Despite the rapid global increase of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, there is currently no effective treatment for patients who have developed severe coronavirus disease 2019 (COVID-19). These severe COVID-19 cases are marked with excess cytokine production and a higher mortality rate. Our previous analysis confirmed that an elevated level of interleukin-6 (IL-6) and C-reactive protein (CRP) are strongly associated with COVID-19 progression.1,2 Thus, it is reasonable to suggest that the inhibition of IL-6 signaling cascade may effectively treat patients with severe SARS-CoV-2 infection. Another potential consideration regarding disease progression is the role of IL-6 gene polymorphisms. The two most extensively studied IL-6 gene promoter polymorphisms, −174G/C (rs1800795) and −572C/G (rs1800797), have been shown to affect both the transcription and secretion level of IL-6.3 Although the role of such polymorphisms have not been studied among COVID-19 patients specifically, it has been demonstrated in other infectious pneumonias.
In this article, we present a systematic review and meta-analysis on the efficacy of anti-IL-6 receptor (anti-IL-6R) antibody in neutralizing IL-6 by evaluating the reduction of the C-reactive protein (CRP) inflammatory marker, clinical outcomes, and the adverse events among severe COVID-19-infected patients. Additionally, a meta-analysis was also performed to estimate the association between IL-6 gene polymorphism with predisposition as well as disease severity of pneumonia.
All meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.4 Records were identified through electronic databases dated up to May 2020 with search terms such as “COVID-19” “SARS-CoV-2”, “IL-6”, “anti-IL-6R”, “Tocilizumab (TCZ), polymorphism”, and “pneumonia” (See Supplementary material). No language restrictions were applied. For TCZ treatment, studies with case-control design evaluating clinical outcomes (i.e., mortality rate, ICU admission, the requirement of mechanical ventilation, and the number of discharged patients) and its adverse events were included. Whereas, for IL-6 gene polymorphisms, studies were included on the basis of the following criteria: (1) aims to evaluate the association between IL-6 gene polymorphisms with predisposition to pneumonia; (2) conducted with a case-control design; and (3) evaluates IL-6 gene polymorphisms in pneumonia patients with or without severe condition (i.e., extra pulmonary bacterial dissemination, sepsis, and multiple organ dysfunction syndrome (MODS)).
Meta-analysis for each gene polymorphism was performed for two or more studies. Genotypic frequency of IL-6 gene polymorphism was tested for deviation from the Hardy–Weinberg equilibrium (HWE) in the control subjects. The associations between IL-6 gene polymorphism with predisposition to pneumonia or severity of pneumonia were calculated by pooled odds ratio (OR) and 95% confidence interval (CI). The Z test was used to evaluate the significance of the pooled effect size. Study heterogeneity was evaluated using Q test and I2 statistic. A significant Q-statistic (p<0.10) indicated heterogeneity