There is growing evidence regarding the imaging findings of coronavirus disease 2019 (COVID-19) in lung ultrasound (LUS), however the use of a combined prognostic and triage tool has yet to be explored.
To determine the impact of the LUS in the prediction of the mortality of patients with highly suspected or confirmed COVID-19.The secondary outcome was to calculate a score with LUS findings with other variables to predict hospital admission and emergency department (ED) discharge.
Material and methodsProspective study performed in the ED of three academic hospitals. Patients with highly suspected or confirmed COVID-19 underwent a LUS examination and laboratory tests.
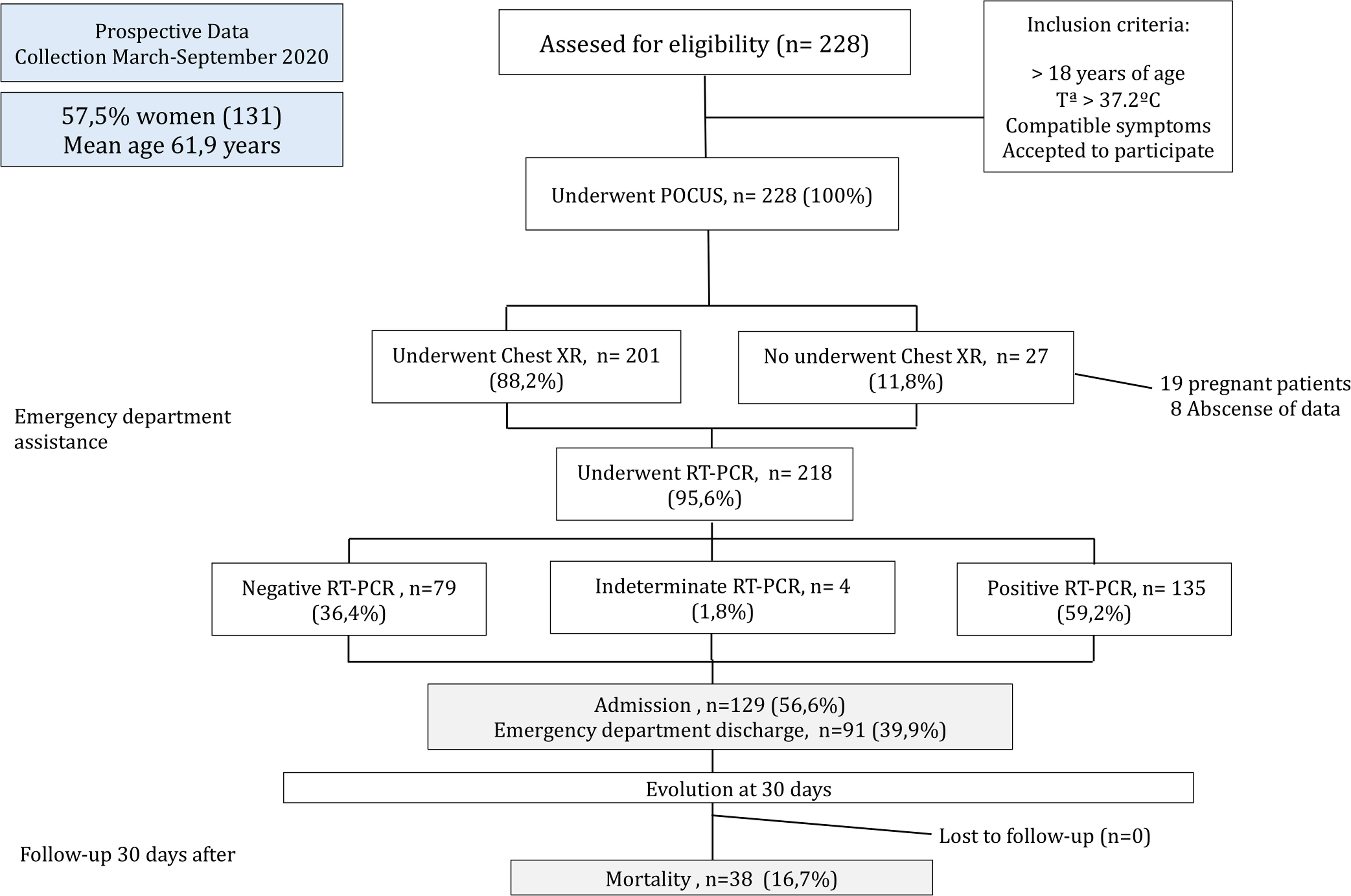
ResultsA total of 228 patients were enrolled between March and September 2020. The mean age was 61.9 years (Standard Deviation – SD 21.1). The most common findings in LUS was a right posteroinferior isolated irregular pleural line (53.9%, 123 patients). A logistic regression model was calculated, including age over 70 years, C-reactive protein (CRP) over 70mg/L and a lung score over 7 to predict mortality, hospital admission and discharge from the ED. We obtained a predictive model with a sensitivity of 56.8% and a specificity of 87.6%, with an AUC of 0.813 [p<0.001].
