Atrial fibrillation (AF) is common in patients admitted with severe COVID-19. However, there is limited data about the management of chronic anticoagulation therapy in these patients. We assessed the anticoagulation and incidence of major cardiovascular events in hospitalized patients with AF and COVID-19.
MethodsWe retrospectively investigated all consecutive patients with AF admitted with COVID-19 between March and May 2020 in 9 Spanish hospitals. We selected a control group of non-AF patients consecutively admitted with COVID-19. We compared baseline characteristics, incidence of major bleeding, thrombotic events and mortality. We used propensity score matching (PSM) to minimize potential confounding variables, as well as a multivariate analysis to predict major bleeding and death.
Results305 patients admitted with AF and COVID-19 were included. After PSM, 151 AF patients were matched with 151 control group patients. During admission, low-molecular-weight heparin was the principal anticoagulant and the incidence of major bleeding and mortality were higher in the AF group [16 (10.6%) vs 3 (2%), p=0.003; 52 (34.4%) vs 35 (23.2%), p=0.03, respectively]. The multivariate analysis showed the presence of AF as independent predictor of in-hospital major bleeding and mortality in COVID-19 patients. In AF group, a secondary multivariate analysis identified high levels of D-dimer as independent predictor of in-hospital major bleeding.
ConclusionsAF patients admitted with COVID-19 represent a population at high risk for bleeding and mortality during admission. It seems advisable to individualize anticoagulation therapy during admission, considering patient specific bleeding and thrombotic risk.
La fibrilación auricular (FA) es frecuente en pacientes ingresados por COVID-19 grave. Sin embargo, los datos sobre el manejo de la anticoagulación crónica en estos pacientes son escasos. Analizamos la anticoagulación y la incidencia de episodios cardiovasculares mayores en pacientes con FA ingresados por la COVID-19.
MétodosRetrospectivamente, se identificaron todos los pacientes con FA ingresados por la COVID-19 entre marzo y mayo de 2020, en 9 hospitales españoles. Se seleccionó un grupo control de pacientes ingresados consecutivamente por la COVID-19 sin FA. Se compararon las características basales, incidencia de hemorragias mayores, episodios trombóticos y mortalidad. Para reducir potenciales factores de confusión se realizó un emparejamiento por puntuación de propensión, así como un análisis multivariante para predecir hemorragia mayor y mortalidad.
ResultadosSe incluyeron 305 pacientes con FA ingresados por la COVID-19. Tras el emparejamiento por puntuación de propensión, 151 pacientes con FA fueron emparejados con 151 controles. Durante el ingreso, la heparina de bajo peso molecular fue el principal anticoagulante y la incidencia de hemorragia mayor y mortalidad fue mayor en el grupo de FA (16[10,6%] vs. 3[2%], p=0,003; 52[34,4%] vs. 35[23,2%], p=0,03, respectivamente). El análisis multivariante demostró la presencia de FA como predictor independiente de sangrados y mortalidad intrahospitalaria en los pacientes con la COVID-19. En el grupo de FA, un segundo análisis multivariante identificó valores elevados de dímero-D como predictor independiente de hemorragia mayor intrahospitalaria.
ConclusionesLos pacientes con FA ingresados por la COVID-19 representan una población de alto riesgo de sangrado y mortalidad durante el ingreso. Parece recomendable individualizar la anticoagulación durante el ingreso, considerando el riesgo específico de sangrado y trombosis.
The coronavirus disease 2019 (COVID-19) pandemic caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) has rapidly spread throughout the world causing significant morbidity and mortality.1 Several studies have repeatedly suggested that pre-existing cardiovascular comorbidities are associated with a worse prognosis COVID-19.2–5 Specifically, atrial fibrillation (AF) has been reported as a common condition in patients admitted with severe forms of COVID-19.3,6,7 It has been suggested that AF might be an independent predictor of mortality for these patients.8 However, underlying aetiology for this high mortality rates are not well established.
On the basis of possible drug-drug interactions, the American Heart Association (AHA) and the European Society of Cardiology (ESC) agree on the recommendation of heparin as the anticoagulant of choice in hospitalized COVID-19 patients with AF who are receiving prior anticoagulation.9,10 Certainly, the systematic anticoagulation with heparin is the most common strategy adopted by the physicians in this clinical scenario. However, there are not data regarding the different anticoagulation therapies in these patients and the incidence of potential outcomes, especially, thrombotic and major bleedings.
The primary aim of this study was to assess the incidence haemorrhagic and thrombotic events, mortality and the anticoagulation regimen in a multicentric cohort of patients with AF admitted with COVID-19. In addition, we pretend to identify clinical or analytical independent predictors of major bleeding and mortality during admission.