across studies, with substantial heterogeneity indicated by an I2 value over 50%. The fixed-effect model (FEM) was used in the absence of heterogeneity, whilst the random-effect model (REM) was implemented if heterogeneity was present. A funnel plot and Begg's test were used to investigate the publication bias if the pooled effect size consisted of 10 or more studies. The value of 0.05 was indicative of the statistical significance. The Newcastle–Ottawa scale (NOS) was used to assess the study quality, in which a score≥7 is considered a good study.5–10
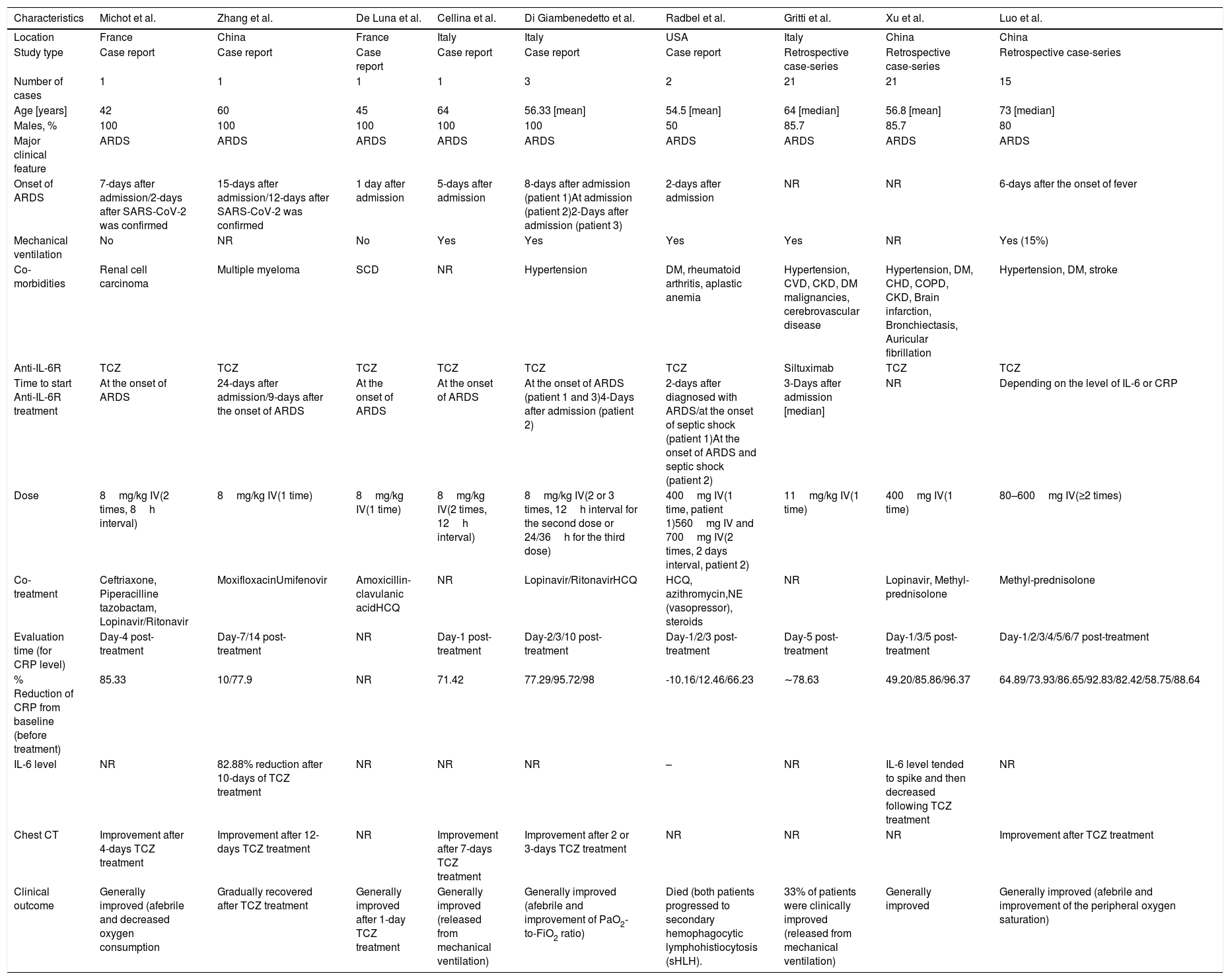
Nine case reports/case-series were included for the analysis on anti-IL-6R antibody treatment (summarized in Table 1) with a total sample of n=66 patients. A large proportion of the samples (89%) were male, with ages ranging from 42 to 73 years old.10–18 All patients developed severe COVID-19, marked by acute respiratory distress syndrome (ARDS) during admission, and more than half of studies reported the use of mechanical ventilators. Hypertension was the most common co-morbidity observed in patients with SARS-CoV-2 infection, followed by diabetes mellitus (DM), cerebrovascular disease, cardiovascular disease (CVD), and chronic kidney disease (CKD). Eight of the studies administered TCZ treatment,11–18 while one utilized Siltuximab.19 One to three times injection of anti-IL-6R antibody was mainly given during the onset of ARDS,11,13–15,18 while the rest were administered several days after the admission/ARDS onset12,15,18,19 or depending on the level of IL-6 or CRP.17 Several additional treatments were given in the studies, including antivirals, antibiotics, corticosteroids, anti-malaria (hydroxychloroquine/HCQ), and vasopressors.
Systematic review of case report and case-series evaluating anti-IL-6R treatment in severe COVID-19.
| Characteristics | Michot et al. | Zhang et al. | De Luna et al. | Cellina et al. | Di Giambenedetto et al. | Radbel et al. | Gritti et al. | Xu et al. | Luo et al. |
|---|---|---|---|---|---|---|---|---|---|
| Location | France | China | France | Italy | Italy | USA | Italy | China | China |
| Study type | Case report | Case report | Case report | Case report | Case report | Case report | Retrospective case-series | Retrospective case-series | Retrospective case-series |
| Number of cases | 1 | 1 | 1 | 1 | 3 | 2 | 21 | 21 | 15 |
| Age [years] | 42 | 60 | 45 | 64 | 56.33 [mean] | 54.5 [mean] | 64 [median] | 56.8 [mean] | 73 [median] |
| Males, % | 100 | 100 | 100 | 100 | 100 | 50 | 85.7 | 85.7 | 80 |
| Major clinical feature | ARDS | ARDS | ARDS | ARDS | ARDS | ARDS | ARDS | ARDS | ARDS |
| Onset of ARDS | 7-days after admission/2-days after SARS-CoV-2 was confirmed | 15-days after admission/12-days after SARS-CoV-2 was confirmed | 1 day after admission | 5-days after admission | 8-days after admission (patient 1)At admission (patient 2)2-Days after admission (patient 3) | 2-days after admission | NR | NR | 6-days after the onset of fever |
| Mechanical ventilation | No | NR | No | Yes | Yes | Yes | Yes | NR | Yes (15%) |