ConclusionsThe combination of LUS, clinical and laboratory findings in this easy to apply “rule of 7” showed excellent performance to predict hospital admission and mortality.
Existe una evidencia creciente con respecto a los hallazgos de imagen de la enfermedad por coronavirus 2019 (COVID-19) en la ecografía pulmonar (LUS), sin embargo, aún no se ha explorado el uso de una herramienta combinada de pronóstico y triaje.
El objetivo principal de este estudio fue determinar el impacto de la LUS en la predicción de la mortalidad de los pacientes con sospecha de afectación pulmonar por COVID-19. El objetivo secundario fue calcular una puntuación con los hallazgos del LUS con otras variables para predecir el ingreso hospitalario y el alta del servicio de urgencias (SU).
Material y métodosEstudio prospectivo realizado en urgencias de tres hospitales académicos, en pacientes con sospecha de COVID-19 o confirmación de esta, a los que se sometió a un examen de LUS y pruebas de laboratorio.
ResultadosSe inscribieron un total de 228 pacientes entre marzo y septiembre de 2020. La edad media fue de 61,9 años (DE 21,1). El hallazgo más común en la LUS fue la irregularidad pleural posteroinferior derecha (53,9%, 123 pacientes). Se calculó un modelo de regresión logística, que incluyó la edad mayor de 70 años, proteína C reactiva (PCR) mayor de 70 mg/L y puntuación de afectación pulmonar mediante LUS score superior a 7 para predecir la mortalidad, el ingreso hospitalario y el alta del SU. Se obtuvo una sensibilidad del 56,8% y una especificidad del 87,6%, con un AUC de 0,813 [p < 0,001] para dicho modelo predictivo, en materia de mortalidad.
ConclusionesLa combinación de LUS, hallazgos clínicos y de laboratorio en esta «regla de 7» de fácil aplicación se mostró de utilidad para predecir el ingreso hospitalario y la mortalidad.
More than a year after the declaration of a global pandemic due to the novel coronavirus disease (COVID-19), with more than 220 million confirmed cases worldwide,1 high income countries continue to explore new tools for an improved diagnosis and treatment protocols while lower income countries are trying to adapt their hospital's capacity in order to prevent a system collapse.2
To date, over one million deaths have been reported globally, of which 55% were reported in the American continent.3 Moreover, 8 out of 10 deaths reported in the United States have been in adults over 65 years old.4 These facts enhance the critical need for an accessible, low cost diagnostic method and an easy-to-use tool for the early risk stratification in COVID-19 patients.5 Lung ultrasound (LUS) qualifies for such a purpose, providing an evidence-based method which will aid to stratify patients depending on their risk of critical care need or death, hence conditioning their need to be admitted or transferred to a better equipped center.6 Due to these characteristics, LUS seems an optimal tool for some special populations,7 such as pregnant women, children and the elderly.
COVID-19 pandemic has been associated with high mortality rates, notably in patients older than 65 year with multimorbidity,8 therefore it is pivotal to keep researching for practical, accessible and easy to use diagnostic and prognostic methods which could determine whether a patient will present complications, thus the need to be closely monitored. This could be a big change in daily practice in some non-hospital settings, such as nursing homes.9
Furthermore, an evidence-based LUS approach could be included in multi-criteria decision analysis systems in order to prioritize patients for hospital admission in low-income areas with limited resources.10
To our best knowledge, the formulation of such an approach is in need for more research. We aimed to determine the impact of LUS, in combination with other clinical variables and laboratory parameters in the prediction of mortality, hospital admission and ED discharge of patients with highly suspected or confirmed coronavirus disease (COVID-19).
Patients and methodsStudy populationThis was a prospective study performed in the emergency department (ED) of three academic hospitals, conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethics Committee of each University Hospital involved. Informed consent was obtained from each enrolled patient.
Patient selectionPatients admitted to the ED with a clinical suspicion of COVID-19 (temperature above 37.2°C, acute respiratory symptoms, gastrointestinal symptoms, or fatigue) requiring X-ray for evaluation were included. We excluded patients<18 years or those who declined to participate. A sample of patients who met these inclusion criteria were enrolled and prospectively studied.
Initial patient assessment and data collectionWe collected the clinical data directly by an individual review of the patient's electronical clinical records from the hospital's database (DXC-HCIS-Healthcare Information System). The clinical data collected through individual review included age, sex, comorbidities, previous treatment, influenza vaccines in the past two years and symptoms.
Laboratory results (hemogram, basic metabolic panel [e.g., glucose, electrolytes, kidney function, liver enzymes], lactate dehydrogenase [LDH], ferritin, C-reactive protein [CRP], procalcitonin, blood gases [lactate and pH], and coagulation [D-dimer, international normalized ratio, partial thromboplastin time, fibrinogen]) were obtained from various hospital data management systems, and information regarding treatment provided during hospital stay was collected from the electronic prescription system.