Patients and methodsPopulation and study designA case–control multicentric study was performed in 9 tertiary referral hospitals from Spain. All patients with previous or newly diagnosed AF admitted with confirmed SARS-CoV-2 infection between March 1st and May 31st, 2020, were retrospectively identified. Confirmed SARS-CoV-2 infection was defined as a positive nasopharyngeal polymerase chain reaction and/or positive serological test. Patients without a previous diagnosis of AF were considered as newly diagnosed AF and all were confirmed by ECG at admission. We included a control group of COVID-19 patients admitted during the same period without AF. The control group was obtained from the admitted COVID-19 patient database of the coordinator centre, which was provided by the Medical Records Department, and the patients were consecutively included by admission date until reaching the same number of AF group, 305 patients. Patients were followed up a mean of 7±1.6 months after hospitalization, by revision of medical digital records or by telephone contact when necessary. The Local Ethics Committee of the coordinator centre approved the study protocol.
Data collection and outcomesBaseline characteristics, hospitalization data and outcomes during admission and follow-up were analyzed. Local electronic medical records served as source data, which were extracted by each centre researcher and centralized to the study coordinator in an anonymized database.
Baseline investigations comprised previous comorbidities, including AF-cardiovascular risk factors with a particular focus on antithrombotic therapy (anticoagulant and antiplatelet). The diagnosis of previous AF was checked in medical records and with ECG, and new onset AF cases were identified with the admission ECG.
During admission, international normalized ratio (INR), platelet count, serum biochemical parameters, antithrombotic therapy, SARS-CoV-2 drugs treatment (hydroxychloroquine, azithromycin, lopinavir/ritonavir, darunavir/cobicistat, remdesivir) and intensive care unit (ICU) admission, were recorded. If different anticoagulant regimens were prescribed during admission, we considered as the principal anticoagulant that prescribed during >50% of the hospitalization period.
Major cardiovascular events during admission and at follow-up were collected. They included major bleeding, death, stroke and venous thromboembolism (VTE). Major bleeding was defined using International Classification of Diseases-10th Revision codes (including lower and upper gastrointestinal bleeding, intracranial haemorrhage, hematuria, hemoptysis and epistaxis) and confirmed by any of the following criteria: (1) haemoglobin drop <7g/dL in needing of any red blood cell transfusion; (2) at least two units of red blood cell transfusion within 48h. If bleeding was suspected without confirmation of an active bleeding source, we considered it as major bleeding if meet criteria (1) or (2). VTE included pulmonary embolism (PE) and deep vein thrombosis (DVT). Synchronously diagnosed DVT and PE in the same patient were considered 1 VTE event. Stroke was considered in case of established or transient ischaemia in brain.
The primary outcome of this study was the incidence of in-hospital major bleeding. Key secondary outcomes were in-hospital mortality and incidence of VTE and stroke. In discharged patients, additional secondary outcomes recorded during follow-up were outpatient mortality, major haemorrhage and thromboembolic events (VTE and stroke).
Statistical analysisNumerical variables are expressed as mean and standard deviation or, for non-normally distributed variables, as median [interquartile range]. Categorical variables are expressed as absolute and relative frequencies and were contrasted with the Pearson's chi-square test or Fisher's exact test in cases where applicability conditions were not met. For the quantitative variables, the Student's t-test was used for independent samples and the Mann–Whitney test was used for those variables with a non-normal distribution. A p value of <0.05 was considered statistically significant. Propensity score matching (PSM) was utilized to control for potential confounding variables. In order to analyze the primary outcome and predictors of it, we performed both multivariate logistic regressions for in-hospital major bleeding: first, in all the COVID-19 patients; and second, only in the AF cohort. In addition, we conducted a multivariate analysis for the key secondary outcome in-hospital mortality in COVID-19 patients. All the multivariate logistic regressions were performed before the PSM. Baseline differences (p<0.10) were introduced in the regression model. Kaplan–Meier survival analysis was performed for mortality during follow-up in the discharged patients and compared with the Log-Rank test. Individuals who experienced the event were censored at their event time. All analyses were conducted using SPSS Statistics (IBM SPSS 24.0, Armonk, NY, USA).
ResultsBasal clinical featuresThree hundred and five patients with previous or newly diagnosed AF admitted with confirmed COVID-19 were included and matched with a control group of COVID-19 patients without AF. Baseline characteristics of both groups are summarized in Table 1. Before matching, patients with AF were significantly older than controls, with a similar sex distribution and more prevalence of previous cardiovascular risk factors. In the AF cohort, most of the patients had permanent AF (61%), with lower proportions of paroxysmal (22%), persistent (5.2%) or newly diagnosed (11.8%). Before admission, most of the patients were on oral anticoagulants (Table A.1). During admission, low-molecular-weight heparin (LMWH) was the most commonly used anticoagulant in AF patients (83%), while DOAC (7.5%) and VKA (2.6%) were used in a minority of patients (Table A.2). After controlling for possible confounding factors using PSM, 151 patients with AF were matched with 151 patients without AF (Table 1). Similar proportions of AF subtypes and cardiovascular risk factors were obtained after matching in the AF cohort, but a higher proportion of cardiac disease in AF patients still remained statistically significant (Table 1). Regarding anticoagulant regimen during admission, there were expected differences after PSM, with a higher proportion of AF patients receiving therapeutic doses of LMWH than non-AF patients.