| Co-morbidities | Renal cell carcinoma | Multiple myeloma | SCD | NR | Hypertension | DM, rheumatoid arthritis, aplastic anemia | Hypertension, CVD, CKD, DM malignancies, cerebrovascular disease | Hypertension, DM, CHD, COPD, CKD, Brain infarction, Bronchiectasis, Auricular fibrillation | Hypertension, DM, stroke |
| Anti-IL-6R | TCZ | TCZ | TCZ | TCZ | TCZ | TCZ | Siltuximab | TCZ | TCZ |
| Time to start Anti-IL-6R treatment | At the onset of ARDS | 24-days after admission/9-days after the onset of ARDS | At the onset of ARDS | At the onset of ARDS | At the onset of ARDS (patient 1 and 3)4-Days after admission (patient 2) | 2-days after diagnosed with ARDS/at the onset of septic shock (patient 1)At the onset of ARDS and septic shock (patient 2) | 3-Days after admission [median] | NR | Depending on the level of IL-6 or CRP |
| Dose | 8mg/kg IV(2 times, 8h interval) | 8mg/kg IV(1 time) | 8mg/kg IV(1 time) | 8mg/kg IV(2 times, 12h interval) | 8mg/kg IV(2 or 3 times, 12h interval for the second dose or 24/36h for the third dose) | 400mg IV(1 time, patient 1)560mg IV and 700mg IV(2 times, 2 days interval, patient 2) | 11mg/kg IV(1 time) | 400mg IV(1 time) | 80–600mg IV(≥2 times) |
| Co-treatment | Ceftriaxone, Piperacilline tazobactam, Lopinavir/Ritonavir | MoxifloxacinUmifenovir | Amoxicillin-clavulanic acidHCQ | NR | Lopinavir/RitonavirHCQ | HCQ, azithromycin,NE (vasopressor), steroids | NR | Lopinavir, Methyl-prednisolone | Methyl-prednisolone |
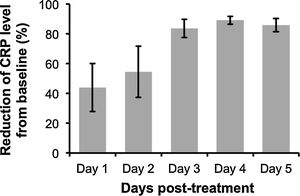
| Evaluation time (for CRP level) | Day-4 post- treatment | Day-7/14 post- treatment | NR | Day-1 post-treatment | Day-2/3/10 post- treatment | Day-1/2/3 post-treatment | Day-5 post- treatment | Day-1/3/5 post-treatment | Day-1/2/3/4/5/6/7 post-treatment |
| % Reduction of CRP from baseline (before treatment) | 85.33 | 10/77.9 | NR | 71.42 | 77.29/95.72/98 | -10.16/12.46/66.23 | ∼78.63 | 49.20/85.86/96.37 | 64.89/73.93/86.65/92.83/82.42/58.75/88.64 |
| IL-6 level | NR | 82.88% reduction after 10-days of TCZ treatment | NR | NR | NR | – | NR | IL-6 level tended to spike and then decreased following TCZ treatment | NR |
| Chest CT | Improvement after 4-days TCZ treatment | Improvement after 12-days TCZ treatment | NR | Improvement after 7-days TCZ treatment | Improvement after 2 or 3-days TCZ treatment | NR | NR | NR | Improvement after TCZ treatment |
| Clinical outcome | Generally improved (afebrile and decreased oxygen consumption | Gradually recovered after TCZ treatment | Generally improved after 1-day TCZ treatment | Generally improved (released from mechanical ventilation) | Generally improved (afebrile and improvement of PaO2-to-FiO2 ratio) | Died (both patients progressed to secondary hemophagocytic lymphohistiocytosis (sHLH). | 33% of patients were clinically improved (released from mechanical ventilation) | Generally improved | Generally improved (afebrile and improvement of the peripheral oxygen saturation) |
ARDS, acute respiratory distress syndrome; CVD, cardiovascular disease; CKD, chronic kidney disease; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; DM, diabetes mellitus; HCQ, hydroxychloroquine; IV, intravenous; NR, not reported; SCD, sickle cell disease. TCZ, Tocilizumab.
