Coronavirus disease-2019 was declared a pandemic in March 2020; since then, different strategies have been developed to control it. One of the main ones is vaccination,1 with the description of side effects being relevant, given their novelty.
A wide variety of reactions have been documented following SARS-CoV-2 vaccination, especially with the use of the mRNA COVID-19 vaccines, Moderna and Pfizer. Local injection site reactions in the form of hives, measles-like rash, erythromelalgia, chilblain-like lesions and pityriasis rosea-like eruptions, as well as systemic reactions have been reported.
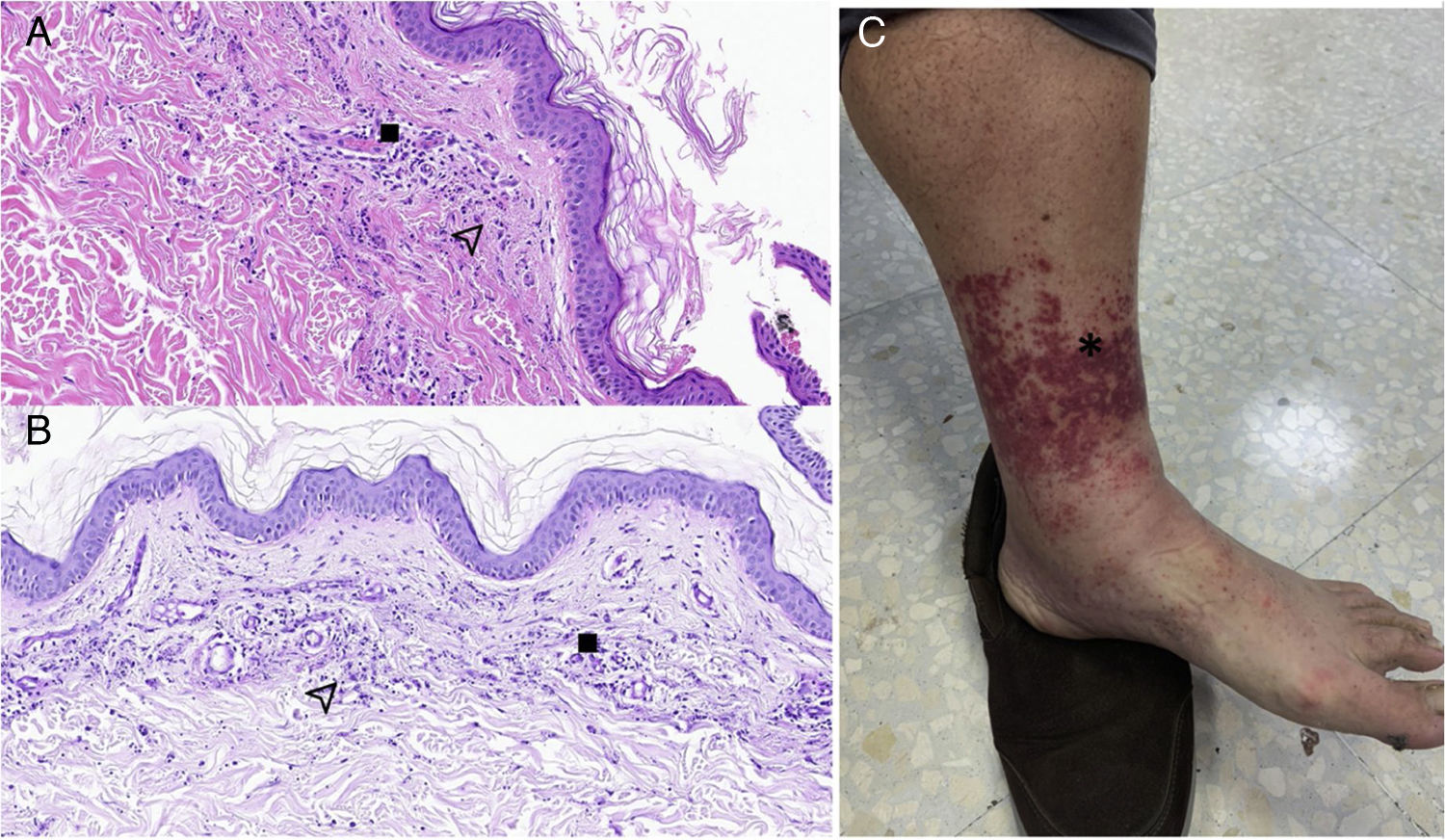
We report the clinical case of a 52-year-old male patient admitted on 4 April 2021 for an 8-day course of an erythematous, non-pruritic rash on both legs with petechiae up to the root of the lower limbs, which did not disappear on diascopy (Fig. 1C). The patient was vaccinated against SARS-CoV-2 with Moderna on 17th February and the second dose was administered on 17th March. The patient denied fever or other symptoms.