Baseline and clinical characteristics during admission of AF and control group patients, before and after propensity score matching.
| Variable | Total(n=610) | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|---|
| AF(n=305) | No-AF(n=305) | p | AF(n=151) | No-AF(n=151) | p | ||
| Baseline characteristics – no. (%) | |||||||
| Age, mean (SD) – yr | 72.9 (14.3) | 79 (10.3) | 66.8 (15.2) | <0.001 | 74.6 (11.0) | 75.1 (12.0) | 0.086 |
| Male sex | 337 (55.2) | 163 (53.4) | 174 (57) | 0.37 | 82 (54.3) | 51 (53.6) | 0.9 |
| HBP | 415 (68) | 244 (80) | 171 (56.1) | <0.001 | 115 (76.2) | 105 (69.5) | 0.19 |
| DM | 194 (31.9) | 117 (38.4) | 77 (25.2) | 0.001 | 46 (30.5) | 46 (30.5) | 1 |
| Dyslipidemia | 216 (35.4) | 123 (40.3) | 93 (30.5) | 0.011 | 63 (41.7) | 8 (38.4) | 0.56 |
| Obesity | 96 (15.7) | 68 (22.3) | 28 (9.2) | <0.001 | 24 (15.9) | 19 (12.6) | 0.41 |
| CKD | 105 (17.5) | 73 (23.9) | 34 (11.1) | <0.001 | 28 (18.5) | 25 (16.6) | 0.65 |
| DBADLa | 204 (33.4) | 133 (43.6) | 71 (23.3) | <0.001 | 46 (30.5) | 50 (33.1) | 0.62 |
| Alcoholism | 29 (4.8) | 18 (5.9) | 11 (3.6) | 0.183 | 8 (5.3) | 8 (5.3) | 1 |
| PVD | 100 (16.4) | 54 (17.7) | 46 (15.1) | 0.382 | 29 (19.2) | 23 (15.2) | 0.36 |
| Hepatopathy | 34 (5.6) | 22 (7.2) | 12 (3.9) | 0.078 | 7 (4.6) | 8 (5.3) | 0.79 |
| Recent strokeb | 5 (0.8) | 5 (1.6) | 0 | 0.06 | 2 (1.3) | 0 | 0.49 |
| Structural heart diseasec | 185 (30.5) | 147 (48) | 38 (12.5) | 0.001 | 69 (46) | 23 (15.3) | 0.001 |
| Mitral stenosisd | 3 (0.5) | 3 (1) | 0 | 0.24 | 1 (0.7) | 0 | 1 |
| Mechanical heart valve | 6 (1) | 5 (1.6) | 1 (0.3) | 0.21 | 3 (2) | 1 (0.7) | 0.62 |
| Prior major bleeding | 39 (6.4) | 28 (9.2) | 11 (3.6) | 0.005 | 8 (5.3) | 8 (5.3) | 1 |
| Prior stroke | 98 (16.1) | 73 (23.9) | 25 (8.2) | <0.001 | 17 (11.3) | 19 (12.6) | 0.72 |
| CHA2DS2-VASc (SD) | 3.5 (2) | 4.4 (1.7) | 2.6 (1.9) | <0.001 | 3.7 (1.6) | 3.3 (1.8) | 0.339 |
| Admission – no. (%) | |||||||
| Creatinine (SD)e | 1.3 (1.1) | 1.4 (1.3) | 1.2 (0.9) | 0.01 | 1.3 (1.16) | 1.3 (0.8) | 0.699 |
| D-dimer (SD)f | 5028 (18501) | 4280 (100001) | 5744 (23963) | 0.334 | 4733 (10192) | 6150 (15308) | 0.352 |
| Platelet count (SD)e | 213529 (91165) | 210767 (91299) | 216291 (91098) | 0.455 | 212490 (82950) | 214880 (91239) | 0.812 |
| ICU admission | 59 (9.7) | 32 (10.5) | 27 (8.9) | 0.493 | 24 (15.9) | 16 (10.6) | 0.174 |
| Hydroxychloroquine | 542 (88.9) | 246 (80.7) | 296 (97) | <0.001 | 131 (86.8) | 144 (95.4) | 0.009 |
| Azithromycin | 511 (83.8) | 217 (71.1) | 294 (96.4) | <0.001 | 108 (71.5) | 147 (97.4) | 0.001 |
| Darunavir/cobicistat | 37 (6.1) | 16 (5.2) | 21 (6.9) | 0.396 | 12 (7.9) | 13 (8.6) | 0.83 |
| Lopinavir/ritonavir | 327 (53.6) | 130 (42.6) | 197 (64.6) | <0.001 | 79 (52.3) | 82 (54.3) | 0.72 |
PSM, propensity score matching; AF, atrial fibrillation; SD, standard deviation; HBP, high blood pressure; DM, diabetes mellitus; CKD, chronic kidney disease; DBADL, dependency for basic activities of daily living; PVD, peripheral vascular disease; ICU, intensive care unit.