Both patient selection and assessment were performed by the treating physician, who notified the sonographers of a potential study subject. To guarantee the sonographers were blinded, they did not have access to the patients’ data.
Ultrasound data collectionPatients underwent chest X-ray or chest Computed Tomography (CT), performed by fellowship-trained radiologists together with a LUS, performed by ultrasound fellowship-trained emergency physicians according to the American College of Emergency Physicians ultrasonographic guidelines, with more than 10 ultrasound exams performed per week, and 5 years of experience in performing and interpreting LUS11. Therefore, an opportunity sampling method was implemented for patient selection.
Participants underwent ultrasonographic measurement of the inferior vena cava (IVC) and a focused cardiac ultrasound (FOCUS). The ultrasound examination of the IVC poses the necessity to consider possible confounding factors. There are situations when the decrease of IVC diameter is not related to progression to hypovolemia, and may be linked to increased abdominal pressure, or an abnormal inspiratory effort. Some specific cardiac and pulmonary conditions determining an obstacle to the venous return or in alveolar hyperinflation, and so on; so, it is essential to consider the clinical settings.
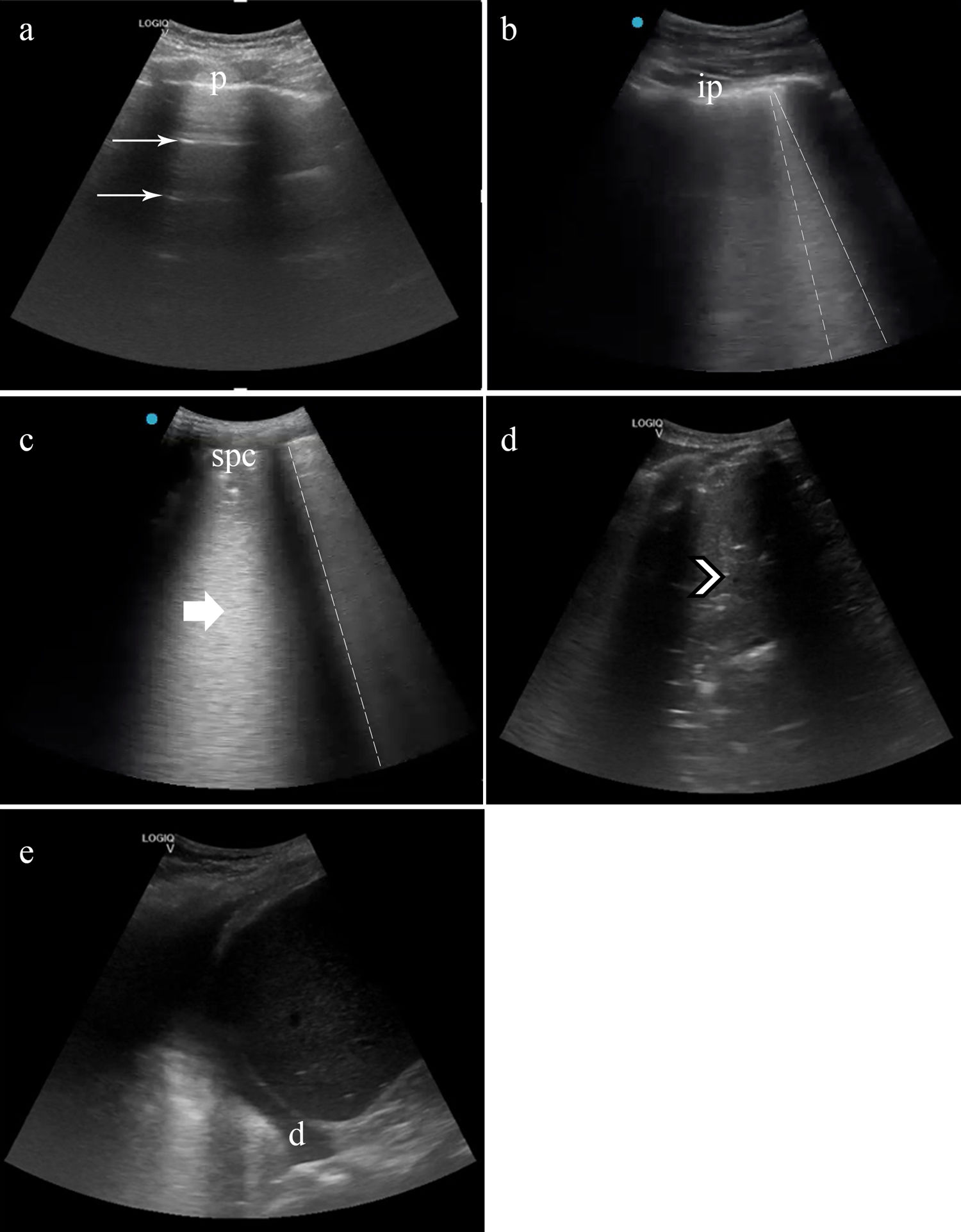
A LUS was performed following a 11-zone protocol (Fig. 1).12 Each intercostal space of the upper and lower parts of the anterior, lateral, and posterior regions of the left and right chest wall was carefully examined. Findings were defined and recorded as follows (Fig. 2)13,14:
- -
A-lines: horizontal reverberation artifacts parallel to the pleural line (Fig. 2a).