(A) Histopathological findings in skin biopsy with haematoxylin-eosin staining. B) Histopathological findings in skin biopsy with PAS.
: deposition of fibrinoid material, isolated microthrombi and prominent endothelium. : leukocytic infiltration with leukocytoclasis and blood extravasation. C) Allergic vasculitis. * Skin lesions compatible with palpable purpura.Laboratory tests showed CRP 1.34 mg/dl, prothrombin time of 16.7 seconds, weak positive lupus anticoagulant with a ratio of 1.34 and D-dimer of 1908 ng/ml, negative direct Coombs test; autoimmunity, anti-beta-2-glycoprotein I and anti-cardiolipin antibodies, homocysteine, sedimentation rate, protein profile, immunoglobulins, 24 h urine and CBC within normal range.
An assessment was requested by the dermatology department, whose clinical impression was allergic vasculitis on the thighs and both ankles, performing a skin biopsy for diagnostic confirmation.
The anatomical pathology findings of the skin biopsy showed an epidermis with orthokeratin and no significant abnormalities. The superficial dermis showed signs of acute vascular damage, with a mild peripheral leukocyte infiltration, leukocytoclasis, blood extravasation, deposition of fibrinoid material, isolated microthrombi and prominent endothelium. No accompanying eosinophils were identified. It was associated with mild oedema of the papillary dermis, with no other findings, and without involvement of the deep dermis (Fig. 1A and B).
Thus, the initial diagnostic suspicion of allergic vasculitis was confirmed, and the lesions resolved with rest without the need for anti-inflammatory or steroid treatment.
The main skin reaction reported so far after administration of the Moderna vaccine was a local delayed skin reaction approximately 7 days after the first dose, in 94% of cases, with this percentage decreasing with the second dose, as well as the extent of the skin lesion. No cases of anaphylaxis or life-threatening cases were reported.2,3
Although most post-vaccination reactions are mild and limited to the site of inoculation, cases of vasculitis have been reported; in particular, allergic vasculitis following influenza vaccination in elderly patients4 and following pneumococcus, chickenpox and hepatitis A vaccination in an immunosuppressed patient.5
Regarding the vaccines developed against the coronavirus, no cases of vasculitis have yet been described after administration, but hypersensitivity with cutaneous vasculitis developed after the above-mentioned vaccines reinforces the role of vaccination against SARS-CoV-2 as a trigger for vasculitis.
The case described was reported to pharmacovigilance because of its temporal relationship with the SARS-CoV-2 vaccination, as it occurred 11 days after the inoculation of the second dose of Moderna.
SARS-CoV-2 vaccines can potentially precipitate cutaneous vasculitis, although well-designed and methodologically sound trials are needed to draw a definitive conclusion.
FundingThis research has not received any grants.
Please cite this article as: Gázquez Aguilera EM, Rodríguez García M, Cantón Yebra MT. Vasculitis cutánea tras vacunación frente a COVID-19. Med Clin (Barc). 2022;158:493–494.