Many of us who have been on the “front line” fighting against COVID-19 have experienced extreme frustration upon seeing many patients die without being able to offer proven treatment. This sentiment may possibly explain the “second pandemic” we have experienced - that of the exponential upsurge of interventions administered on a “compassionate use” basis. Tocilizumab, a monoclonal antibody capable of blocking the effect of interleukin (IL)-6 by binding to both soluble and membrane bound receptors, was one of the most widespread “compassionate use” interventions in Spain and the rest of the world. The rationale for its prescription is that elevated IL6 levels correlate with disease severity,1,2 consequently their blockade could be an effective treatment against COVID-19.3–5 A further justification is that its half-life (according to its SmPC) is 7–10 days, implying that even if it is administered early, it could have an effect during the advanced phase of the disease.
One of the first studies of tocilizumab in COVID-19 was published by Xu et al.6 and included 21 patients. The results reported after the administration of the drug were impressive: decrease in the C-reactive protein concentration, in the temperature and in the oxygen requirements, increase in oxygen saturation, disappearance of lung tomographic lesions in 90% of patients and 100% survival. Subsequently, case series,7 observational studies8,9 and even a few meta-analyses10,11 seemed to confirm the good results. Additionally, the positive perception of many doctors and patients expressed in webinares and WhatsApp groups exponentially increased confidence in this intervention. However, on 17 June 2020, the first alert appeared in a press release; an Italian randomised clinical trial (RCT) reported that early administered tocilizumab is unable to prevent clinical worsening of the disease.12 On 29 June the unwanted news resounded - another press release stated that the first global RCT, phase III, with more than 400 patients, had not found significant differences between the intervention and the placebo in terms of cure and mortality.13 Following that, three RCTs reported neutral effects of the intervention.14–16 However, in early 2021 another two RCTs (REMAP-CAP and RECOVERY) showed very positive results with the aforementioned intervention.17,18
At the time of writing this narrative review, the end of COVID-19 does not seem ‘nigh’ painting a picture that is difficult to visualise, so this dizzying history of tocilizumab leads us to ask three questions: 1) if tocilizumab can, in theory, effectively modulate the immune response, why have some RCTs had negative results? 2) how can multiple observational studies show positive results and yet some RCTs do not? 3) given the dissimilar results reported by the RCTs, what should we do?
Effective modulation of the immune responseIf tocilizumab can, in theory, effectively modulate the immune response, why have some RCTs had negative results?The pathophysiology of severe infections is complex and involves networks of interactions that are often resistant to a single ultraspecific intervention. The specific blockade of a single mediator or receptor might not be sufficient in complex diseases such as COVID-19 since living organisms, in order to resist the environmental changes to which they are continuously exposed, have complex and highly interconnected pathophysiological pathways. For example, the general attributes of cytokines include: pleiotropy (different effects of a cytokine when acting on different cells); redundancy (several cytokines can exert the same effect); synergism (two or more cytokines cooperate together to produce an enhancing effect); and antagonism (inhibit or block the effects of other cytokines). These characteristics may explain why blocking one specific pathway or structure is rarely successful at deconstructing the entire network (there are pathways capable of compensating or replacing the loss of a pathway).19 In the past, in other critical illnesses such as sepsis, attempts have been made to block certain molecules or structures and the results have been disappointing.20–23
Therapeutic target. For a drug to be effective, the patient must have the therapeutic target. The term “cytokine storm” was recently shown to be inaccurate given that blood concentrations of IL6, IL8, and TNFα in patients with acute respiratory distress syndrome (ARDS) associated with SARS-CoV-2 were less than or equal to that in ARDS of other aetiologies, in septic-shock without ARDS, in trauma or in out-of-hospital cardiac arrest.24 Furthermore, Han et al.25 compared 102 COVID-19 patients with 45 healthy controls. Although they reported that the IL6 concentration between both groups was different, the confidence intervals of each group overlapped, which allows the conclusion to be reached that a percentage of COVID-19 patients did not have the treatment target (it is assumed that the normal concentration of one variable is not a therapeutic target). It is noteworthy that none of the abovementioned RCTs required the IL6 levels to be elevated prior to prescribing the drug.12–16 However, in Spain the IL6 blood concentration is often used to prescribe tocilizumab. But a recent RCT sub-study, namely COVACTA, demonstrated that IL6 does not predict the response to tocilizumab; therefore, it should not be taken into consideration when deciding whether or not to prescribe the aforementioned blocker.26 Finally, in three RCTs12,14,15 the inflammation was surrogated by some markers (Table 1); however, the relationship between the inflammatory state, the variables required for its definition, and the response to tocilizumab is not clear.