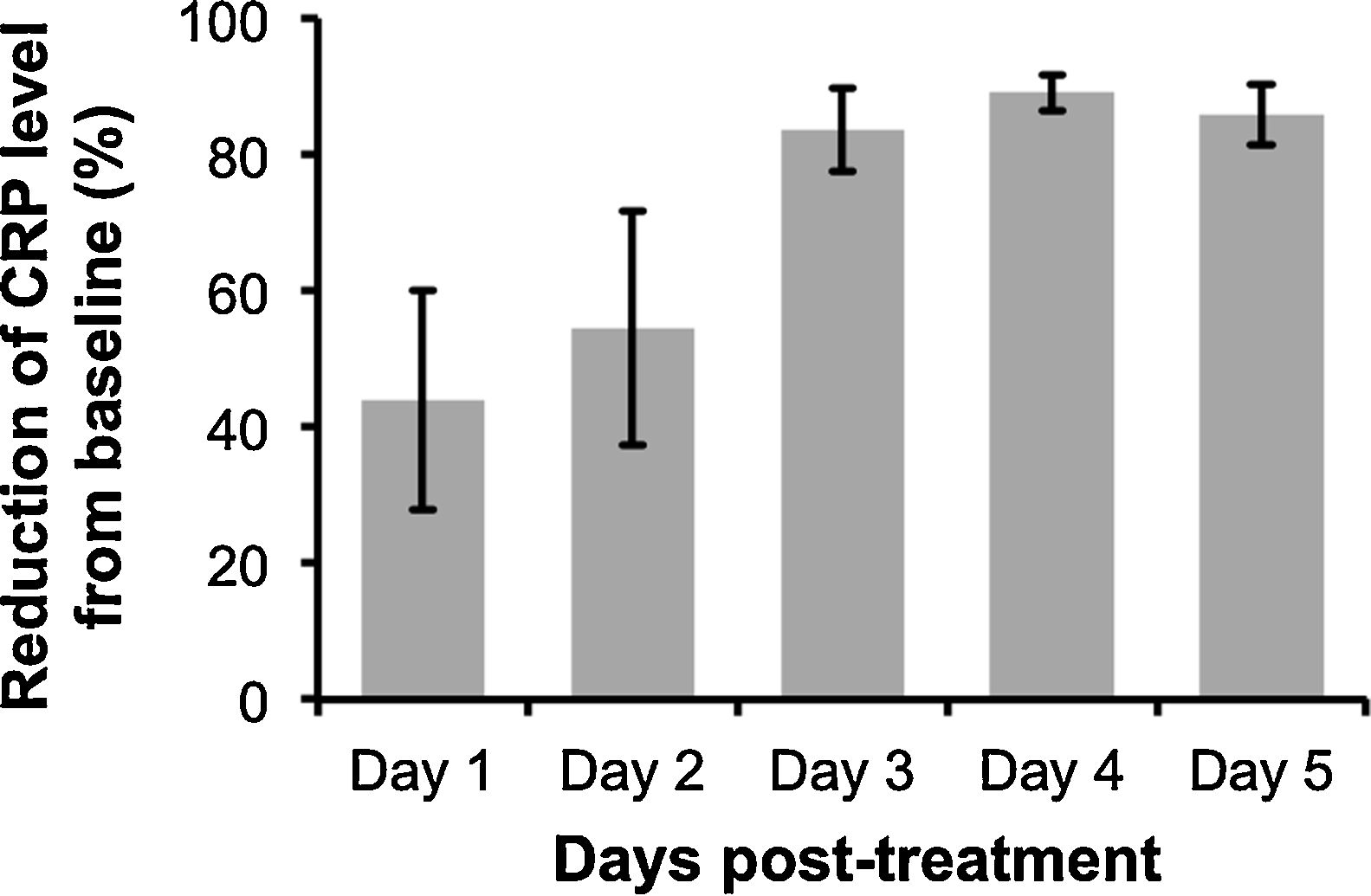
The analysis revealed that despite some variability in the levels of CRP post-treatment with anti-IL-6R antibody, peak CRP reduction was observable at 3 to 4-days after the administration (Fig. 1). Additionally, anti-IL-6R antibody treatment also resulted in the suppression of IL-6 levels12,16 and remarkable reduction of COVID-19 severity characterized by the improvement of chest CT and its symptoms. However, as reported by Radbel et al.,18 adverse secondary hemophagocytic lymphohistiocytosis (sHLH) occurred despite the lowered CRP levels, indicating the potential risk of side effects with this treatment. Thus, further studies evaluating efficacy and safety of anti-IL-6R antibody in treating COVID-19-infected patients is indispensable.
