To analyze the incidence of Covid-19 in patients who are chronic users of hydroxychloroquine.
Patients and methodsCross-sectional retrospective observational multicenter study in health areas and districts from Castilla La-Mancha and Andalucia. Of the 4451 participants included in the first recruitment, 3817 with valid data were selected. The main variable of the study is the presence or absence of Covid-19 infection by clinical, serological or polymerase chain reaction diagnosis. Sociodemographic and clinical variables and treatment and concomitant comorbidities were recorded.
Results169 (4,45%) patients had Covid-19 infection, of which 12 (7.1 %) died and 32 (18.9%) required hospital admission. Previous respiratory pathology was related to Covid-19 infection (P < .05). Maculopathy appears in 5.3% of patients and is significantly related to the dose of hydroxychloroquine consumed (P < .05).
ConclusionThere is no relationship between chronic use of hydroxychloroquine and the incidence of Covid-19.
Analizar la incidencia de la enfermedad del coronavirus 19 (COVID-19) en pacientes consumidores crónicos de hidroxicloroquina.
Pacientes y métodosEstudio multicéntrico observacional retrospectivo transversal en Áreas de Salud de Castilla La-Mancha y distritos sanitarios de Andalucía. De los 4.451 participantes incluidos en el primer reclutamiento se seleccionaron 3.817 sujetos con datos válidos. La variable principal del estudio ha sido la presencia o ausencia de infección por la COVID-19 por diagnóstico clínico, serológico o por reacción en cadena de la polimerasa. Se registraron variables sociodemográficas, clínicas y tratamientos y comorbilidades concomitantes.
ResultadosCiento sesenta y nueve (4,45%) pacientes presentaron infección por la COVID-19, de los cuales fallecieron 12 (7,1%) y 32 (18,9%) requirieron ingreso hospitalario. La enfermedad respiratoria previa se relacionó con la infección por la COVID-19 (p < 0,05). La maculopatía aparece en un 5,3% de los pacientes y está relacionada significativamente con la dosis de hidroxicloroquina consumida (p < 0,05).
ConclusiónNo existe relación entre consumo crónico de hidroxicloroquina e incidencia de la COVID-19.
Chloroquine and hydroxychloroquine (HCQ) are drugs widely used in the treatment of various diseases such as acute or chronic rheumatoid arthritis and lupus erythematosus due to their immunomodulatory effect. HCQ is also used for the prophylaxis and treatment of uncomplicated malaria caused by sensitive plasmodium species, as an alternative to chloroquine. The off-label use of HCQ is also quite widespread in diseases such as antiphospholipid syndrome and different dermatological conditions.
The off-label use of HCQ in COVID-19 has presented various ethical dilemmas; using the fight against the pandemic as the most prominent all-justifying factor has led to undermining evidence-based decision making.1,2 Following its widespread use for the treatment of COVID-19 in healthcare facilities around the world, the Infectious Diseases Society of America has recommended that HCQ only be used in the context of a clinical trial.3
Given the immediate need for effective treatments for the management of the COVID-19 infection, published data have raised great expectations about these drugs However, the limited level of evidence adds to the uncertainties about aspects of the disease itself, and its different stages of severity, which may determine the efficacy or the benefit-risk balance of the treatment depending on the stage when it is used.