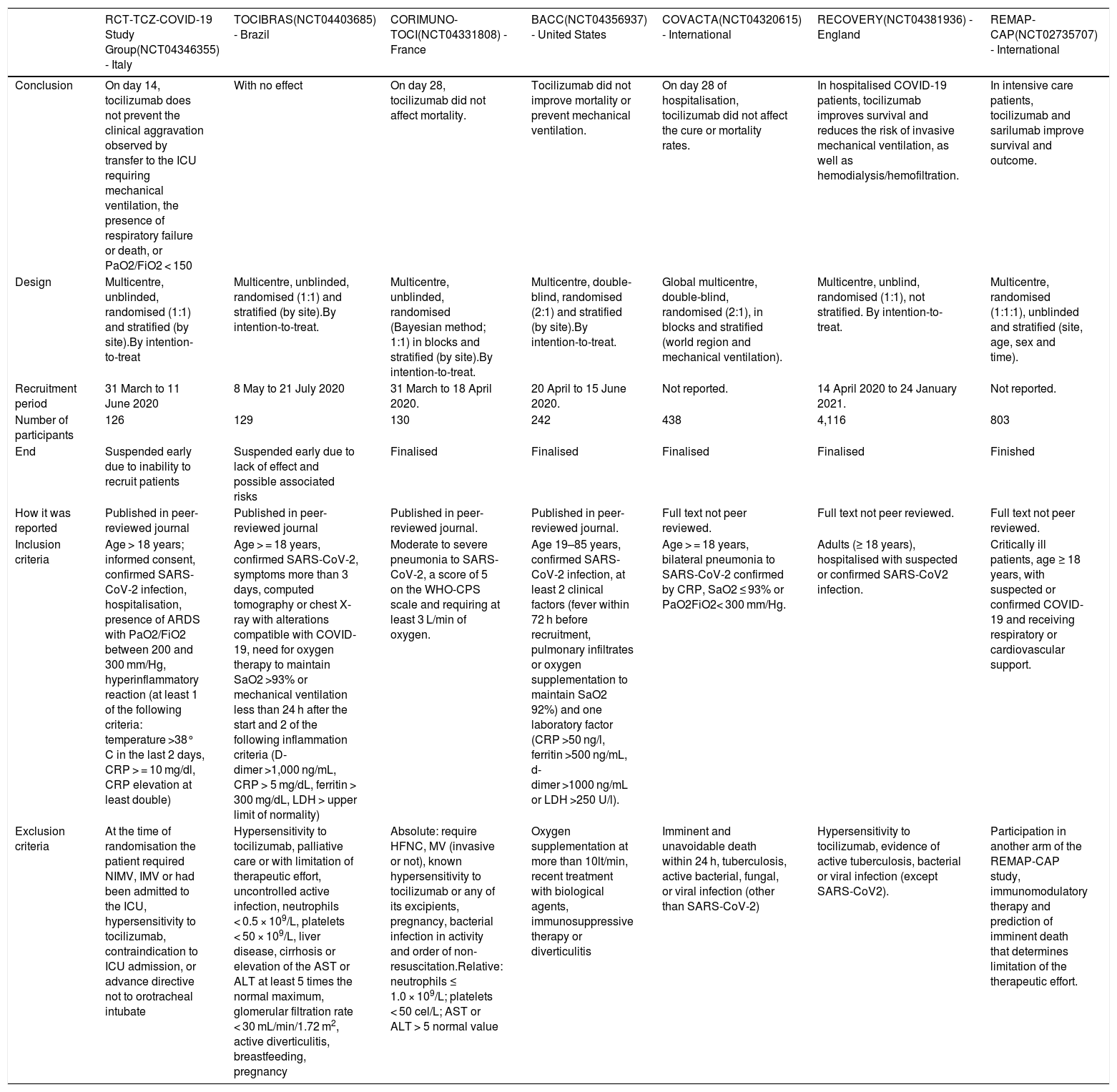
Randomized, controlled studies comparing tocilizumab versus placebo.