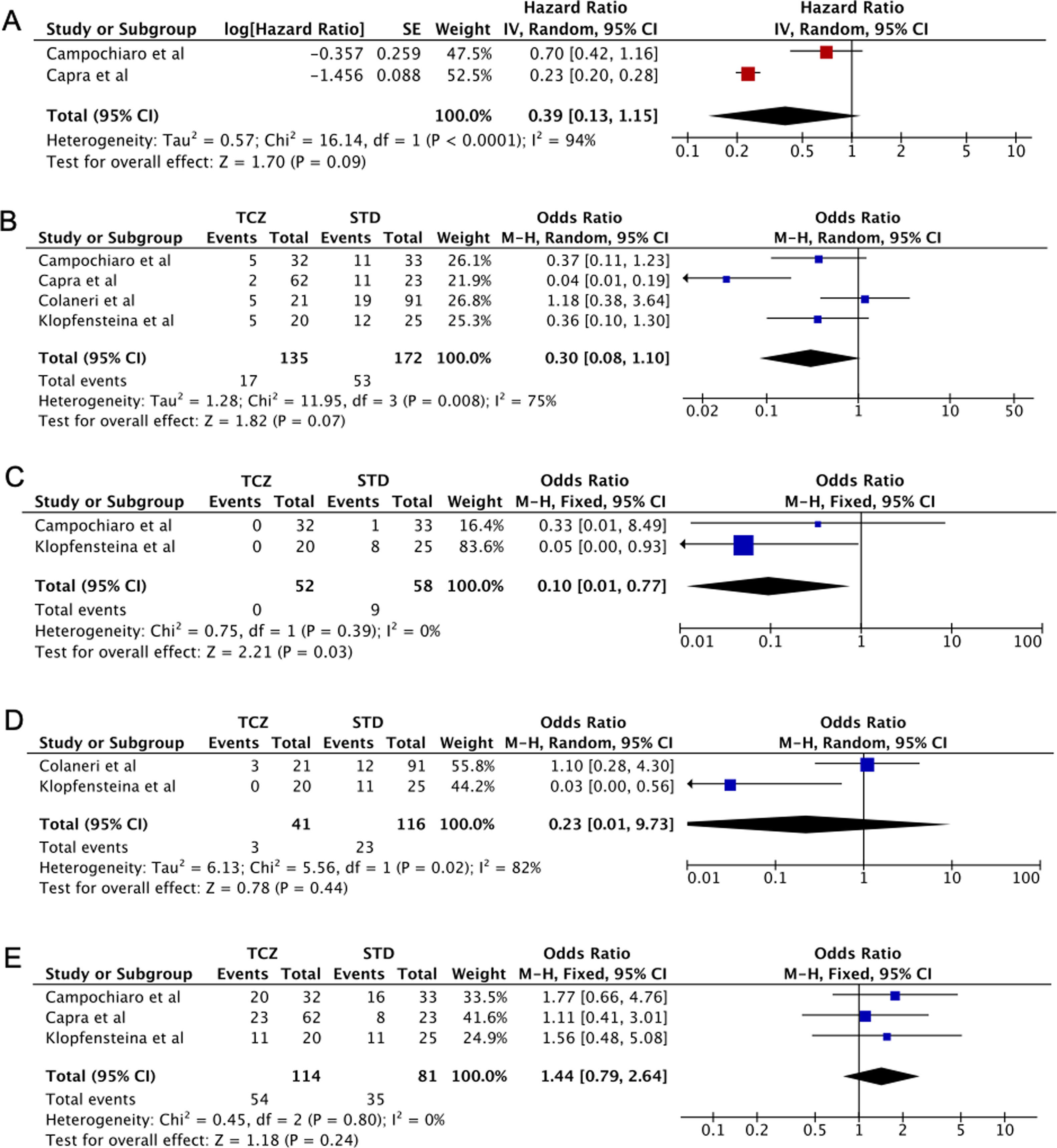
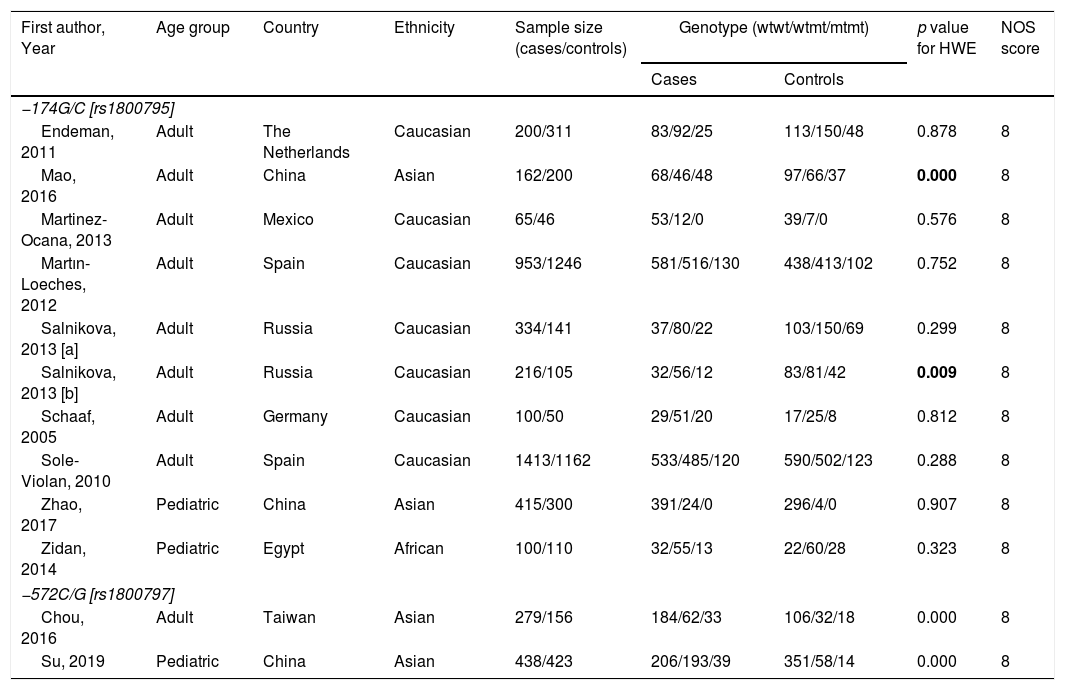
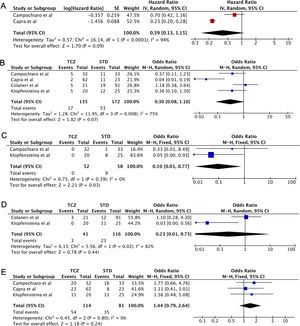
Five case-control studies evaluating TCZ treatment in severe COVID-19 were initially included20–24; followed by the exclusion of one study in which the control group displayed milder clinical presentation24 (Table 2). No statistical significance was observed between the pooled mortality rates of the TCZ and standard treatment (STD) groups, which may be due to the heterogeneity between studies. However, it can be noted that relative to STD treatment, TCZ treatment was marginally associated with lower mortality rate (HR=0.39, 95%CI 0.01–0.77, p=0.09, Fig. 2A; OR=0.30, 95%CI 0.08–1.10, p=0.07, Fig. 2B). In a study conducted by Sciascia et al.,25 TCZ treatment was shown to increase the likelihood of survival among severe COVID-19 patients (Table 2).
