To characterize health care-related adverse events in patients with SARS-CoV-2 infection who died in a tertiary hospital.
MethodsThis is a retrospective, observational study, that included patients who died at HUGTiP hospital between 16 March and 10 April 2020. Data was extracted from the electronic medical record.
ResultsThe median age of the 164 SARS-CoV-2 infected patients who died in the center in the study period was 77.5 years and >90% of patients had ≥1 comorbidity. Forty point two percent of patients had at least ≥1 health care-related adverse event. Twenty three point eight of patients had an adverse drug reaction, the leading cause of adverse events in patients who died. Of patients who died in intensive care units, the frequency of problems related to mechanical ventilation was 8.8%.
ConclusionsAlthough the case fatality rate associated with the adverse events detected was very low, close monitoring of potential health care-related adverse events, especially drug reactions, as the therapeutic management of the disease remains unclear.
Caracterizar los eventos adversos relacionados con la asistencia sanitaria en pacientes infectados por SARS-CoV-2 fallecidos en un hospital de tercer nivel.
MétodosEstudio observacional retrospectivo en el que se incluyeron los pacientes fallecidos en el centro entre el 16 de marzo y el 10 de abril de 2020. La información fue extraída desde la historia clínica electrónica.
ResultadosLa mediana de edad de los 164 pacientes analizados fue de 77,5 años. Más de 9 de cada 10 pacientes fallecidos presentaban al menos una comorbilidad. El 40,2% de los pacientes presentó al menos un evento adverso (EA) asociado a la atención sanitaria. Un 23,8% de los pacientes presentó alguna reacción adversa a medicamentos, constituyendo la primera causa de EA entre los pacientes fallecidos. Entre los pacientes que fallecieron en unidades de cuidados intensivos, los problemas relacionados con la ventilación mecánica han aparecido con una frecuencia del 8,8%.
ConclusionesA pesar de que la letalidad asociada a los EA detectados fue muy reducida, es fundamental establecer una vigilancia estrecha de los posibles EA asociados a la asistencia sanitaria, especialmente los farmacológicos, dado que se trata de una enfermedad con un manejo terapéutico incierto.
On 31 December 2019, the Wuhan Municipal Wuhan Municipal Health and Sanitation Commission (Hubei Province, China) informed of a cluster of 27 cases of pneumonia of unknown aetiology. On 7 January 2020, the Chinese authorities identified a new type of virus from the family Coronaviridae, as the causative agent of the outbreak which was named SARS-CoV-2.1 By mid-April 2020, almost three million people worldwide had been reported as infected, of whom approximately 115,000 had died.2
With seroprevalence data of 5% in the Spanish territory, most of the COVID-19 cases reported so far are mild or asymptomatic cases.3 The prevalence of underlying disease is 49% in non-hospitalised cases, 79% in hospitalised cases, 81% in those admitted to the ICU, and 95% in the deceased. 87% of patients who die are over 70 years old.4 The clinical treatment of a large number of the hospitalised patients entails dealing not only with this new infectious entity, but also with the patient’s comorbidities and their being advanced in years.
Hospital mortality committees are interested in evaluating mortality related to healthcare, which in itself implies it is potentially preventable, as an indicator of possible healthcare deficits.5 The objective of this study was to analyse the adverse events related to healthcare in patients with SARS-CoV-2 infection who died in a tertiary hospital during the first weeks of the pandemic.
Material and methodDesign and study populationThis is a retrospective observational study, which analysed data from patients infected with SARS-CoV-2 (positive PCR analysis) and who died during hospitalisation of the Germans Trias i Pujol University Hospital. The study period ranges from the death of the first patient infected by SARS-CoV-2 in the hospital (16 March), until 10 April 2020. Patients with compatible symptoms but with a negative PCR result were not included in the analysis, nor were those hospitalised patients who, being PCR positive, did not die during the study period.
Information sources and variables of the studyThe data were collected from the patients’ electronic medical records. The primary variables were classified as: sociodemographic, comorbidities at the time of admission, duration of hospital admission, complications associated with the infection, and cause of death. Also adverse events potentially related to health care were collected from the medical record: adverse drug reactions, problems with respirators, venous thromboembolic complications, pressure ulcers and problems in the treatment of blood glucose. The review of each medical record was carried out by at least two members of the mortality committee involved in this study.