| RCT-TCZ-COVID-19 Study Group(NCT04346355) - Italy | TOCIBRAS(NCT04403685) - Brazil | CORIMUNO-TOCI(NCT04331808) - France | BACC(NCT04356937) - United States | COVACTA(NCT04320615) - International | RECOVERY(NCT04381936) - England | REMAP-CAP(NCT02735707) - International | |
|---|---|---|---|---|---|---|---|
| Conclusion | On day 14, tocilizumab does not prevent the clinical aggravation observed by transfer to the ICU requiring mechanical ventilation, the presence of respiratory failure or death, or PaO2/FiO2 < 150 | With no effect | On day 28, tocilizumab did not affect mortality. | Tocilizumab did not improve mortality or prevent mechanical ventilation. | On day 28 of hospitalisation, tocilizumab did not affect the cure or mortality rates. | In hospitalised COVID-19 patients, tocilizumab improves survival and reduces the risk of invasive mechanical ventilation, as well as hemodialysis/hemofiltration. | In intensive care patients, tocilizumab and sarilumab improve survival and outcome. |
| Design | Multicentre, unblinded, randomised (1:1) and stratified (by site).By intention-to-treat | Multicentre, unblinded, randomised (1:1) and stratified (by site).By intention-to-treat. | Multicentre, unblinded, randomised (Bayesian method; 1:1) in blocks and stratified (by site).By intention-to-treat. | Multicentre, double-blind, randomised (2:1) and stratified (by site).By intention-to-treat. | Global multicentre, double-blind, randomised (2:1), in blocks and stratified (world region and mechanical ventilation). | Multicentre, unblind, randomised (1:1), not stratified. By intention-to-treat. | Multicentre, randomised (1:1:1), unblinded and stratified (site, age, sex and time). |
| Recruitment period | 31 March to 11 June 2020 | 8 May to 21 July 2020 | 31 March to 18 April 2020. | 20 April to 15 June 2020. | Not reported. | 14 April 2020 to 24 January 2021. | Not reported. |
| Number of participants | 126 | 129 | 130 | 242 | 438 | 4,116 | 803 |
| End | Suspended early due to inability to recruit patients | Suspended early due to lack of effect and possible associated risks | Finalised | Finalised | Finalised | Finalised | Finished |
| How it was reported | Published in peer-reviewed journal | Published in peer-reviewed journal | Published in peer-reviewed journal. | Published in peer-reviewed journal. | Full text not peer reviewed. | Full text not peer reviewed. | Full text not peer reviewed. |
| Inclusion criteria | Age > 18 years; informed consent, confirmed SARS-CoV-2 infection, hospitalisation, presence of ARDS with PaO2/FiO2 between 200 and 300 mm/Hg, hyperinflammatory reaction (at least 1 of the following criteria: temperature >38° C in the last 2 days, CRP > = 10 mg/dl, CRP elevation at least double) | Age > = 18 years, confirmed SARS-CoV-2, symptoms more than 3 days, computed tomography or chest X-ray with alterations compatible with COVID-19, need for oxygen therapy to maintain SaO2 >93% or mechanical ventilation less than 24 h after the start and 2 of the following inflammation criteria (D-dimer >1,000 ng/mL, CRP > 5 mg/dL, ferritin > 300 mg/dL, LDH > upper limit of normality) | Moderate to severe pneumonia to SARS-CoV-2, a score of 5 on the WHO-CPS scale and requiring at least 3 L/min of oxygen. | Age 19–85 years, confirmed SARS-CoV-2 infection, at least 2 clinical factors (fever within 72 h before recruitment, pulmonary infiltrates or oxygen supplementation to maintain SaO2 92%) and one laboratory factor (CRP >50 ng/l, ferritin >500 ng/mL, d-dimer >1000 ng/mL or LDH >250 U/l). | Age > = 18 years, bilateral pneumonia to SARS-CoV-2 confirmed by CRP, SaO2 ≤ 93% or PaO2FiO2< 300 mm/Hg. | Adults (≥ 18 years), hospitalised with suspected or confirmed SARS-CoV2 infection. | Critically ill patients, age ≥ 18 years, with suspected or confirmed COVID-19 and receiving respiratory or cardiovascular support. |