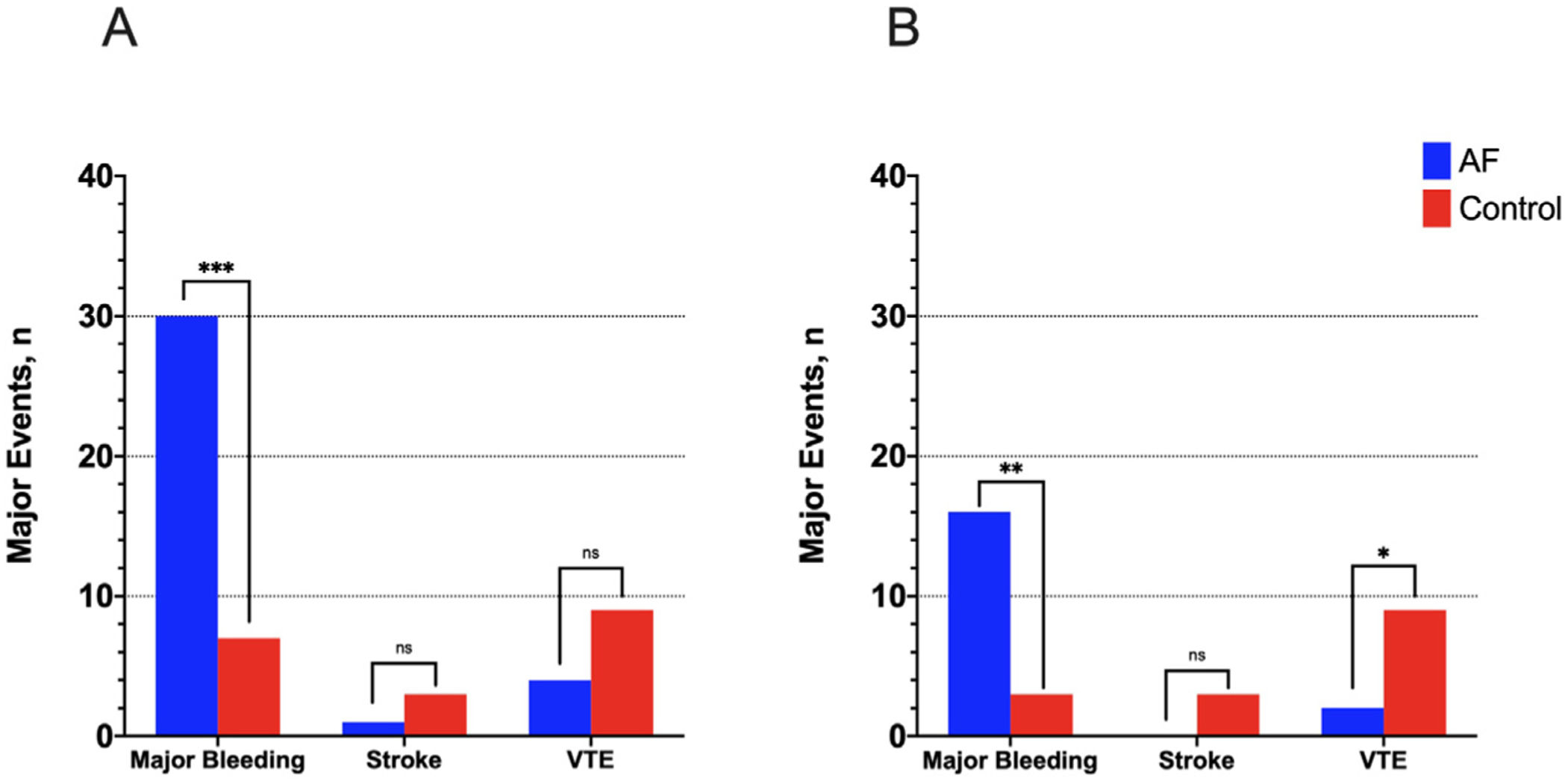
The primary outcome in-hospital major bleeding occurred in 37 (6%) patients: 30 (9.8%) and 7 (2.3%) in the AF and control groups, respectively (OR: 4.64, 95% CI 2.00–10.74, p<0.001). This finding was consistent after the PSM [16 (10.6%) vs 3 (2%), OR: 5.85, 95% CI 1.67–20.51, p=0.003] regardless of baseline and clinical comorbidities (Fig. 1). The list of major bleeding episodes in AF patients is summarized in Table A.3. AF patients with a major bleeding event during admission were compared with those with no major bleeding (Table 2). Dyslipidemia and obesity were more common in AF patients with in-hospital major bleeding (OR: 2.43, 96% CI 1.12–5.24, p=0.021; OR: 2.61, 95% CI 1.19–5.73, p=0.014, respectively). No major bleeding occurred in patients treated with DOACs. The multivariate analysis identified AF as independent predictor of in-hospital major bleeding in all the COVID-19 patients of the study (OR: 3.51, 95% CI 1.49–8.32, p=0.004), as well as prior major bleeding, ICU admission and diabetes mellitus (Table 3). The variable “prior anticoagulant therapy” was closely related to the presence of AF and therefore was excluded of the regression model. A secondary multivariate analysis was performed only in the AF cohort, showing high D-dimer levels as an independent predicting factor of major bleeding during admission (OR: 1.03, 95% CI 1.003–1.06, p=0.03) (Table A.4).