There are no published clinical trials with HCQ and chloroquine, although there are data in vitro and a review on the role of chloroquine in the management of SARS-CoV-2 infection.4 HCQ appears to be effective in limiting the replication of SARS-CoV-2 in vitro, through the inhibition of nucleic acid synthesis, viral proteins glycosylation, virus assembly, transport of new virus particles, and viruses release.5 This justifies its use in regards to the objective to gather information on clinical efficacy in patients in the context of clinical trials. Following this, a synergistic effect has been observed in vitro when combined with azithromycin.6
Both chloroquine and HCQ have the ability to prolong the QT interval, which can lead to a severe interaction when administered together with other drugs that cause the same effect, including azithromycin.7 There is currently very limited information about the safety of their combined administration,8 and its use outside the context of a clinical trial is not recommended.3,9
The joint use of HCQ with azithromycin is based on the greater negativisation results of the nasopharyngeal viral load, although it can cause QT interval lengthening, hypoglycemia, neuropsychiatric effects and idiosyncratic hypersensitive reactions,10 therefore, it is necessary to assess the benefit-risk ratio in each patient and exercise extreme caution. A systematic review11 concluded that it does not produce clinical benefits, indicating that the use of HCQ does not decrease the viral load and increases mortality from all causes among acute hospitalised patients who have been administered this drug. In a study published in non-hospitalised COVID-19 patients with early symptoms, treated with HCQ, no reduction in the severity of symptoms was observed.12 Recently, a study has been published regarding the use of HCQ with or without associated azithromycin in patients admitted with mild-moderate symptoms. No improvement was observed in the clinical condition after 15 days compared to standard treatment, while an elevation and prolongation of the QT interval and elevated liver enzymes were observed.13 In another study in hospitalised patients with mild-moderate COVID-19, the use of HCQ did not produce greater negative seroconversion than patients on standard treatment, while the occurrence of adverse events was higher.14
Currently, the Food and Drug Administration has revoked the authorisation for the use of HCQ in patients with COVID-19, due to the null benefit-risk balance that this drug presents in these patients.15
It has been queried whether the use of non-steroidal anti-inflammatory drugs and treatment with antihypertensive drugs such as angiotensin converting enzyme inhibitors (ACEIs) and/or angiotensin-II receptor antagonists (ARA-II) could be a severe risk factor, which could even include mortality for hospitalised patients infected with COVID-19.16–18 However, to date there are no clinical data that support a greater severity in the evolution of the infection in patients treated with these anti-inflammatory and antihypertensive drugs. The recommendations are based mainly on experimental findings, without evidence of a real clinical effect in humans.
Putting to one side the beneficial effect of HCQ in patients with acute illness due to COVID-19, the objective of this study was to verify the possible protective effect of HCQ on the incidence of COVID-19 in chronic HCQ users. At the same time, the influence that respiratory comorbidities, diabetes, obesity and hypertension could have in these patients has been evaluated, as well as the concomitant consumption of HCQ with other drugs such as: ACEI, ARA-II and/or non-steroidal anti-inflammatory drugs (NSAIDs).
Patients and methodsThis is an anonymised cross-sectional retrospective observational multicentre study.
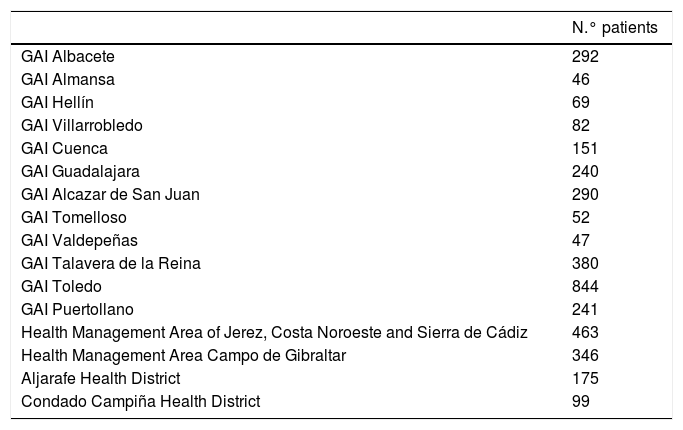
The study population included all patients over 18 years of age who used HCQ chronically for the indications approved in the summary of product characteristics and some off-label indications, such as antiphospholipid syndrome and different dermatological conditions. The patients in the study (Table 1) are under the Integrated Care Management groups (Gerencias de Atencion Integrada — GAI) of Albacete, Almansa, Hellín, Villarrobledo, Cuenca, Guadalajara, Alcázar de San Juan, Tomelloso, Valdepeñas, Talavera, Toledo, Puertollano, Jerez Health Management Area, Costa Noroeste and Sierra de Cádiz, Campo de Gibraltar Health Management Area, Aljarafe Health District and Condado Campiña Health District.
Number of patients according to health areas.
| N.° patients | |
|---|---|
| GAI Albacete | 292 |
| GAI Almansa | 46 |
| GAI Hellín | 69 |
| GAI Villarrobledo | 82 |
| GAI Cuenca | 151 |
| GAI Guadalajara | 240 |
| GAI Alcazar de San Juan | 290 |
| GAI Tomelloso | 52 |
| GAI Valdepeñas | 47 |
| GAI Talavera de la Reina | 380 |
| GAI Toledo | 844 |
| GAI Puertollano | 241 |
| Health Management Area of Jerez, Costa Noroeste and Sierra de Cádiz | 463 |
| Health Management Area Campo de Gibraltar | 346 |
| Aljarafe Health District | 175 |
| Condado Campiña Health District | 99 |
GAI: Integrated Care Management.