Statistic analysisThe characteristics of the patients included in the study were described using absolute and relative frequencies for the qualitative variables and mean and standard deviation for the quantitative parametric variables, or with median and interquartile range for quantitative nonparametric variables. Next, a comparison was made between the characteristics of cases in which therapeutic limitation was applied upon admission with those that it was not. The χ2 test (or Fisher's test when the application requirements were not met) was used to assess the distribution of qualitative variables.
Confidentiality and data protectionThis study was carried out following the basic ethical principles contained in the Declaration of Helsinki (Fortaleza, October 2013) and it was evaluated and approved by the hospital's Research Ethics Committee.
The project researchers collected the data from the patients’ electronic medical records. The information was analysed via an anonymised database. Patient data was processed in accordance with the Personal Data Protection Law of the European Regulation (EU) 2016/679 and its transposition in the Organic Law 3/2018.
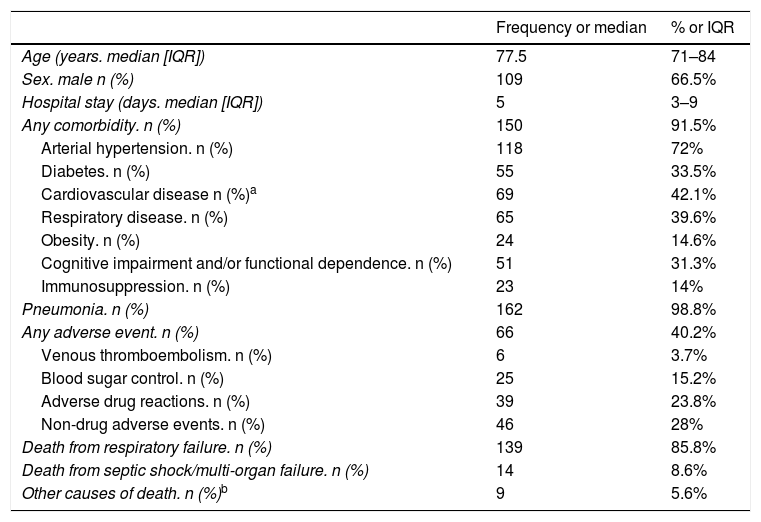
ResultsA total of 164 patients with SARS-CoV-2 infection died in the hospital during the study period. The characteristics of these patients are shown in Table 1. 73.3% of the patients died in conventional hospitalisation units (including the Short Stay Unit in the emergency department), 19.4% in critical care units and 7.3% in the emergency cubicles. 4.3% of the patients died in the external hospital facilities equipped and enabled for the pandemic. Almost 90% of the deaths were recorded in five departments: Infectious Diseases (26.2%), Intensive Medicine (18.3%), Internal Medicine (18.3%), Pulmonology (15.2%) and Acute Geriatrics Unit (11.6%). The median hospital stay was 5 days (IQR 3–9).
Characteristics of patients infected with SARS-CoV-2, who died during hospitalisation during the study period (n = 164).