Incidence of major cardiovascular events. Incidence of major cardiovascular events in patients with and without AF in the whole sample (panel A) and after propensity score matching analysis (panel B). Note the significant difference between both groups in major bleeding, with a similar rate of AF-related thromboembolic complications. (ns: non significant; *** means p<0.001; ** means p<0.01 and * means p<0.05).
Baseline, clinical characteristics and endpoints during admission of AF patients depending on the presence of major bleeding during admission.
| Variable | Total(n=305) | No bleeding(n=275) | Bleeding(n=30) | p |
|---|---|---|---|---|
| Baseline characteristics – no. (%) | ||||
| Age, mean (SD) – yr | 79 (10.3) | 79.2 (10.3) | 77 (9.2) | 0.263 |
| Male sex | 163 (53.4) | 144 (52.4) | 19 (63.3) | 0.253 |
| HBP | 244 (80) | 216 (78.5) | 28 (93.3) | 0.055 |
| DM | 117 (38.4) | 102 (37.1) | 15 (50) | 0.167 |
| Dyslipidemia | 123 (40.3) | 105 (38.2) | 18 (60) | 0.021 |
| Obesity | 68 (22.3) | 56 (20.4) | 12 (40) | 0.014 |
| CKD | 73 (23.9) | 62 (22.5) | 11 (36.7) | 0.085 |
| DBADLa | 133 (43.6) | 123 (44.7) | 10 (33.3) | 0.232 |
| Alcoholism | 18 (5.9) | 15 (5.5) | 3 (10) | 0.402 |
| PVD | 54 (17.7) | 47 (17.1) | 7 (23.3) | 0.448 |
| Hepatopathy | 22 (7.2) | 19 (6.9) | 3 (10) | 0.464 |
| Recent strokeb | 5 (1.6) | 4 (1.5) | 1 (3.3) | 0.406 |
| Structural heart diseasec | 147 (48) | 131 (47.6) | 16 (53.3) | 0.462 |
| Mitral stenosisd | 3 (1) | 2 (0.7) | 1 (3.3) | 0.268 |
| Mechanical heart valve | 5 (1.6) | 4 (1.5) | 1 (3.3) | 0.406 |
| Prior major bleeding | 28 (9.2) | 23 (8.4) | 5 (16.7) | 0.173 |
| Prior stroke | 73 (23.9) | 65 (23.6) | 8 (26.7) | 0.712 |
| CHA2DS2-VASc (SD) | 4.4 (1.7) | 4.3 (1.7) | 4.6 (1.4) | 0.423 |
| Anticoagulant | 234 (76.7) | 207 (75.3) | 27 (90) | 0.07 |
| Antiplatelet | 38 (12.5) | 33 (12) | 5 (16.7) | 0.557 |
| Atrial fibrillation | ||||
| Newly diagnosed | 36 (11.8) | 34 (12.4) | 2 (6.7) | 0.519 |
| Paroxysmal | 67 (22) | 61 (22.2) | 6 (20) | |
| Persistent | 16 (5.2) | 13 (4.7) | 3 (10) | |
| Permanent | 186 (61) | 167 (60.7) | 19 (63.3) | |
| Admission – no. (%) | ||||
| Creatinine (SD)e | 1.06 (0.8–1.48) | 1.4 (1.3) | 1.6 (1.1) | 0.347 |
| D-dimer (SD)f | 4280 (10001) | 3936 (8281) | 7657 (20255) | 0.065 |
| Platelet count (SD)e | 210767 (91299) | 210145 (90115) | 216466 (103030) | 0.719 |
| ICU admission | 32 (10.5) | 27 (9.8) | 5 (16.7) | 0.222 |
