The cytokine storms (CS) mediated by overproduction of proinflammatory cytokines have been observed in a large population of critically ill patients infected with COVID-19.1
In previous studies, severe patients that have been hospitalized for COVID-19 have had laboratory results that show an increased level of cytokines, specifically interleukin 6 (IL-6). Tocilizumab is a recombinant humanized monoclonal antibody that has an antagonist effect on the IL-6 receptor, and could play a role in treatment for severely ill patients with COVID-19.2 Corticosteroids such as methylprednisolone (MP) are the conventional agents used to treat CS, but usually are related with risk of side effects, the most common the secondary bacterial superinfection. There are observational studies on the frontline in China and Italy that suggest the use of methylprednisolone was associated with better clinical outcomes in severe patients with COVID-19 pneumonia.3,4
This was a single centre observational study. We report the outcomes of patients tretaed with tocilizumab plus glucocorticoids in severe and critical severe COVID-19 patients between March 26 and April 17, 2020 in a Intensive Care Unit (ICU) Hospital in Barcelona, Spain. We included patients with high suspicion of CS (persisten fever, increase in inflammatory parameters (CPR, d-dimer, ferritine), and excluded patients with confirmed bacterial superinfection at the start of the tretament.
All patients enrolled met the severe or critical severe criteria defined by the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (6th interim edition) sponsored by National Health Commission of the People's Republic of China.5
The diagnose of severity was defined if any of the following conditions was met1: respiratory rate ≥30breaths/min 2; SpO2≤93% while breathing room air3; PaO2/FiO2≤300mmHg. A critical case was diagnosed if any of1: respiratory failure which requiring mechanical ventilation2; shock3; combined with other organ failure, need to be admitted to ICU.
A single dose of tocilizumab was administered in 21 patients and two doses in 4 patients. Methylprednisolone were adminstered 1mg/kg/day during the inflammatory phase. Afther that, decreasing doses were administered.
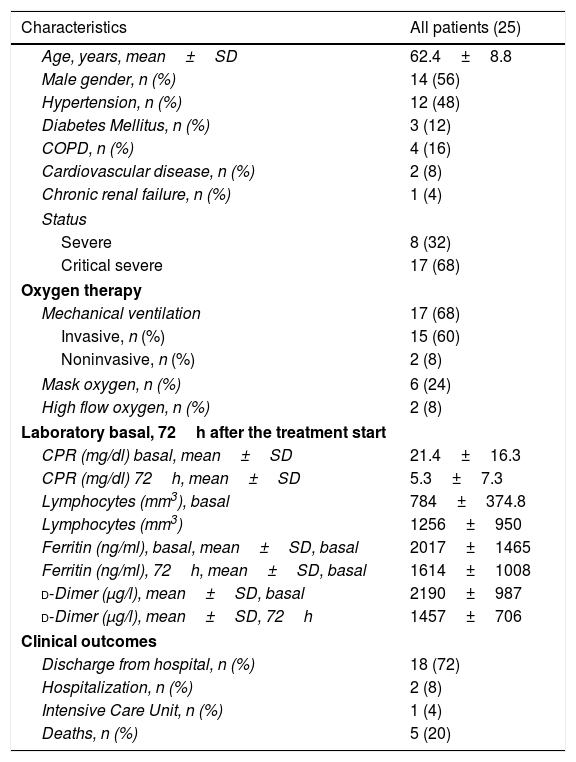
Twenty five patients (14 males and 11 females) with COVID-19 were included in this study. The characteristics of patients, status, laboratory and clinical outcomes are summarized in Table 1. The media age of the patients was 62.4 years. Eight (32%) patients were severe ill, and 17 were critical severe ill (68%), 15 of them with invasive mechanical ventilation, 2 with non invasive ventilation and 8 with high doses of oxigen (O2 mask more than 50% FiO2, or high flow oxygen) at the start of the treatment.
Clinical characteristics of the patients.
| Characteristics | All patients (25) |
|---|---|
| Age, years, mean±SD | 62.4±8.8 |
| Male gender, n (%) | 14 (56) |
| Hypertension, n (%) | 12 (48) |
| Diabetes Mellitus, n (%) | 3 (12) |
| COPD, n (%) | 4 (16) |
| Cardiovascular disease, n (%) | 2 (8) |
| Chronic renal failure, n (%) | 1 (4) |
| Status | |
| Severe | 8 (32) |
| Critical severe | 17 (68) |
| Oxygen therapy | |
| Mechanical ventilation | 17 (68) |
| Invasive, n (%) | 15 (60) |
| Noninvasive, n (%) | 2 (8) |
| Mask oxygen, n (%) | 6 (24) |
| High flow oxygen, n (%) | 2 (8) |
| Laboratory basal, 72h after the treatment start | |
| CPR (mg/dl) basal, mean±SD | 21.4±16.3 |
| CPR (mg/dl) 72h, mean±SD | 5.3±7.3 |
| Lymphocytes (mm3), basal | 784±374.8 |
| Lymphocytes (mm3) | 1256±950 |
| Ferritin (ng/ml), basal, mean±SD, basal | 2017±1465 |
| Ferritin (ng/ml), 72h, mean±SD, basal | 1614±1008 |
| d-Dimer (μg/l), mean±SD, basal | 2190±987 |
| d-Dimer (μg/l), mean±SD, 72h | 1457±706 |
| Clinical outcomes | |
| Discharge from hospital, n (%) | 18 (72) |
| Hospitalization, n (%) | 2 (8) |
| Intensive Care Unit, n (%) | 1 (4) |
| Deaths, n (%) | 5 (20) |
COPD: chronic obstructive pulmonary disease; CPR: C-reactive protein; SD: standard deviation.
The body temperature of 19 patients (76%) returned to normal in the first 72h after receiving the treatment. The total number of lymphocytes increases in 17 (68%) patients and CRP decreased significantly in 92% patients at 72h after the start of treatment. At the same time this was related to a clinical improvement of most patients (Appendix A).
There were 8 (32%) patients with subsequent bacterial superinfection, all these patients with long ICU admission. The median of days of hopitalization was 25 (8–52) days.
At this time (May 31, 2020), 18 (72%) patients were discharged from hospitalization, 2 patients remain hospitalized, 1 patient in ICU, and 5 deaths (20%).
The time that the anti-inflammatory treatment with tocilizumab was started since the hospital admission was variable among patients, the treatment were started earlier in patients who survived than those who died (7.6±5 vs. 13.6±7.7 days; p: 0.03).
In our study we observed a significant decrease in fever, CPR, d-dimer, ferritine, and an increase in the total number of lymphocytes at 72h after ther start the anti-inflammatory treatment in critical COVID patients.
The reported mortality in critically ill patients infected by COVID-19 is high between 18 and 66%.5 For this reason, pharmacological strategies should be sought to mitigate the inflammatory phase.
Although data from several ongoing randomized, controlled trials will soon provide more evidence regarding the different treatments, the outcomes observed in this report specifically the improvements in the inflamamatory parameter, with a 72% of the patients discharge from the hospitalization and overall mortality of 20%, suggest that tocilizumab plus glucocorticoids appears to be an effective treatment option in COVID-19 patients to attenuate the CS.
FundingDr. Jiménez-Brítez has a grant for research (Becas Carlos Antonio López).