Patients were selected using the primary care computer systems and the Health System prescription billing programmes. Both databases were crossed, and all patients who, between 01 January and 28 February 2020, withdrew HCQ containers from the chemist, were screened, verifying that the HCQ treatment had lasted for at least 6 months. The initial sample consisted of 4451 patients, leaving 3817 patients in the final sample after excluding those who did not meet some of the inclusion criteria, or who had not consumed the drug for a minimum of 6 months, or who had died for reasons aside from COVID-19. The time period studied to confirm whether these patients have suffered from COVID-19 infection was from 01 March to 30 June 2020. During this period, all the patients continued to consume HCQ on a regular basis, as well as the rest of the drugs that were object of study herein.
The number of patients were collected from the medical history processing system of the various participating areas, using either the ICD or the CIAP code: V01.82 (exposure to SARS-associated coronavirus), 079.82 (SARS-associated coronavirus infection), 480.3 (pneumonia due to SARS-associated coronavirus) and A77.01 (coronavirus infection, unspecified, with no diagnostic group).
The main study variables were:
- •
Diagnosis of absence of active COVID-19 infection confirmed by polymerase chain reaction (PCR) or serological tests.
- •
Diagnosis of absence of past COVID-19 infection by serological tests.
- •
Diagnosis of absence of active COVID-19 infection due to the absence of clinical symptoms of acute respiratory infection.
- •
HCQ dosage.
COVID-19 with mild symptoms was defined as the appearance of symptoms such as cough, fatigue, and fever, while severe symptoms were the presence of dyspnea, decreased saturation, and radiologically confirmed bilateral pneumonia.
As secondary variables, data was collected regarding: age, sex, patient at home or institutionalised, treatment with ACEI or ARA-II, chronic treatment with NSAIDs for at least 3 months, comorbidities affecting the respiratory system (chronic obstructive pulmonary disease [COPD], asthma, emphysema, etc.), obesity (body mass index >30 kg/m2), presence or not of: smoking, diabetes, hypertension and comorbidities such as acute myocardial infarction (AMI), stroke, immunosuppression and cancer-chemotherapy.
With the data obtained, a bivariate analysis of the sample’s categorical variables using proportions was performed, followed by the calculation of a hypothesis contrast between proportions for non-parametric independent samples (Chi squared).
The data was entered, stored and analysed using the SPSS 22.0 program.
The study was classified as EPA-OD (PAS-OD post-authorisation study — other design) by the Spanish Agency for Medicines and Medical Devices (AEMPS) with the code FTCHID-2020-04 and approved by the Medicinal Products Research Ethics Committee of the Albacete Integrated Care Management, with code 2020-24 (EPA-OD).
The study was carried out in accordance with the principles derived from the Declaration of Helsinki (Revision of Fortaleza, 2013), the good clinical practice guidelines and current legal regulations (Biomedical Research Act 14/2007).
All the information obtained from the study participants was treated confidentially, complying with Organic Law 3/2018, on the Protection of Personal Data with its last update on 25 July 2019.
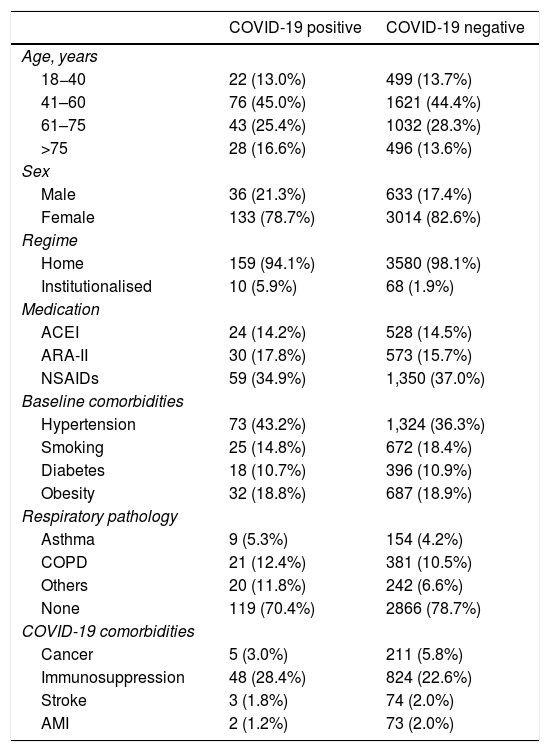
ResultsThe final sample studied was 3817 patients (Table 2), of which 3148 (82.5%) were females and 669 were males (17.5%). Of these 3817 patients, only 78 (2.0%) were in an institutionalised regime. Age was divided into 4 ranges: 18–40 years (13.6%), 41–60 years (44.5%), 61–75 years (28.2%) and over 75 years (13.7%).