Characteristic of retrospective case-control and prospective cohort studies included in the analysis of anti-IL-6R treatment in severe COVID-19.
| Author | Location | No. of TCZ/STD treated patients | TCZ eligibility criteria | Therapy | Outcome at days | Survival rate (HR, 95% CI) | Mortality | Required IMV | ICU admission | Discharge | Adverse effect* | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCZ | STD | TCZ | STD | TCZ | STD | TCZ | STD | TCZ | STD | |||||||
| Campochiaro et al. | Italy | 32/33 | 2x Positive RT-PCR of SARS-CoV-2 on nasopharyngeal swab; hyper-inflammation (CRP, ≥100mg/L or r ferritin≥900ng/mL); severe respiratory involvement (chest X-ray/CT, SaO2≤92%, PaO2:FiO2≤300mmHg) | STD: HCQ, lopinavir/ritonavir, ceftriaxone, azithromycin, anti-coagulation prophylaxisTCZ: STD+TCZ 400mg IV (1 time, 24h interval for the second dose) | 28 | HR for death 0.44, 95% CI 0.167–1.184, p=0.122 | 5/32 | 11/33 | 0/32 | 1/33 | – | – | 20/32 | 16/33 | 4/32a5/32b | 4/336/33 |
| Capra et al. | Italy | 62/23 | Confirmed SARS-CoV-2, and one of the following criteria: RR≥30 breaths/min, SpO2≤93%, PaO2/FiO2≤300mmHg, severe respiratory involvement by chest X-ray | STD: HCQ, lopinavir, ritonavirTCZ: STD+TCZ 400mg IV or 324mg SC (1 time) | 35 | HR for death 0.035, 95% CI 0.004–0.347, p=0.004 | 2/62 | 11/23 | – | – | – | – | 23/62 | 8/23 | – | – |
| Colaneri et al. | Italy | 21/91 | Confirmed SARS-CoV-2, CRP>5mg/dl, PCT<0.5ng/mL, PaO2:FiO2<300; ALT<500U/L | STD: HCQ, azithromycin, prophylactic dose of low weight heparin, and methylprednisoloneTCZ: STD+TCZ 400mg IV | 7 | – | 5/21 | 19/91 | – | – | 3/21 | 12/91 | – | – | 0/21b | 0/91 |
| Klopfensteina et al. | France | 20/25 | Confirmed SARS-CoV-2; failure of standard treatment, oxygen therapy≥5l/min, >25% of lung damages on chest computed tomography (CT) scan, and ≥ 2 parameters of inflammation (high level of ferritin, CRP, D-dimers, lymphopenia, and LDH) | STD: HCQ, lopinavir-ritonavir, antibiotics, corticosteroidsTCZ: STD+TCZ (1 or 2 doses) | 11 | – | 5/20 | 12/25 | 0/20 | 8/25 | 0/20 | 11/25 | 11/20 | 11/25 | – | – |
| Quartuccio et al. | Italy | 42/69 | Confirmed SARS-CoV-2; level of CRP and IL-6 | STD: antivirals, antimalarials, glucocorticoids, antibiotics, LMWHTCZ: STD+TCZ 8mg/kg IV single infusion | 12 | – | 4/42 | 0/69** | – | – | – | – | – | – | – | – |
| Author | Location | No. of patients | TCZ eligibility criteria | Therapy | Outcome (HR, 95% CI) | ||
|---|---|---|---|---|---|---|---|
| Adverse effect | Clinical improvement | Survival rate | |||||
| Morena et al. | Italy | 51 | Confirmed SARS-CoV-2, age ≥ 18 years, RR ≥ 30min–1, SpO2<93%, PaO2/FiO2 < 250mmHg, IL-6 plasma level > 40pg/mL. | TCZ 400mg IV or 8mg/kg (1 time, 12h interval for the second dose) | Increased AST/ALT (29%), Bacteremia (27%) | HR 67% (95% CI 56–68)Clinical improvement based on severity or discharge, 30 days follow up | Mortality rate 27%, 30 days follow up |
| Sciascia et al. | Italy | 56 | Confirmed SARS-CoV-2, SpO2<93%, PaO2/FiO2<300mmHg, CRP or D-dimer>10× normal values, LDH>2× the upper limits, ferritin>1000ng/mL | TCZ 8mg/kg IV or 324mg SC (1 or 2 doses) | No adverse effect was reported | – | TCZ increased survival rate, HR 2.2 (95% CI 1.3–6.7), p<0.05, Survival rate according to D-dimer levels, 14 days follow up |
TCZ, Tocilizumab; STD, Standard treatment; *adverse effects including secondary infectiona or severe hepatic injury/increase ALT/ASTb; **milder clinical presentation; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; CT, computerized tomography; FiO2, fraction of inspired oxygen (FiO2); HCQ, hydroxychloroquine; ICU, intensive care unit; IV, intravenous; IMV, invasive mechanical ventilation; LDH lactate dehydrogenase; PaO2, partial pressure of oxygen; PCT, procalcitonin; RT-PCR, reverse transcription polymerase chain reaction; SC, subcutaneous, SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
(A) Forest plot of studies reporting hazard ratio (HR) that investigates the mortality rate between Tocilizumab (TCZ) group and standard treatment (STD) group. (B–E) Forest plot of pooled studies evaluating mortality rate, invasive mechanical ventilation (IMV) requirement, ICU admissions, and the number of discharged patients between Tocilizumab (TCZ) group and standard treatment (STD) group, respectively.