- -
B-lines: hyperechoic vertical artifacts that arise from the pleural line, extending to the bottom of the screen without fading that erases the A-line artifact.
- -
Irregular pleural line: indented or broken pleural line (Fig. 2b).
- -
Small consolidations: consolidations (hypoechoic areas) smaller than 1cm in diameter, surrounded by a hyperechoic artifact tail (Fig. 2b).
- -
Lobar consolidations: larger (over 1cm) consolidation areas with or without the presence of air bronchograms (Fig. 2d).
- -
Pleural effusion: a typically anechoic space between the parietal and visceral pleura (Fig. 2e).
Lung ultrasound in patients with COVID-19 and lung score. (a) A lines: pattern of horizontal (thin arrow) lines parallel to pleura (p). (b) Focal B lines. Pattern of vertical lines that reach the depth of field ant start from the pleura line (dashed line). The pleural line is fragmented, like irregular pleura (ip). (c) Confluent B lines. In the form of a “white lung” (thick arrow) the B lines (dashed line) converge. The pleural line increases her irregularity, generating a subpleural consolidation (spc). (d) If the subpleural consolidation progresses, or in superinfection cases, translobar consolidations appear (arrowhead), achiving a look liver tissue-like. Pleural effusion could appear in severe cases (d). Lung score: We summed every area's points, obtaining the patient's lung score, ranging from 0 to 33. *: Irregular pleural lines and focal B lines=1 point, **: Confluent B lines=2 point, ***: Subpleural or lobar consolidation or pleural effusion=3 points.
A compatible LUS exam was considered a pattern of B-lines, isolated or confluent, irregular pleural lines, and/or subpleural consolidations.
The examinations were performed using a GE LOGIQ e ultrasound system fitted with a phased and curvilinear array transducer (1.5–4.5MHz) (General Electrics Healthcare, Madrid, Spain) as a cart-based device, a Butterfly IQ (Butterfly Network, Guilford, CT, USA) as a hand-held device and using a SonoSite Edge II with a phased and curvilinear array transducer at another site involved.
The sonographers were blinded to the patient's past medical history, vital signs, symptoms, laboratory measurements and therapy. The results of the ultrasound were recorded in the patient's medical history, and this information was available to the treating physician, who adjusted the therapy based on these findings. However, the analytical and X-ray request depended on the clinical criteria of the treating physician, who in no case was previously aware of the ultrasound results. Only in the case of pregnant women was this information provided to avoid taking a chest X-ray.
Outcome measures and definitionsThe main purpose of this study was to describe and correlate the LUS findings of the disease in patients with COVID-19 admitted to the ED with prognosis. The primary outcome was to determine the impact of the LUS and mortality of patients with highly suspected or confirmed coronavirus disease (COVID-19). The secondary outcome was to calculate a score with LUS findings in combination with other variables (laboratory markers, physical exam, demographics) to predict Hospital admission and emergency department (ED) discharge.
We defined a confirmed case as any patient with clinical symptoms and positive RT-PCR, and a high suspicion case as any patient with negative RT-PCR but compatible clinical symptoms and typical X-ray, CT scan or LUS.
We developed a numeric lung score based on the pathological findings in each lung echographic area, taking as a reference Soldati et al. score.15 Although we differentiated eleven lung echographic areas (Fig. 1) and gave each pathological finding a different score (Fig. 2):
- •
Irregular pleural lines and focal B lines: 1 point
- •
Confluent B lines: 2 points
- •
Subpleural or lobar consolidation or pleural effusion: 3 points
We summed every area's points, obtaining the patient's lung score, ranging from 0 to 33.
Statistical analysisBaseline characteristics are presented as mean and standard deviation (SD) for continuous variables and count and proportions for categorical variables. For group comparisons, we used a t-test for continuous variables and the chi-squared or Fisher's exact test for categorical variables. The correlations between continuous variables were tested using Spearman's rho test for categorical variables. Mean values were reported, along with 95% confidence intervals (CIs). Statistical significance was set at p<0.05.
Then a logistic regression analysis was performed to obtain a model that could predict the outcome variables. Statistical analyses were conducted with IBM SPSS software v20.0 (SPSS Inc., Chicago, IL, USA).
Our aim was to determine the impact of LUS, in combination with other clinical variables and laboratory parameters in the prediction of mortality, hospital admission and ED discharge of patients with highly suspected or confirmed COVID-19.