Sociodemographic and clinical characteristics of the study population.
| COVID-19 positive | COVID-19 negative | |
|---|---|---|
| Age, years | ||
| 18−40 | 22 (13.0%) | 499 (13.7%) |
| 41–60 | 76 (45.0%) | 1621 (44.4%) |
| 61–75 | 43 (25.4%) | 1032 (28.3%) |
| >75 | 28 (16.6%) | 496 (13.6%) |
| Sex | ||
| Male | 36 (21.3%) | 633 (17.4%) |
| Female | 133 (78.7%) | 3014 (82.6%) |
| Regime | ||
| Home | 159 (94.1%) | 3580 (98.1%) |
| Institutionalised | 10 (5.9%) | 68 (1.9%) |
| Medication | ||
| ACEI | 24 (14.2%) | 528 (14.5%) |
| ARA-II | 30 (17.8%) | 573 (15.7%) |
| NSAIDs | 59 (34.9%) | 1,350 (37.0%) |
| Baseline comorbidities | ||
| Hypertension | 73 (43.2%) | 1,324 (36.3%) |
| Smoking | 25 (14.8%) | 672 (18.4%) |
| Diabetes | 18 (10.7%) | 396 (10.9%) |
| Obesity | 32 (18.8%) | 687 (18.9%) |
| Respiratory pathology | ||
| Asthma | 9 (5.3%) | 154 (4.2%) |
| COPD | 21 (12.4%) | 381 (10.5%) |
| Others | 20 (11.8%) | 242 (6.6%) |
| None | 119 (70.4%) | 2866 (78.7%) |
| COVID-19 comorbidities | ||
| Cancer | 5 (3.0%) | 211 (5.8%) |
| Immunosuppression | 48 (28.4%) | 824 (22.6%) |
| Stroke | 3 (1.8%) | 74 (2.0%) |
| AMI | 2 (1.2%) | 73 (2.0%) |
NSAIDs: non-steroidal anti-inflammatory drugs; ARA-II: angiotensin II receptor antagonists; COVID-19: coronavirus 19 disease; COPD: chronic obstructive pulmonary disease; AMI: acute myocardial infarction; ACEI: angiotensin converting enzyme inhibitors.
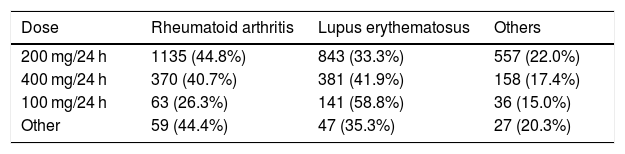
79.6% of the patients had been treated with HCQ for the indications described in the summary of product characteristics (42.6% for rheumatoid arthritis or polyarthritis and 37.0% for lupus erythematosus). The rest, 20.4% of the patients, took HCQ for off-label use (indications not on the SmPC).
The most prescribed dose of HCQ was 200 mg/24 h in 66.4% of patients. Table 3 shows the dose distribution according to the different diagnoses. In the ‘other dosages’ variable, which only represented 3.5%, regimes with weekend breaks, 300 mg/24 h, or alternate days were included.
The adverse reactions produced by the chronic use of HCQ included QT interval abnormalities in 42 patients (1.1%), ophthalmological disorders, mainly maculopathy, in 202 patients (5.3%) and other adverse reactions in 155 patients (4.1%). When relating the ophthalmological adverse effects with the dose of HCQ consumed, differences were found between consuming the dose of 200 mg/24 h and the appearance of adverse effects (p < 0.05) (58.9% vs. 66.9%).