| Hydroxychloroquine | 246 (80.7) | 223 (81.1) | 23 (76.7) | 0.56 |
| Azithromycin | 217 (71.1) | 194 (70.5) | 23 (76.7) | 0.482 |
| Darunavir/cobicistat | 16 (5.2) | 15 (5.5) | 1 (3.3) | 1 |
| Lopinavir/ritonavir | 130 (42.6) | 118 (42.9) | 12 (40) | 0.76 |
| LMWH | 253 (82.9) | 228 (82.9) | 25 (83.3) | 0.953 |
| Therapeutic dose | 79 (25.9) | 71 (25.8) | 8 (26.7) | 0.92 |
| Prophylactic dose | 174 (57) | 157 (57.1) | 17 (56.7) | 0.964 |
| DOAC | 23 (7.5) | 23 (8.4) | 0 | 0.145 |
| VKA | 8 (2.6) | 7 (2.5) | 1 (3.3) | 0.568 |
| No anticoagulation | 21 (6.9) | 17 (6.2) | 4 (13.3) | 0.138 |
| Endpoints during admission | ||||
| Stroke | 1 (0.3) | 1 (0.4) | 0 | 1 |
| Venous thromboembolism | 4 (1.3) | 3 (1.1) | 1 (3.3) | 0.341 |
| Exitus | 116 (38) | 103 (37.5) | 13 (43.3) | 0.529 |
SD, standard deviation; HBP, high blood pressure; DM, diabetes mellitus; CKD, chronic kidney disease; DBADL, dependency for basic activities of daily living; PVD, peripheral vascular disease; ICU, intensive care unit; LMWH, low-molecular-weight heparin; DOAC, direct oral anticoagulant; VKA, vitamin K antagonist.
Multivariate analysis. Independent predicting factors of in-hospital major bleeding in COVID-19 patients.
| Variable | Odds ratio | 95% CI | p | |
|---|---|---|---|---|
| Lower | Upper | |||
| Atrial fibrillation | 3.51 | 1.49 | 8.32 | 0.004 |
| Prior major bleeding | 2.83 | 1.06 | 7.56 | 0.038 |
| ICU admission | 3.06 | 1.27 | 7.37 | 0.013 |
| DM | 2.01 | 1.01 | 4.03 | 0.048 |
CI, confidence interval; DM, diabetes mellitus; ICU: intensive care unit.
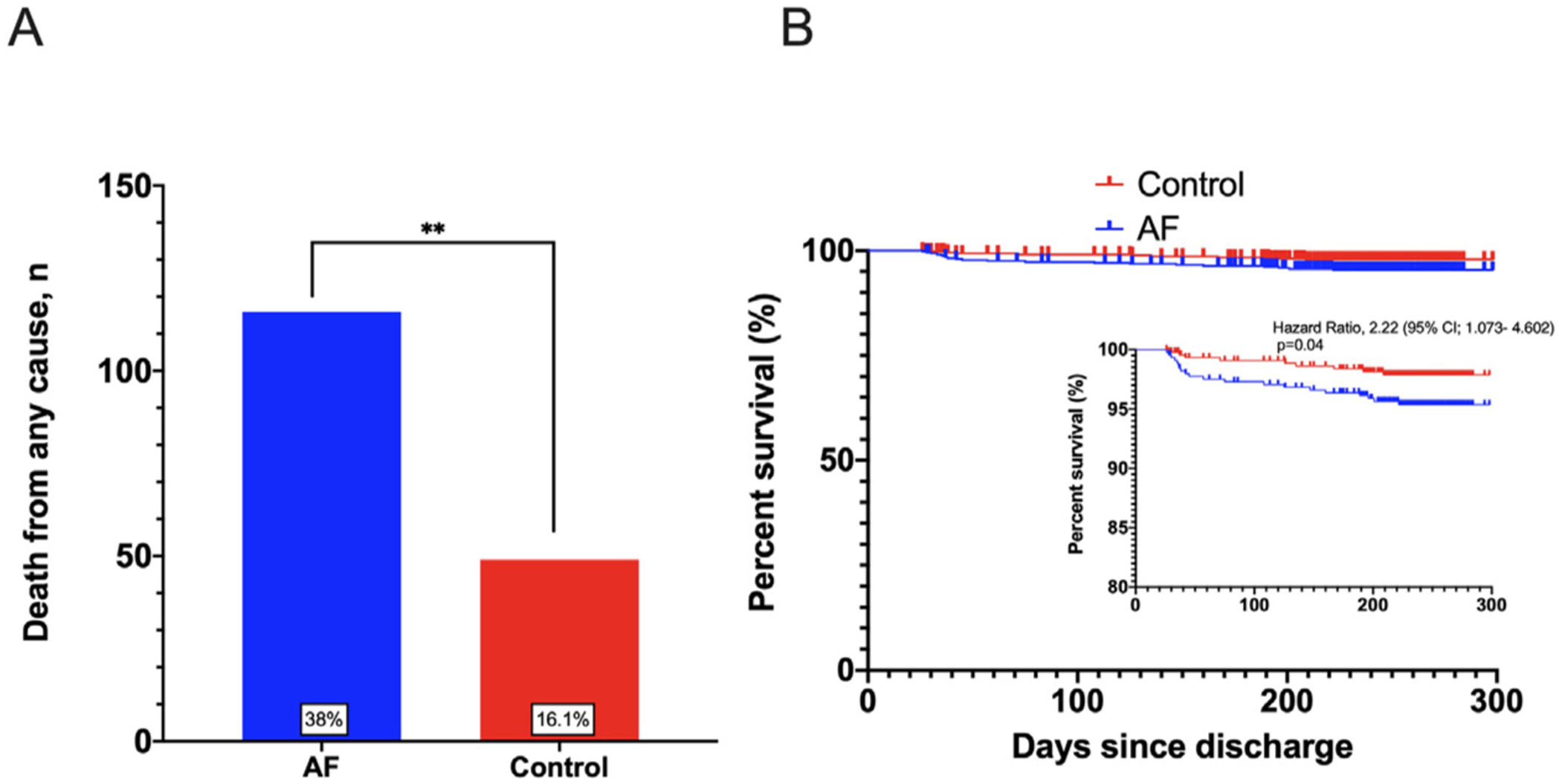
The incidence of the key secondary outcome in-hospital mortality was 165 (27%) and it was significantly higher in the AF group before and after PSM [116 (38%) vs 49 (16.1%), OR: 3.21, 95% CI 2.19–4.70, p<0.001; 52 (34.4%) vs 35 (23.2%), OR: 1.74, 95% CI 1.05–2.87, p=0.03, respectively] (Fig. 2A and Table A.5). Multivariate analysis showed the presence of AF as an independent predicting factor of in-hospital mortality (OR: 1.88, 95% CI 1.19–2.94, p=0.006) as well as male sex, older age, chronic kidney disease (CKD), D-dimer and absence of anticoagulation during admission (Table 4). However, the presence of major bleeding during admission as well as the anticoagulant therapy with LMWH were not independently associated with the increased mortality in AF patients.