| Exclusion criteria | At the time of randomisation the patient required NIMV, IMV or had been admitted to the ICU, hypersensitivity to tocilizumab, contraindication to ICU admission, or advance directive not to orotracheal intubate | Hypersensitivity to tocilizumab, palliative care or with limitation of therapeutic effort, uncontrolled active infection, neutrophils < 0.5 × 109/L, platelets < 50 × 109/L, liver disease, cirrhosis or elevation of the AST or ALT at least 5 times the normal maximum, glomerular filtration rate < 30 mL/min/1.72 m2, active diverticulitis, breastfeeding, pregnancy | Absolute: require HFNC, MV (invasive or not), known hypersensitivity to tocilizumab or any of its excipients, pregnancy, bacterial infection in activity and order of non-resuscitation.Relative: neutrophils ≤ 1.0 × 109/L; platelets < 50 cel/L; AST or ALT > 5 normal value | Oxygen supplementation at more than 10lt/min, recent treatment with biological agents, immunosuppressive therapy or diverticulitis | Imminent and unavoidable death within 24 h, tuberculosis, active bacterial, fungal, or viral infection (other than SARS-CoV-2) | Hypersensitivity to tocilizumab, evidence of active tuberculosis, bacterial or viral infection (except SARS-CoV2). | Participation in another arm of the REMAP-CAP study, immunomodulatory therapy and prediction of imminent death that determines limitation of the therapeutic effort. |
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BACC: Boston Area COVID-19 Consortium (BACC) Bay tocilizumab Trial; CORIMUNO-TOCI: Cohort Multiple Randomized Controlled Trials Open-label of Immune Modulatory Drugs and Other Treatments in COVID-19 Patients –tocilizumab Trial–; COVACTA: a study to evaluate the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia; LDH: lactate dehydrogenase; NCT: number clinical trial; PaO2/FiO2: ration between partial pressure of oxygen and fraction of inspired oxygen; CRP: C-reactive protein; SaO2: arterial oxygen saturation; TOCIBRAS: Safety and efficacy of tocilizumab in moderate to severe COVID-19 with inflammatory markers; ICU: Intensive Care Unit.
Timing of the intervention. The administration of tocilizumab is an allegedly immunodulatory intervention, that is, it would influence the host response, but not necessarily the viral activity. Theoretically, the patients should have a sufficiently strong inflammatory response so that their prognosis is influenced by it; but without having passed the point of “no return” in which the adverse event (e.g. death) is inevitable. These two characteristics were met by the patients in the RCTs: REMAP-CAP (xx% invasive mechanical ventilation or not, mean XX days from symptom onset and mean 2 days from hospitalisation); and RECOVERY (54% invasive mechanical ventilation or not, mean 9 days from symptom onset and mean 2 days from hospitalisation), wherein they showed that tocilizumab was associated with an improvement in prognosis.31,32 Patients with very mild symptoms or a very short time of evolution may not benefit from this intervention, since their prognosis could be mainly influenced by viral action. To this day, the ideal time to apply this intervention is unknown.