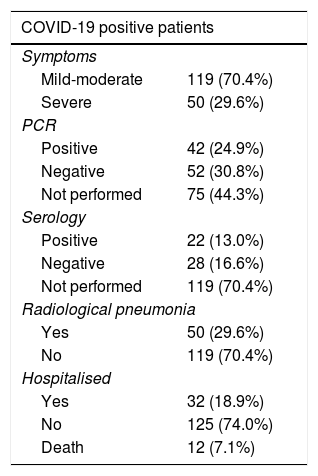
Of the 3817 patients, 169 (4.45%) were diagnosed as COVID-19, either based on clinical criteria (positive registration with the ICD and CIAP codes described above), PCR or serology.
As shown in Table 4, of the 169 diagnosed patients, 70.4% had mild-moderate symptoms, 42 showed a positive PCR result, while only 22 had a positive serological test result and 29.6% of the patients presented bilateral pneumonia by radiography. 18.9% of the patients had to be hospitalised and in 12 patients the cause of death was attributed to infection by COVID-19.
Clinical characteristics of COVID-19 patients.
| COVID-19 positive patients | |
|---|---|
| Symptoms | |
| Mild-moderate | 119 (70.4%) |
| Severe | 50 (29.6%) |
| PCR | |
| Positive | 42 (24.9%) |
| Negative | 52 (30.8%) |
| Not performed | 75 (44.3%) |
| Serology | |
| Positive | 22 (13.0%) |
| Negative | 28 (16.6%) |
| Not performed | 119 (70.4%) |
| Radiological pneumonia | |
| Yes | 50 (29.6%) |
| No | 119 (70.4%) |
| Hospitalised | |
| Yes | 32 (18.9%) |
| No | 125 (74.0%) |
| Death | 12 (7.1%) |
COVID-19: coronavirus disease 19; PCR: polymerase chain reaction.
Of the patients diagnosed with COVID-19, 78.7% were females, 70.4% were in the age group between 40 and 75 years and more than 94% lived at home, with very few patients residing in an institutionalised regime. Of the COVID-19 patients who died, 58.3% were males and 41.7% females, 75% were over 75 years old, 16.6% between 61 and 75 years old and 8.4% between 41 and 60 years.
Regarding the pharmacological treatments used by these patients, 14.2% regularly used ACEIs, 17.8% ARA-II and 34.9% NSAIDs. To compare the benefit-risk relationship of taking these drugs with the evolution of the disease, a comparison was made between the proportion of consumption of these treatments by the group diagnosed with COVID-19 and the group not diagnosed, and no relationship was found between consumption of these drugs and COVID-19 infection, or between mild-moderate and severe symptoms.
Our results confirm that the presence of some type of chronic respiratory disease is related to the appearance of COVID-19 infection (p < 0.05) (29.6% vs. 21.3%). However, the relationship between smoking and the presence of COVID-19 (14.8%–18.4%) did not show differences.
Within the group of COVID-19 patients, 43.2% had hypertension, 10.7% diabetes and 18.8% obesity. When comparing hypertension, diabetes, and obesity with the non-COVID-19 group of patients, no statistically significant relationship was found, so these factors cannot be considered as risk factors in our sample.
Among the seemingly obvious comorbidities that would aid a worse prognosis of coronavirus disease such as stroke, AMI and cancer-chemotherapy, is immunosuppression at 28.4%, which appeared more frequently in HCQ consumer patients (65% of the patients did not present any of these comorbidities).
In the period from 1 March 2020 to 31 July 2020, 73,320 patients with the ICD and CIAP coronavirus codes were registered in the Sescam primary care databases. This data, referring to 2,032,863 patients from Castilla La Mancha (National Institute of Statistics, January 2019) represents a 3.6% incidence of COVID-19. Of these 73,320 patients, a total of 2440 patients were registered as deaths, which represents 3.3% deaths. In the studied sample of chronic HCQ consumer patients, 169 patients with COVID-19 were found, representing an incidence of 4.40%, and of these 12 patients died, representing 7.1%. Comparing mortality in the group of COVID-19 patients treated with HCQ versus COVID-19 patients from the general population, no significant differences were observed.