ResultsA total of 228 patients were enrolled between March and September 2020 (Fig. 3). The mean age was 61.9 years (SD 21.1), and 131 (57.5%) patients were women. 92 patients (40.4%) of the patients had hypertension, most receiving angiotensin-converting-enzyme inhibitors or angiotensin receptor blocker therapy. The most common presenting symptom was dyspnea (82.9%) and fever (86.6%). The patients were normotensive and had low oxygen saturation (93.9%, SD 5.8, with a respiratory rate of 15rpm (SD 4.1). The mean lymphocyte count was 1.561×109 (SD 1.5), CRP was 57.2mg/L (SD 86.3, Normal Value: 0–10mg/L), and LDH was 281.2U/L (SD 184.6; NV: 140–280U/L) at admission (see Table 1).
Demographics and clinical characteristics of patients included (N=228).
| Demographics | |
| Gender (female) – N (%) | 131 (57.5) |
| Age (years) mean (SD) | 61.9 (21.2) |
| Age>70 years – N (%) | 84 (36.8) |
| Past medical history – N (%) | |
| Pulmonary disease | 53 (23.2) |
| Diabetes mellitus | 42 (18.4) |
| Hypertension | 92 (40.4) |
| Obesity | 29 (12.7) |
| Previous NSAID therapy | 17 (7.5) |
| Previous ACEi/ARB therapy | 55 (24.1) |
| Symptoms | |
| Dyspnea – mean (SD) | 82.9 (130.7) |
| Fever – mean (SD) | 86.6 (129.4) |
| Myalgias – N (%) | 87 (38.2) |
| Gastrointestinal symptom – N (%) | 32 (14) |
| Cough – N (%) | 128 (56.1) |
| Chest pain – N (%) | 39 (17.1) |
| Anosmia/ageusia – N (%) | 15 (6.6) |
| Physical exam | |
| SBP (mmHg) mean (SD) | 129.5 (22.8) |
| DBP (mmHg) mean (SD) | 76.5 (13.3) |
| Respiratory rate (rpm) mean (SD) | 15.6 (4.1) |
| Temperature (°C) mean (SD) | 39.9 (4.3) |
| SO2 (%) mean (SD) | 93.9 (5.9) |
| Laboratory results – mean (SD) | |
| WBC×10^9/L | 7617.1 (3714.3) |
| Lymphocite×10^9/L | 1561.6 (1520.1) |
| LDH – U/L | 281.2 (184.6) |
| pO2 – mmHg | 75.2 (41.5) |
| pCO2 – mmHg | 31.3 (13.9) |
| D-dimer – ng/mL | 3595.8 (13,750.7) |
| PCT – ng/mL | 1.6 (9.8) |
| C-reactive protein – mg/L | 57.2 (86.3) |
| Troponin I – ng/mL | 73.2 (544.3) |
| NT-proBNP – pg/mL | 1443.9 (3752.1) |
| Ferritin – ng/mL | 508.3 (861.4) |
| C-reactive protein>70mg/L – N (%) | 72 (39.6) |
| SARS-CoV-2 (PCR) test – N (%) | 218 (95.6) |
| Positive | 135 (59.2) |
| Negative | 79 (34.6) |
| Indeterminate | 4 (1.8) |
| Follow-up – N (%) | |
| Admission | 129 (56.6) |
| Discharge from E.D. | 91 (39.9) |
| Mortality | 38 (16.7) |
ACEi: angiotensin-converting-enzyme inhibitors. ARB: angiotensin receptor blockers; E.D.: Emergency Department; LDH: lactate dehydrogenase; NT-ProBNP: N-terminal pro-brain natriuretic peptide; PCR: polymerase chain reaction; PCT: procalcitonin; SD: standard deviation.
In the group of patients who died, the mean lung score was 13.9 (SD 7.1), CRP of 122.8mg/L (SD 102.3) and age of 81.8 years (SD 12.9) in comparison with a lung score of 9.1 (7.3), CRP of 43.3 (SD 75.9) and age of 57.9 (SD 20.1) in the group of patients who survived (p<0.001).
In the group of patients were admitted to the hospital, the mean lung score was 12.9 (SD 6.9), CRP of 86.4mg/L (SD 96.6) and age of 70.2 years (SD 18.3) in comparison with a lung score of 6.0 (6.2), CRP of 15.2 (SD 42.4) and age of 51.0 (SD 19.4) in the group of patients who were discharged from the ED (p<0.001).
Imaging modalities: chest X-ray and ultrasound studiesAll the included patients underwent a LUS study, and almost all of them had a chest X-ray (see Table 2). The most frequent pattern in the chest X-ray were an interstitial pattern (31.1% [71 patients out of 201]), and ground-glass opacities (GGOs) (30.3% [69 patients out of 201]). 37.7% (86 patients out of 228) of them had a normal chest X-ray. 37.7% (86 patients of 201) of patients had a completely normal chest X-ray. 27 patients in whom no chest X-ray data was available (did not undergo chest X-ray due to clinical circumstances-pregnancy, availability, etc.- or because there is no data in the clinical system). Some radiographs were delayed and those not performed at the time of ultrasound were not considered.