This analysis also showed that invasive mechanical ventilation (IMV) was required less in the TCZ group (OR=0.10, 95%CI 0.01–0.77, p=0.03, Fig. 2C). No statistical difference was observed in terms of ICU admissions, the number of discharged patients, and the adverse effects of treatment (bacteremia and an elevated level of AST/ALT) between the two groups (Fig. 2D, E, Supplemental Fig. 1, respectively). Interestingly, however, Morena et al.26 demonstrated that 67% of patients administered with TCZ showed an improvement in their clinical severity class. Thus, the administration of TCZ seems beneficial in lowering the mortality rate and increased favorable clinical outcomes in patients with severe SARS-CoV-2 infection. However, additional data are still required to understand the effect of TCZ in treating patients with severe and critically ill COVID-19.
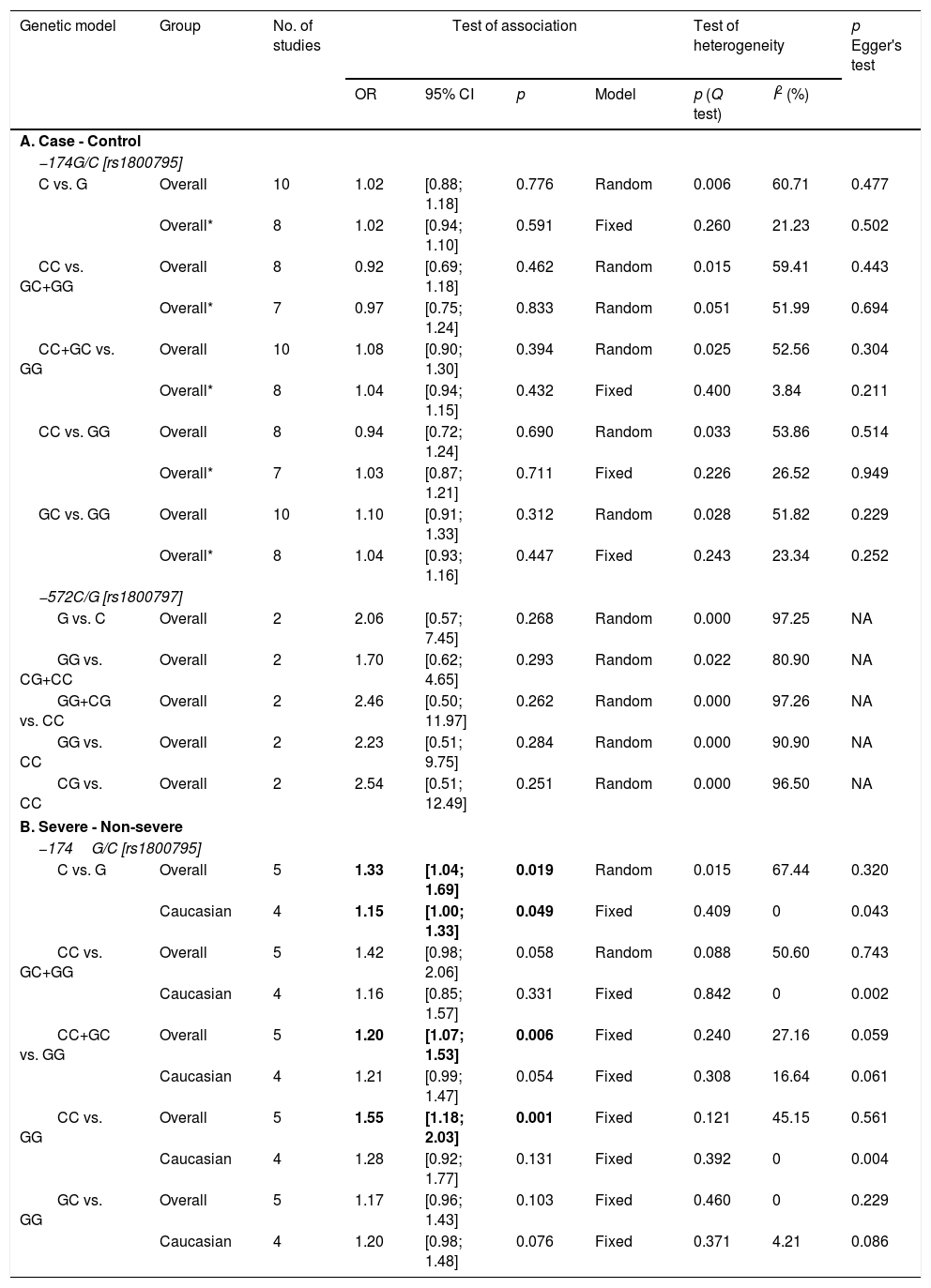
For the analysis on IL-6 gene polymorphisms and pneumonia, 24 articles were found using the aforementioned search strategy. Irrelevant articles were subsequently excluded, leaving a total of 11 eligible studies. The total sample included for analysis were 3958 cases and 3671 controls; 717 cases and 579 controls for IL-6 –174G/C and –572C/G polymorphisms, respectively27–30 (Supp. Refs. 1–7). To assess the association between IL-6 –174G/C with pneumonia severity, 671 severe and 2910 non-severe cases were examined29 (Supp. Ref. 3,6]) The characteristics of the included studies are shown in Table 2. All but four of the studies30 (Supp. Ref. 2,3,5) did not comply with the HWE (p<0.05). Overall, a lack of association between IL-6 −174G/C and −572C/G polymorphisms with pneumonia predisposition was observed in all genetic models (Table 3). Additionally, results remained insignificant following subgroup analysis based on ethnicity and age (data not shown).
The characteristics of included studies on IL-6 gene polymorphism and pneumonia.