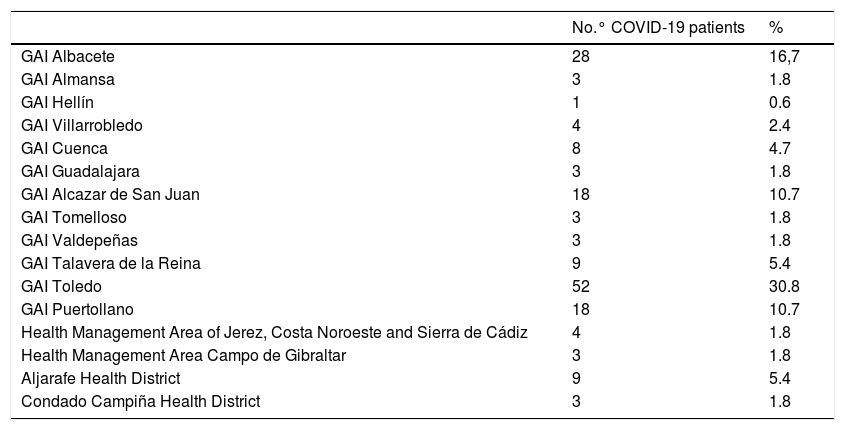
When the sample was stratified into 2 groups (Table 5), Castilla La-Mancha (CLM) and Andalusia, the sample of CLM patients was 2,734, of which 150 patients were diagnosed with COVID-19, and the Andalusian sample was 1083 patients of which 19 were diagnosed with COVID-19. Once the sample was separated, the incidence in CLM became 5.49%, while that of Andalusia was just 1.75%. This data confirmed the differences in terms of incidence that have been observed in the studies nationwide.
Number of COVID-19 patients according to health areas and health districts.
| No.° COVID-19 patients | % | |
|---|---|---|
| GAI Albacete | 28 | 16,7 |
| GAI Almansa | 3 | 1.8 |
| GAI Hellín | 1 | 0.6 |
| GAI Villarrobledo | 4 | 2.4 |
| GAI Cuenca | 8 | 4.7 |
| GAI Guadalajara | 3 | 1.8 |
| GAI Alcazar de San Juan | 18 | 10.7 |
| GAI Tomelloso | 3 | 1.8 |
| GAI Valdepeñas | 3 | 1.8 |
| GAI Talavera de la Reina | 9 | 5.4 |
| GAI Toledo | 52 | 30.8 |
| GAI Puertollano | 18 | 10.7 |
| Health Management Area of Jerez, Costa Noroeste and Sierra de Cádiz | 4 | 1.8 |
| Health Management Area Campo de Gibraltar | 3 | 1.8 |
| Aljarafe Health District | 9 | 5.4 |
| Condado Campiña Health District | 3 | 1.8 |
GAI: Integrated Care Management.
The COVID-19 pandemic has been a challenge for health systems and biomedical research worldwide, establishing a race against time to find a treatment, whether preventive or curative, to alleviate the effects of the disease. Drugs that inhibit the interleukin pathway (tocilizumab), or antivirals such as remdesivir are being investigated. Recently, the Convacta trial with tocilizumab has been suspended for not improving the clinical condition of the patient, nor for reducing mortality (Roche press release dated 29 July 2020). Remdesivir, for its part, has not yet provided clear data on its efficacy, offering only a small reduction in days of hospitalization.19 There are currently numerous vaccines being developed, of which some are more promising than others. However, we must not forget the importance of meeting the deadlines since these vaccines are going to be administered to healthy subjects, and the minimum requirements are that they demonstrate real and lasting efficacy over time and, above all, that they do not present safety problems. Obviously, if we lag in time the long-term safety problems will probably go unnoticed. Another of the drugs widely studied and used off-label has been HCQ. Studies have shown its inefficacy in the acute phase of the disease and the appearance of safety problems especially of the heart.13 In view of these results, we decided to change the problem perspective and study the possible protective effect that HCQ produces in patients who consume it chronically for various diseases.
This study may present certain limitations, the main one being the absence of a control group, although we think that this fact does not invalidate the results obtained. Although it has not been a study objective, it is important to consider how health professionals have dealt with the study patients with COVID-19 symptoms. At the time of diagnosis, these patients were already taking the drug, which was the main recommendation at that time of the pandemic, so the therapeutic approach in these patients could not have been easy.