Multivariate analysis. Independent predicting factors of in-hospital mortality in COVID-19 patients.
| Variable | Odds ratio | 95% CI | p | |
|---|---|---|---|---|
| Lower | Upper | |||
| Atrial fibrillation | 1.88 | 1.19 | 2.94 | 0.006 |
| Age | 1.06 | 1.04 | 1.08 | 0.01 |
| Male sex | 1.74 | 1.14 | 2.64 | 0.01 |
| D-dimer | 1.02 | 1.01 | 1.04 | 0.002 |
| CKD | 2.31 | 1.43 | 3.72 | 0.001 |
| Anticoagulation during admission | 0.026 | |||
| LMWHa | 1 | – | – | – |
| No anticoagulation | 3.80 | 1.40 | 10.30 | 0.01 |
| DOAC | 1.59 | 0.62 | 4.06 | 0.33 |
| VKA | 0.27 | 0.03 | 2.36 | 0.24 |
CI, confidence interval; CKD, chronic kidney disease; DOAC, direct oral anticoagulant; VKA, vitamin K antagonist.
Finally, there were no differences between both groups in the other key secondary outcomes of stroke and VTE events during admission. Nevertheless, there was a trend towards a lower incidence in AF group (Fig. 1).
Events at follow upRespectively, 189 (62%) and 256 (83.9%) patients with and without AF were discharged after the COVID-19 admission. In the AF group, DOAC therapy was the most frequent anticoagulant prescribed at discharge (45.7%), followed by AVK (14.5%) and LMWH (9.6%); up to 30.2% were not prescribed any anticoagulant. In the control group, most of the patients were not anticoagulated after discharge (97.3%).
During a mean follow-up period of 7±1.6 months, incidence of outpatient major bleeding was similar in the AF group compared with control group (4.8% vs 2.7%, p=0.256), as well as stroke (1.6% vs 1%, p=0.65) and VTE events (0% vs 0.8%, p=0.51). Interestingly, a significantly higher outpatient mortality rate was still present among patients with AF (Fig. 2B). A secondary multivariate analysis was performed in the AF cohort for 6-month mortality, showing the male sex, age, dependency for basic activities of daily living and CKD as independent predictors of higher mortality in AF patients (Table A.6).
DiscussionPatients with previous or newly diagnosed AF, admitted with SARS-CoV-2 infection, present an increased risk of major bleeding, as well as an extremely high rate of mortality during admission and at follow up after discharge, with a low rate of thromboembolic events, which was similar to the control group. These worst outcomes seem to be independent of previous clinical status, cardiovascular risk factors or prior cardiovascular and bleeding events.