Intervention dose. The currently recommended dose is 8 mg/kg of body weight administered intravenously over one hour. However, this dose is an extrapolation from the recommended dose for patients with rheumatoid arthritis.27 With COVID-19, especially in the most severe patients who present septic shock, there are substantial pharmacodynamic and pharmacokinetic changes that could modify the recommended dosage.
Expectations exceed realities. Although some RCTs do not show an effect on mortality or cure rate, they do report beneficial effects of tocilizumab in other “weaker” events. For example, COVACTA demonstrated positive effects in some secondary episodes such as number of hospitalisation days or length of stay in the intensive care unit.13 It is important to highlight that these last two episodes are subjective since they depend on the decision of the doctor treating the patient. Although the study is double-blind, it is relatively easy to identify patients clinically who are receiving tocilizumab, and this can cause a follow-up bias. It is also important to note that the beneficial effects being associated exclusively with relatively “weak” events is observed in widely-accepted and routinely-used interventions, such as oseltamivir for influenza pneumonia or steroids for septic shock.28–30
Sample size. One of the obligatory characteristics of RCTs is to calculate the sample size a priori based on the type I, type II error and the magnitude of the effect to be detected. The reason for this obligation is the optimisation of resources and the protection of patients. Very small sample sizes could be insufficient to draw valid conclusions, and excessive sample sizes could unnecessarily expose patients to the risk of receiving an experimental intervention. In two of the seven RCTs reported,12,14 patient recruitment stopped before reaching the desired sample size.
Non-interchangeable groups. One of the main characteristics of RCTs is treatment randomisation and the extreme care that must be taken when performing it (concealing the randomisation and stratification sequence, for example). This is to achieve the valued objective of generating groups that only differ from each other in the intervention, thereby allowing direct comparison. Randomisation seeks to distribute both the measurable variables (e.g. age, comorbidities, etc.) and the non-measurable variables (e.g. genetic polymorphisms, cytokine concentrations, etc.) equally among the different groups. However on occasions there are difficulties. For example, in the CORIMUNO-TOCI study16 the groups differed in the percentage of patients who received antiviral agents, and adjuvant treatments (steroids and immunomodulators), in the presence of chronic kidney damage and in some baseline lab test variables (e.g. C-reactive protein, d-dimer, etc.). In the COVACTA study,13 the groups were differentiated by race and adjuvant treatments (corticosteroids, antivirals and convalescent plasma). The effect of these differences on the final outcome of the RCTs is unknown; however, it is indisputable that they constitute a bias and suggest the possible existence of differences in unobserved variables.