The first conclusion we can draw from our data is that chronically consumed HCQ does not protect against COVID-19 infection. In our study group, the incidence of coronavirus was 4.4%, compared to 3.3% in the data obtained from patients classified with ICD or CIAP codes, and this difference was not significant.
At the beginning of the pandemic, it was speculated that the use of drugs that inhibit the renin-angiotensin system and NSAIDs could be a risk factor for coronavirus infection, and with a worse prognosis in the case of being infected. At a later date it was published that the consumption of these drugs does not worsen the course of the disease,18 and that they do not favour infection. This fact has been confirmed in our study, since there are no differences between the group that consumed these drugs and those that did not take them in terms of the incidence of COVID-19 infection.
Although the comorbidities such as obesity, diabetes and hypertension do not seem to be related to a higher incidence or prognosis of COVID-19, hypertension is present in a greater proportion of these patients (p = 0.08). The same occurs with immunosuppression, which does not reach statistical significance but however it appears in a higher percentage in COVID-19 patients. These data coincide with the results obtained in the study on comorbidities present in patients who died from COVID-19 in Aragon.20 Isolated comorbidities could explain some deaths, but the presence of several comorbidities and polypharmacy would be true risk factors to explain the lethality of this disease.
From our results, we can conclude that the presence of underlying respiratory disease (asthma, COPD, emphysema, etc.), but not smoking, is related to the increased incidence of COVID-19 in our group of patients. This data supports the use of masks in the group of respiratory patients, despite recent debates as to whether this type of patient should be exempt from the use of a mask.
An aspect not related to COVID-19, although of utmost importance, is the significant off-label use of HCQ (up to 20% in our study group) not indicated in the summary of product characteristics. This carries implications, since this is a drug that presents safety problems, both when used alone and concomitantly with other drugs that lengthen the QT interval. It is worth noting how these HCQ users are monitored for any ophthalmological adverse effects. Maculopathy is the main clinical manifestation, appearing in 5.2% of the study patients, coinciding with existing papers in this regard.21
The sample comes from 2 clearly differentiated areas, such as CLM and Andalucía Oeste. The results of the incidence of COVID-19 in our sample show a difference between both areas, 5.49% versus 1.75% respectively. These data coincide with the incidence data that have been offered throughout the pandemic period, in which CLM has been one of the regions most affected by the disease, while Andalusia (and in general the South of the Spanish peninsula) has been much less affected.
In summary, this multicentre study has found that the chronic use of HCQ does not provide benefits to patients. This result corroborates with the numerous studies that have confirmed the little or no benefit of HCQ when used as a treatment in acute patients, whether associated with azithromycin or not.
All these results obtained in the commented studies are disappointing to the health systems. They highlight how extremely difficult it will be to establish protocols of action in the event of a new wave of infections, as the only drugs that have shown a favourable safety and efficacy profile to date are corticosteroids.22
AuthorshipThe following people have contributed to the review of the data: Sonia Martínez Cruz, Julia de Fez Herráiz, Adriana Arcega Baraza, María Teresa González Zarca, Carolina Payá Giner, Ángel Fernández Mistal, María Dolores Pérez Pacheco, Macarena Flores Dorado, Piedad Reguera Martínez, Olga Rojas Corrales, María Isabel Ibarra Lorente, María Elena Carretero Albiñana, María Isabel Tofiño González, Belén de la Hija Díaz, Beatriz de la Calle Riaguas, Juan José Navarro Agüera, Rosario Lara Olivares, Dolores Caniego Rodrigo, Yolanda González Gero, Inmaculada Casa Hidalgo, Aina Tomás Luiz, Eva María García Martínez, Nuria Monteagudo Martínez, Ismael Pérez Alpuente, Francisco Tomás Pagán Núñez, Ana Ramírez Córcoles, Rocío Pardo Sánchez, Palmira Quero González, Rocío Peña Pou, Alejandro Rodríguez Delgado, Francisco M. Ferrer Soler y Alejandro Rodríguez Delgado.
Conflict of interestsThe authors declare that they have no conflict of interest.
Please cite this article as: Tejada Cifuentes F, Lloret Callejo Á, Tirado Peláez MJ, Rubio Pulido O, Ruiz-Morote Aragón M, Fernández Urrusuno R, et al. Incidencia de la COVID-19 en pacientes en tratamiento crónico con hidroxicloroquina. Med Clin (Barc). 2021;156:166–171.