Although in our study we describe a high incidence of major bleeding in patients with AF, we did not identify a statistical association between any anticoagulant and major bleeding events. However, we recognize that a relevant proportion of these patients received therapeutical doses of LMWH, with a residual use of DOACs. In fact, we did not observe major bleeding episodes in patients treated during admission with DOACs, although significant differences could not be demonstrated due to the retrospective and not randomized design of our study.
Regarding mortality, here we suggest that AF involves an independent poor prognosis in COVID-19 patients in the same way to other recent studies.8,11 However, due to the previously mentioned limitations, we cannot confirm if the high proportion of major bleeding observed in AF patients with COVID-19 is related to their high mortality rate.
Several studies have been reported AF as a frequent condition in patients admitted with severe forms of COVID-193,6–8 and it has lately been associated with an increased risk of unfavourable outcomes in COVID-19 patients.11 A remarkable finding of our research was that the absence of anticoagulation during admission was independently associated with a higher mortality in patients with COVID-19, but not with thrombotic events. Although a proper anticoagulation and a lower mortality in COVID-19 patients has been established before,12–14 our data suggest that some of these deaths might be attributable to an advanced COVID-19 disease or extreme fragility that prevent physicians from anticoagulating them.
Although patients with AF are a population at high risk for worse outcomes of COVID-19, the risk of bleeding events and the safest anticoagulation therapy has not been established yet. On the basis of possible drug-drug interactions between DOAC and VKA with empiric COVID-19 pharmacotherapy and their potential consequences, the AHA and the ESC both agree on the recommendation of heparin as the anticoagulant of choice in hospitalized COVID-19 patients with AF, who are receiving prior anticoagulation.9,10 In the present study, most of the patients with AF were treated with therapeutic doses of LMWH during admission, with low levels of continuation of prior anticoagulation with DOAC and VKA. This strategy has been previously named as bridging therapy and is associated with higher risk of bleeding with no significant difference in mortality or thrombotic events, especially in the setting of perioperative invasive procedures.15–18
In line with this information, here we describe a high incidence of major bleeding in AF patients receiving full LMWH dosing during admission, before and after controlling for confounders with PSM, with low levels of thrombotic events. In contrast, control group patients without AF showed lower incidence of major bleeding before and after PSM, with a higher proportion of patients receiving a prophylactic LMWH dose.
Another important finding of our study was the independent association of AF with in-hospital major bleeding in COVID-19 patients, something that has not been previously described. This novel finding should be taken into account and it suggests that precise management of anticoagulation might reduce the risk of bleeding. Additionally, we observed that high levels of D-dimer were strongly associated with high risk of major bleeding in AF patients. Interestingly, a common clinical approach in COVID-19 patients with elevated D-dimer levels is to intensify the anticoagulant doses of heparin, something that might aggravate haemorrhagic complications, especially in AF patients.
DOACs have repeatedly been found to be safer and more effective than VKA antagonists in the treatment of nonvalvular AF, especially in older patients.19,20 Taking these data into account, and the low and similar rate of thromboembolic events amongst our study groups, a change in the anticoagulation strategy in the COVID-19 patients with AF might be considered, giving a more important role to DOACs. Moreover, most of the drugs with potential interaction with DOACs have been proven to be ineffective against COVID-19, so are used less and less.
Al-Samkari et al.21 analyzed the rate of bleeding and thrombotic complications in a large multicentre cohort of critically ill and noncritically ill COVID-19 patients, showing a major bleeding rate of 2.3%. Here, we describe a higher incidence of major bleeding events among patients with AF. Although the control group patients of the present study were very similar to the patients of their study, certainly, patients with AF in our study were older, had more comorbidities and the majority of them were treated with LMWH at therapeutic dose, than those of Al-Samkari study. All these facts are likely related with this higher bleeding rate. Anyway, these potential confounding variables were controlled with PSM; hence, the anticoagulation regimen seems to be playing an important role.
In summary, patients with prior or newly diagnosed AF admitted with COVID-19 represent a population at high risk for major bleeding and mortality during the hospitalization. It seems critical to individualize anticoagulation therapy during admission, considering patient specific risks for bleeding and VTE.
LimitationsThe non-randomized nature of the study limits the conclusions about the influence of the anticoagulation therapy and outcomes. This bias is partially controlled with propensity score matching study that have achieved very similar study groups. Larger and randomized studies are required to better clarify this issue. Data on the severity of the SARS-CoV-2 infection were not fully collected. The high mortality rate is in many cases more due to the COVID-19 infection rather than cardiovascular events. However, this is similar for both PSM groups and differences related to the AF condition are still present.
Authors’ contributionsAll of the authors had access to the data and participated in the preparation of the manuscript.
Ethical considerationsThe study protocol was approved by the Local Ethics Committee of the coordinator centre.
FundingNone declared.
Conflict of interestsThe authors declare that there is no conflict of interest.