| Frequency or median | % or IQR | |
|---|---|---|
| Age (years. median [IQR]) | 77.5 | 71–84 |
| Sex. male n (%) | 109 | 66.5% |
| Hospital stay (days. median [IQR]) | 5 | 3–9 |
| Any comorbidity. n (%) | 150 | 91.5% |
| Arterial hypertension. n (%) | 118 | 72% |
| Diabetes. n (%) | 55 | 33.5% |
| Cardiovascular disease n (%)a | 69 | 42.1% |
| Respiratory disease. n (%) | 65 | 39.6% |
| Obesity. n (%) | 24 | 14.6% |
| Cognitive impairment and/or functional dependence. n (%) | 51 | 31.3% |
| Immunosuppression. n (%) | 23 | 14% |
| Pneumonia. n (%) | 162 | 98.8% |
| Any adverse event. n (%) | 66 | 40.2% |
| Venous thromboembolism. n (%) | 6 | 3.7% |
| Blood sugar control. n (%) | 25 | 15.2% |
| Adverse drug reactions. n (%) | 39 | 23.8% |
| Non-drug adverse events. n (%) | 46 | 28% |
| Death from respiratory failure. n (%) | 139 | 85.8% |
| Death from septic shock/multi-organ failure. n (%) | 14 | 8.6% |
| Other causes of death. n (%)b | 9 | 5.6% |
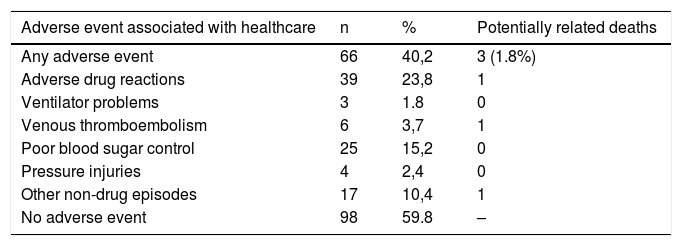
40.2% of the patients presented at least one adverse event associated with health care (Table 2). 23.8% of the patients presented an adverse drug reaction (ADR), constituting the first cause of adverse events among deceased patients. One death was potentially ADR-related. This patient came another hospital due to a psoas hematoma after anticoagulation therapy with enoxaparin. Death was due to an acute myocardial infarction after DIVAS was performed in our hospital.
Distribution of patients with adverse events and potentially related deaths associated with healthcare among SARS-CoV-2 infected patients who died during hospitalisation during the study period.
| Adverse event associated with healthcare | n | % | Potentially related deaths |
|---|---|---|---|
| Any adverse event | 66 | 40,2 | 3 (1.8%) |
| Adverse drug reactions | 39 | 23,8 | 1 |
| Ventilator problems | 3 | 1.8 | 0 |
| Venous thromboembolism | 6 | 3,7 | 1 |
| Poor blood sugar control | 25 | 15,2 | 0 |
| Pressure injuries | 4 | 2,4 | 0 |
| Other non-drug episodes | 17 | 10,4 | 1 |
| No adverse event | 98 | 59.8 | – |
Of the 34 patients who were intubated and received invasive mechanical ventilation, 8.8% presented ventilator-related problems: poor administration of the programmed tidal volume and loss of PEEP in two patients, and tension pneumothorax caused by excess positive pressure in fibrotic lung in one patient. These problems did not in themselves evoke death, but they were contributing factors to the worsening of the patients’ respiratory conditions.
Among the 164 deceased patients, six cases (3.7%) of high suspicion or certainty of venous thromboembolism were detected, of which four occurred in critical care units and two in the emergency department or the conventional hospital ward. All of them had received suitable thromboprophylaxis in accordance with the protocols. One case of suspected massive pulmonary embolism was considered to be directly related to the patient’s death. During hospital admission, 15.2% of the patients presented some degree of difficulty in controlling blood sugar levels (either hypoglycemia or hyperglycemia). Blood sugar level control problems appeared both in patients with and without a previous diagnosis of diabetes (13/25 and 12/25 respectively). The blood sugar alterations were related to the use of high doses of bolus corticosteroids in 12 patients and to the use of hydroxychloroquine or chloroquine in 3 patients. A causal relationship with patient death has not been established in any of the cases.
Pressure injuries were detected in 2.4% of the patients. Of these four cases, three patients were partially or totally care-dependent regarding basic activities of daily living. The median age of the patients with pressure injuries was 79 years and the median length of stay was 14.5 days (IQR: 7–77).
DiscussionFour out of every ten patients in this study suffered an adverse event associated with healthcare, with adverse drug reactions highlighted as the most frequent factor. Despite this, the lethality associated with the adverse events detected was very low.