Imaging modalities (chest X-ray and point-of-care ultrasound) findings of patients included (N=228).
| Imaging modalities | |
|---|---|
| Chest X-ray – N (%) | 201 (88.2) |
| Normal | 86 (37.7) |
| Ground-glass opacity (GGO) | 69 (30.3) |
| Interstitial pattern | 71 (31.1) |
| Unilobar | 18 (7.9) |
| Multilobar | 11 (4.8) |
| Bilateral | 85 (37.3) |
| Point-of-care ultrasonography (POCUS) results | 228 (100) |
| Pleural effusion | 31 (13.6) |
| Right posteroinferior confluent B-lines | 77 (33.8) |
| Left posteroinferior confluent B-lines | 82 (36) |
| Right posteroinferior focal B-lines | 86 (37.7) |
| Left posteroinferior focal B-lines | 76 (33.3) |
| Right posteroinferior irregular pleural B-lines | 123 (53.9) |
| Right posterosuperior irregular pleural B-lines | 86 (37.7) |
| Left posteroinferior irregular pleural B-lines | 120 (52.6) |
| Left posterosuperior irregular pleural B-lines | 89 (39) |
| Right posteroinferior subpleural consolidation | 63 (27.6) |
| Left posteroinferior subpleural consolidation | 66 (28.9) |
| Lung score>7 | 127 (55.7) |
| Lung score>10 | 93 (40.8) |
Regarding the LUS, the most common findings were right posteroinferior isolated irregular pleural lines (53.9%, 123 patients), followed by left posteroinferior isolated irregular pleural lines (52.6% [120 patients out of 228]). 13.6% (31 patients out of 228) patients had pleural effusion.
Primary outcome: correlation between lung score and mortalityThe primary goal of this investigation was to describe and correlate the LUS findings of the disease in patients with COVID-19 admitted to the ED.
Setting the lung score threshold at 7 points, we found a sensitivity of 76.3% and a specificity of 48.4% (p=0.005), the specificity increased to 63.7% but with a decrease of the sensitivity to 63.7% (p=0.002) if the lung score was set at 10 points.
An analysis of receiver operating characteristic curves (Fig. 3) showed the area under the curve [AUC] for age over 70 years, CRP over 70mg/L and lung score over 7.
We generated a logistic regression model using age over 70 years, CRP over 70mg/L and lung score over 7 to predict mortality, hospital admission and discharge from the ED.
Globally, using these three parameters (age over 70 years, CRP over 70mg/L and lung score over 7) we obtained a predictive model with a sensitivity of 56.8% and specificity of 87.6%, with an AUC of 0.813 (p<0.001) to predict mortality. A sensitivity of 88.5% and specificity of 53.3%, with an AUC of 0.769 (p<0.001) to predict hospital admission, and a sensitivity of 61.5% and specificity of 89.2%, with an AUC of 0.813 (p<0.001) to predict discharge from ED (Fig. 4).
Receiver operating characteristic (ROC) curve for predicting mortality. Orange line=reference line; blue line=only LUS score>7; red line=LUS score>7+CRP>70 mg/L; green line=LUS score>7+CRP>70 mg/L+age>70. (1) All patients with clinical COVID19 compatible. Receiver operating characteristic (ROC) curve for predicting mortality according to lung ultrasonography (LUS) score above 7 [area under the curve (AUC) of 59.9%, p=0.064], with also CRP above 70 [AUC of 69.3%, p≤0.001] and adding Age above 70 [AUC of 74.3%, p<0.001]. (2) Patients with clinical COVID19 compatible and positive RT-PCR. Receiver operating characteristic (ROC) curve for predicting mortality according to lung ultrasonography (LUS) score above 7 [area under the curve (AUC) of 54%, p=0.557], with also CRP above 70 [AUC of 68.9%, p=0.006] and adding Age above 70 [AUC of 75%, p<0.001]. (3) Patients with clinical COVID19 compatible and negative RT-PCR. Receiver operating characteristic (ROC) curve for predicting mortality according to lung ultrasonography (LUS) score above 7 [area under the curve (AUC) of 63.0%, p=0.154], with also CRP above 70 [AUC of 70.8%, p=0.022] and adding Age above 70 [AUC of 71.9%, p=0.016]. (4) Patients with clinical COVID19 compatible, chest X-ray COVID19 compatible and negative RT-PCR. Receiver operating characteristic (ROC) curve for predicting mortality according to lung ultrasonography (LUS) score above 7 [area under the curve (AUC) of 66.7%, p=0.230], with also CRP above 70 [AUC of 80.6%, p=0.028] and adding Age above 70 [AUC of 52.8%, p=0.841].
