survival was found in recipients with higher bone marrow fibrosis (MF). MF 0–1: normal-scarce reticulin fibers (n=19). MF 2–3: moderate-abundant reticulin fibers (n=8). Median of follow-up for MF 0–1 was 828 (102–1496) days and for MF 2–3 778 (158–1436) days.
In general, we observed clusters of erythroid and myeloid cells, as well as patchy megakaryocyte precursors with immature hyperlobulated nucleus. Furthermore, in 19 (70.37%) samples with non-or a low number of reticulin fibers were observed, while in the other 8 (29.63%) a diffuse reticulin pattern was found, scored as MF ≥2, producing disturbance in the bone marrow architecture and a smaller space for hematopoietic cells.
DiscussionHematopoietic stem cell transplantation is now a standard procedure in the treatment of many hematologic and non-hematologic diseases, knowledge and expertise has grown revealing many complexities on the dynamics of generating a new hematopoietic system and the corresponding immune changes that in successful cases lead to a normalized immunohematopoiesis; we have previously reported these changes in our patients.12 However, studies documenting remodeling changes in bone marrow architecture in the context of clinical post-transplant outcome are scarce.
It has been documented in adult subjects, especially those aged >65 years, that a hypocellular BM can be observed and it correlates to the normal aging process, consisting predominantly of mature fat cells with an increased number of fibroblasts and collagen fibers, while in individuals younger than 30 years of age,13 normocellular BM was observed, whereas the overall number of capillaries and sinusoids was higher in infancy than in adulthood.14 For the purposes of histological analysis in our report, cellularity parameters were fixed according to expected-age cellularity.
Histological evaluation revealed that although patients who received HSCT had a good clinical outcome and normal peripheral blood counts, the BM biopsy exhibited a decreased cellularity adjusted to age in 40% of the cases, with half of the age-expected cellularity. Normal peripheral blood count parameters found in our group can be explained by the fact that on histopathology examination these cells had an appropriated maturation sequence, so that even although bone marrow cellularity was reduced, production was sufficient to maintain a normal CBC and to support the immune reconstitution within physiologic ranges.12,15
Novel allotransplant approaches are based on the concept that intensive and toxic cytoreductive conditioning therapy can be replaced by non-myelotoxic immunosuppression and that HSCT allografts create their own bone marrow space16 through limited GvHD reactions that, in addition, are responsible for the control of certain hematological malignancies through graft vs. tumor effect.17,18
At the time of BM biopsy our HSCT patients were clinically healthy and had a normal CBC; however, BM histological architecture did not fully recover to age-expected cellularity during the follow-up period up to 700 days. The analysis of the percentage cellularity, adjusted for age,11 found a lower BM cellularity in 40% of our patients. Despite this, properly ordered maturation sequence of the three hematopoietic cell lines accompanied a normal peripheral blood count. Remarkably, no differences in BM histology were documented when comparing the grade of fibrosis, indicating that the successful engraftment of hematopoietic stem cells is sufficient to compensate the production of hematologic cells, despite the alteration of bone marrow architecture.19
The WBC count of our recipients was significantly lower in those with increased BM fibrosis (p=0.015), but that had no impact in clinical outcomes, with no differences in the number of infections between groups. Although previous studies found an increased need for blood transfusion in this group,8 this was not significant (p=0.804) in our patients.
In our study, recipients with lower grades of fibrosis had higher overall survival than those with diffuse fibrosis, although it was not statistical significant (p=0.180), Fig. 1. Previous studies also documented higher survival in patients with lower grades of fibrosis6,8 underscoring the importance of this finding and its potential prognostic implications in the follow-up of HSCT recipients, however fibrosis could improve with the administration of pharmacologic agents, as recently shown.20
There are limitations in our study, including a small number of patients in both, autologous and allogeneic groups and heterogeneous disease categories, also bone marrow was studied once; however, the findings are relevant as they highlight important aspects of the dynamics of transplant recovery after RIC and the complex interaction between BM histological and functional recovery.
In conclusion, the findings on BM histological architecture in post-HSCT recipients conditioned with a RIC protocol reflect a considerable capacity to maintain normal peripheral blood cell counts despite a decreased BM cellularity secondary to age-related changes, hematologic disease, its treatment and subsequent challenges after conditioning regimen and hematopoietic grafting.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingNo financial support was provided.
Conflict of interestThere are no conflicts of interest to report.