The median age of the SARS-CoV-2 infected patients who died at the hospital during the first weeks of the pandemic was 77.5 years. 75% of the patients were over 71 years old. Two thirds of the deceased were males. More than nine out of ten of these patients had some comorbidity. The most frequently detected was arterial hypertension (72%) followed by cardiovascular disease (42.5%). These data are consistent with the recent systematic review by Tian et al., which concludes that the presence of comorbidities such as hypertension, coronary heart disease and diabetes are associated with a significantly higher risk of death among patients with COVID-19.6
The prevalence of health care-related adverse events found in this study (40.2% of patients) is substantially higher than published data on adverse events in hospitalised patients. Previous studies that quantify adverse events using the tool Global Trigger Tool show lower prevalence figures for adverse events in the United States (33.2%)7 and Switzerland (20.5%).8 Unlike these studies, our work focuses on the analysis of subjects treated during a pandemic, with very high levels of care burden. Furthermore, the thoroughness with which the review of the medical records was carried out may be related to the detection of a greater number of adverse events. Finally, the increased prevalence of adverse drug reactions during hospitalisation (23.8% of patients) has also been higher than that published previously under non-pandemic conditions (15%).9 This has contributed substantially to the final count of adverse events from any cause. Only one patient died as a result of an adverse drug reaction (an outpatient who came from another hospital). This prevalence (0.6%) is very similar to that obtained in a study conducted in our hospital with data from patients who died due to adverse drug reactions during 2015.10
The problems related to mechanical ventilation have appeared infrequently (8.8%) among patients admitted to intensive care units. In none of the three cases were these problems the direct cause of the patient's death. In the care of COVID-19 patients, non-specific respirators of critical units have had to be used, mainly taken from the operating rooms of the hospital, and which were designed for other performances. Although it would not have affected the number of deaths, it is likely that the use of more appropriate respirators for ventilation of the critical patient would have reduced the percentage of adverse events due to this cause.
Venous thromboembolic disease (VTD) has generally led to a significant increase in morbidity and mortality in patients infected with SARS-CoV-2, especially among those who had to be admitted to critical care units.11 Due to the characteristics of our study where no data comparison was made with that of non-deceased patients, it was not possible to determine whether the incidence of thromboembolic complications in deceased patients was higher than other patients. In the majority of the cases, the personnel administered correct thromboprophylaxis, in accordance with the hospital protocols of the VTD committee and the COVID-19 hospital group of clinical experts, based on the recommendations of scientific societies.12 Although generalised thromboprophylaxis at the beginning of the pandemic was controversial, all the admitted patients presented severe disease due to COVID-19, with the resultant immobility and respiratory failure for most, leading to the consensus for thromboprophylaxis. Later, in the second half of the study period, the association between COVID-19 and VTD was already well characterized,13 therefore, thromboprophylaxis was recommended in all cases of COVID-19 that required hospitalisation, except in those cases in which it was contraindicated. All thromboembolic complications were detected and treated appropriately, including for those patients whose outcome was death.
This study has been carried out by members of the mortality committee, a multidisciplinary team that analyses the information from different points of view. The medical record of all the patients included in the study was reviewed by at least two of the group’s researchers. Among the limitations it should be noted that in times of such critical care overload as that experienced during the pandemic, the accuracy of data recording during the patient’s clinical progression, and the completeness of the epicrisis reports by health professionals may have been reduced. Given that the study includes the first deceased patients cared for during the first weeks of the pandemic (March to early April 2020), the results will probably be different from those obtained about patients who died subsequently, mainly due to the progressive advance in knowledge and treatment of the disease and the changes in the hospital occupancy rate. This analysis is a crude analysis and merely descriptive, which represents a substantial limitation in data extrapolation. Lastly, an inherent limitation of the study design to be noted is the absence of cases of patients infected with SARS-CoV-2 who survived hospitalisation.
From the analysis of the data of deaths from SARS-CoV-2 in the first weeks of the pandemic, we can derive the need for close monitoring of possible adverse reactions to the drugs administered without clear evidence of efficacy against the infection, and in relation to a disease for which therapeutic treatment is still uncertain.
Authorship/collaboratorsGM and EM have contributed equally as first authors.
Conflict of interestsThe authors declare that they have no conflict of interest.
Please cite this article as: Mena G, Montané E, Rodríguez M, Beroiz P, López-Núñez JJ, Ballester M. Caracterización y eventos adversos relacionados con la asistencia sanitaria en pacientes infectados por el SARS-CoV-2 fallecidos en un hospital de tercer nivel. Med Clin (Barc). 2021;156:277–280.