In addition to the particularities or shortcomings of the cited designs, the possibility also exists that, despite the existence of a good pathophysiological theory to support the intervention and an intense perception of its usefulness, the intervention simply does not work in some conditions due to reasons that may never be known. This should not be viewed as outstanding because, in reality, it is the core of the rationale for doing a clinical trial: we believe that the new intervention may work (which is why it is ethical to do it!) but we are not sure (which is why it is ethical do it!). It is not the first, nor will it be the last time, that a clinical trial in advanced phases has failed; in fact, it happens relatively frequently. Two well-known examples are lidocaine to prevent arrhythmias in acute myocardial infarction and hormone replacement in postmenopausal women to reduce the risk of secondary ischemic events. In both interventions, the initial perception was very good and various observational studies were published in their favour, including some RCTs with intermediate episodes in the case of replacement therapy. However, the RCTs with harder episodes and the meta-analyses of the RCT have demonstrated the futility or even the harm of both interventions and nowadays they are not applied routinely.31,32
Discordance between observational studies and randomised controlled trialsHow can multiple observational studies show positive results and yet some RCTs do not?As previously mentioned, the dissociation between observational studies and RCTs should not surprise us as it has been common knowledge for years. The main explanation may lie in the effect of confounding factors. RCTs are the best instrument to assess the efficacy of an intervention. They are superior to observational studies for this specific purpose because they have characteristics that protect them from certain biases that frequently affect observational studies. For example, random treatment assignment tends to produce equal groups in everything except the intervention; not only in variables that we know matter but also in others that we ignore their role or simply do not know that they exist (e.g. genetic polymorphisms). In observational studies, depending on the intervention evaluated, it is the doctor, the environment (availability) or even the patient himself who assigns the intervention, and this tends to produce very different groups. An attempt is made to reduce this confusion using strategies such as restriction or semi-restriction (e.g. cases and controls) or multivariate regression analysis (e.g. COX). Describing the multivariate analysis process in detail is beyond the scope of this review; however, it is worth noting that it is not always transparent and has been know to be influenced by the investigator. Furthermore, the final result can be adjusted only for variables that have been observed and measured; however, there are always latent or unrecorded variables that could be very relevant contributory factors. Recently, studies in which propensity scores9 are applied to match the groups (semi-restriction) or to adjust in the analysis have gained notoriety. There is no doubt that they provide some advantages over the more traditional models, but the completeness of the adjustment is still uncertain.
Behaviour in the face of mixed resultsGiven the divergent results reported by the RCTs, what should we do?The development of knowledge and science is characterised by being repetitive, heterogeneous and on multiple occasions contradictory. Despite having well-designed and high-quality clinical studies, there are often differing results simply due to chance, differences in populations, or because they have been conducted over different periods of time (medicine is continually advancing; two similar studies, but performed in different periods of time, may differ in the treatment of complications, in the diagnostic methods applied, in the adjuvant treatments, etc.). For all these reasons, it is the doctor or the investigator who must perform a critical analysis and consider whether it “makes sense to compare” the different studies with each other. If the conclusion is ‘yes’, that is that they are comparable studies, then it should be remembered that the scientific evidence, by consensus, is organised in a pyramid, with the apex being the maximum quality of information to evaluate efficacy, the large clinical trials and the RTC meta-analyses.33 The meta-analyses imply a robust, reproducible and internationally accepted methodology based on the precept of including all the studies related to the topic in question; furthermore, of almost greater importance than the statistical analysis itself is the quality of the preceding systematic review. Additionally, it should be noted that we refer to an RTC meta-analysis, as there are meta-analyses of observational studies or others that combine observational studies and RTCs whose evidence is hierarchically lower than that of an RTC (for example the meta-analyses by Xu et al.6 and Soraya et al.11 previously cited). Finally, the results of the meta-analyses must also undergo an evaluation process before incorporating their recommendations into the clinical practice guidelines.
All in all, the severity of the COVID-19 pandemic has pushed the scientific community towards rallying forward as never before with the aim of finding effective treatments urgently. The situation and the need for effective treatment has been so critical that the search for “shortcuts” such as the use of treatments under the label of “compassionate treatment” is understandable. Simultaneously, we had to get used to hearing the latest information through alternative channels such as press releases, articles published on platforms that were not peer-reviewed or webinares. The short, but very intense history of tocilizumab applied to COVID-19 patients shows that science requires a scientific and time-consuming method to develop. However understandable the application of “shortcuts” in science may be, it does not seem to be appropriate as it implies putting patients at risk. After months of intense research and contradictory results between observational studies and the RCTs, and even between different RCTs, it appears that tocilizumab would have a beneficial effect in the subgroup of patients who are more severely ill and within a relatively short time since the onset of symptoms.
Conflict of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sancho M, Muñiz J, Cardinal-Fernández P. Tocilizumab en el paciente con COVID-19. Med Clin (Barc). 2021;156:402–406.