We used a predictive logistic regression model using lung score>7, obtaining an AUC of 0.784, [95% CI 0.724–0.845, p<0.001]. Setting the lung score threshold at 7 points, we found a sensitivity of 72.9% and a specificity of 66.7% (p<0.001), which changed to sensitivity of 58.9% and specificity of 82.8% (p<0.001) if the lung score was 10 points.
Globally, using the three parameters (age over 70 years of age, CPR over 70mg/L and lung score over 7) predicted hospital admission. We obtained a predictive model with a sensitivity of 88.5% and specificity of 53.3%, with an AUC of 0.769 (p<0.001)
Correlation between lung score and discharge from the EDWe used a predictive logistic regression model using lung score>7, obtaining an AUC of 0.800, CI 0.740–0.859, p<0.001. Setting the lung score threshold at 7 points, we found a s sensitivity of 70.3% and a specificity of 73% (p<0.001), which increased to sensitivity of 85.7% and decreased specificity of 58.4% (p<0.001) if the lung score was 10 points.
DiscussionEasy to access and reliable diagnostic methods which can predict prognosis in COVID-19 are vital in non-hospital settings and areas with limited resources. Some studies start to point out that LUS could be a first-line diagnostic tool alternative to conventional chest X ray and CT scan, including the critically ill patients, where some LUS scores have already been suggested.16,17 Other studies have started to use multi-criteria system analysis in hospital settings to prioritize admissions.10
According to the Centers for Disease Control and Prevention (CDC), the total number of excess deaths from January to October 2020 among 75–84 years of age was 94,646. Overall, numbers of deaths among persons this aged was 21.5% above average. Although not only SARS-COV2 contributes to this data, during this period can make a special contribution. Examined by race and ethnicity, the largest increase was in Hispanic people (53.6%).18
Our investigation aimed to create a score which could correlate with mortality, so it could be put into practice in non- hospital settings and lower income areas and help prioritize which patients needed transferring to a medical facility for closer monitoring. Some studies have used lung ultrasound as a triage for symptomatic patients outside hospital settings.15
The combined score we produced includes three factors – age, CRP and LUS findings – which could be obtained in many settings. Regarding age, it is well known that it is a negative prognostic factor in COVID-19 patients, rising to a 32.5% mortality rate in severe cases.19 Nevertheless, age only is a lacking method of triage, for the older global population greatly exceeds the health systems’ capacity.
Moreover, Chinese studies CRP has been found to be an independent predictor of adverse outcomes with a high sensitivity and specificity.20 Elevation of CRP has also been positively correlated with chest CT findings and severity of COVID-19.21
Concerning LUS examination, some authors claim it can replace the stethoscope – which is challenged as efficient in viral pneumonias–, a way to guarantee both patients’ right to be thoroughly examined and the health care providers safety.22
There is growing literature suggesting LUS as safe and easily accessible tool, which helps in early correct diagnosis, appropriate management and a positive correlation with prognosis.23–26
To the best of the authors’ knowledge, this is the first time that age, CRP and LUS findings have been combined in a score which can predict hospital admission and mortality. We believe this approach is a simple and reproducible one, which can be put into use in a varied range of settings, inside and outside hospitals. Future research could further explore the clinical implications and prognosis of scores such as the one we propose.
The findings in our study should be interpreted with its limitations. First, the sample size was a medium one, and it included patients with sufficient severity of symptoms to consult in the ED. Further studies should explore the role of combined Scores outside a Hospital setting and with a greater sample size. Second, our study did not take into account other factors (e.g., therapy against COVID-19 or symptomatic therapy) in the association between our score and hospital admission rate and mortality. Thirdly, in a pandemic situation, the high prevalence of respiratory infection attributed to SARS-CoV-2 could have generated a bias because ultrasound findings are not apt to determine etiology, so all findings compatible with COVID-19 were considered as such. Another limitation is the chance of misdiagnosis, since there might be other diseases mimicking COVID-19 or even more, the performance of the RT-PCR is not 100% accurate, this could be a challenge to interpret the LUS findings. This limitation was minimized given the patients were followed-up by reviewing their electronic history, and any complications were recorded. Moreover, LUS findings might become paramount in negative or indeterminate PCR patients, for it could indicate a change in patient diagnosis or therapy.27 On the other hand, the treating physician knows the LUS findings; so this could modified the management. Finally, our score needs an experienced sonographer to evaluate LUS findings.
ConclusionThe combination of LUS, clinical and laboratory findings in this easy to apply “rule of 7” (LUS score>7, age>70 year-old and CRP>70mg/L) showed a good to excellent performance to predict hospital admission and mortality.
Authors’ contributionsAll authors read and approved the final manuscript. All authors have contributed to this work. Conception and design: YTC, AGR. Analysis and interpretation: YTC, AGR. Data collection: YTC, PRF, RFF, AGR. Writing the article: AAM. Critical revision of the article: YTC, PRF, RFF, AAM, AGR, JMRR, PLS, RMB. Final approval of the article: YTC, PRF, RFF, AGR, AAM, JMRR, RMB, PLS. Statistical analysis: YTC. Overall responsibility: YTC.
Availability of data and materialThis work, figures and tables, have not been previously published and reproduced from another source.
Ethics approvalWe certify that this research was conducted in conformity with ethical principles of our institution. This study was carried out in strict compliance with the principles of the Declaration of Helsinki.
Consent to participateUnder the exceptional circumstances generated by the COVID-19 pandemic, the urgent need to obtain feasible data related to this new disease, and the noninterventional and retrospective nature of the project, the requirement that written patient consent be obtained to be included in the study was waived. All patients were codified by investigators of the participating centers before entering their data into the general database, thereby ensuring patient anonymity to investigators analyzing the database. This study was carried out in strict compliance with the principles of the Declaration of Helsinki. The authors designed the study, gathered and analyzed the data, vouched for the data and analysis, wrote the article, and decided to publish.
Data access and responsibilityThe principal investigator, Yale Tung Chen, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
FundingThis work has not been supported by public grants or financial support. No sources of funding were used to assist in the preparation of this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestEach author certifies that he has no commercial associations that might pose a conflict of interest in connection with the submitted article.










![Receiver operating characteristic (ROC) curve for predicting mortality. Orange line=reference line; blue line=only LUS score>7; red line=LUS score>7+CRP>70 mg/L; green line=LUS score>7+CRP>70 mg/L+age>70. (1) All patients with clinical COVID19 compatible. Receiver operating characteristic (ROC) curve for predicting mortality according to lung ultrasonography (LUS) score above 7 [area under the curve (AUC) of 59.9%, p=0.064], with also CRP above 70 [AUC of 69.3%, p≤0.001] and adding Age above 70 [AUC of 74.3%, p<0.001]. (2) Patients with clinical COVID19 compatible and positive RT-PCR. Receiver operating characteristic (ROC) curve for predicting mortality according to lung ultrasonography (LUS) score above 7 [area under the curve (AUC) of 54%, p=0.557], with also CRP above 70 [AUC of 68.9%, p=0.006] and adding Age above 70 [AUC of 75%, p<0.001]. (3) Patients with clinical COVID19 compatible and negative RT-PCR. Receiver operating characteristic (ROC) curve for predicting mortality according to lung ultrasonography (LUS) score above 7 [area under the curve (AUC) of 63.0%, p=0.154], with also CRP above 70 [AUC of 70.8%, p=0.022] and adding Age above 70 [AUC of 71.9%, p=0.016]. (4) Patients with clinical COVID19 compatible, chest X-ray COVID19 compatible and negative RT-PCR. Receiver operating characteristic (ROC) curve for predicting mortality according to lung ultrasonography (LUS) score above 7 [area under the curve (AUC) of 66.7%, p=0.230], with also CRP above 70 [AUC of 80.6%, p=0.028] and adding Age above 70 [AUC of 52.8%, p=0.841]. Receiver operating characteristic (ROC) curve for predicting mortality. Orange line=reference line; blue line=only LUS score>7; red line=LUS score>7+CRP>70 mg/L; green line=LUS score>7+CRP>70 mg/L+age>70. (1) All patients with clinical COVID19 compatible. Receiver operating characteristic (ROC) curve for predicting mortality according to lung ultrasonography (LUS) score above 7 [area under the curve (AUC) of 59.9%, p=0.064], with also CRP above 70 [AUC of 69.3%, p≤0.001] and adding Age above 70 [AUC of 74.3%, p<0.001]. (2) Patients with clinical COVID19 compatible and positive RT-PCR. Receiver operating characteristic (ROC) curve for predicting mortality according to lung ultrasonography (LUS) score above 7 [area under the curve (AUC) of 54%, p=0.557], with also CRP above 70 [AUC of 68.9%, p=0.006] and adding Age above 70 [AUC of 75%, p<0.001]. (3) Patients with clinical COVID19 compatible and negative RT-PCR. Receiver operating characteristic (ROC) curve for predicting mortality according to lung ultrasonography (LUS) score above 7 [area under the curve (AUC) of 63.0%, p=0.154], with also CRP above 70 [AUC of 70.8%, p=0.022] and adding Age above 70 [AUC of 71.9%, p=0.016]. (4) Patients with clinical COVID19 compatible, chest X-ray COVID19 compatible and negative RT-PCR. Receiver operating characteristic (ROC) curve for predicting mortality according to lung ultrasonography (LUS) score above 7 [area under the curve (AUC) of 66.7%, p=0.230], with also CRP above 70 [AUC of 80.6%, p=0.028] and adding Age above 70 [AUC of 52.8%, p=0.841].](https://static.elsevier.es/multimedia/23870206/0000015900000001/v2_202301300833/S2387020622003011/v2_202301300833/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