| First author, Year | Age group | Country | Ethnicity | Sample size (cases/controls) | Genotype (wtwt/wtmt/mtmt) | p value for HWE | NOS score | |
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | |||||||
| −174G/C [rs1800795] | ||||||||
| Endeman, 2011 | Adult | The Netherlands | Caucasian | 200/311 | 83/92/25 | 113/150/48 | 0.878 | 8 |
| Mao, 2016 | Adult | China | Asian | 162/200 | 68/46/48 | 97/66/37 | 0.000 | 8 |
| Martinez-Ocana, 2013 | Adult | Mexico | Caucasian | 65/46 | 53/12/0 | 39/7/0 | 0.576 | 8 |
| Martın-Loeches, 2012 | Adult | Spain | Caucasian | 953/1246 | 581/516/130 | 438/413/102 | 0.752 | 8 |
| Salnikova, 2013 [a] | Adult | Russia | Caucasian | 334/141 | 37/80/22 | 103/150/69 | 0.299 | 8 |
| Salnikova, 2013 [b] | Adult | Russia | Caucasian | 216/105 | 32/56/12 | 83/81/42 | 0.009 | 8 |
| Schaaf, 2005 | Adult | Germany | Caucasian | 100/50 | 29/51/20 | 17/25/8 | 0.812 | 8 |
| Sole-Violan, 2010 | Adult | Spain | Caucasian | 1413/1162 | 533/485/120 | 590/502/123 | 0.288 | 8 |
| Zhao, 2017 | Pediatric | China | Asian | 415/300 | 391/24/0 | 296/4/0 | 0.907 | 8 |
| Zidan, 2014 | Pediatric | Egypt | African | 100/110 | 32/55/13 | 22/60/28 | 0.323 | 8 |
| −572C/G [rs1800797] | ||||||||
| Chou, 2016 | Adult | Taiwan | Asian | 279/156 | 184/62/33 | 106/32/18 | 0.000 | 8 |
| Su, 2019 | Pediatric | China | Asian | 438/423 | 206/193/39 | 351/58/14 | 0.000 | 8 |
| First Author, Year | Age group | Country | Ethnicity | Sample Size (Severe/Non-severe) | Genotype (GG/GC/CC) | p value for HWE | NOS score | |
|---|---|---|---|---|---|---|---|---|
| Severe | Non-severe | |||||||
| −174G/C [rs1800795] | ||||||||
| Mao, 2016 | Adult | China | Asian | 188/200 | 56/37/95 | 68/46/48 | 0.000 | 8 |
| Schaaf, 2005 | Adult | Germany | Caucasian | 25/75 | 3/15/7 | 26/36/13 | 0.929 | 8 |
| Sole-Violan, 2010 [a] | Adult | Spain | Caucasian | 159/817 | 73/68/18 | 392/341/84 | 0.441 | 8 |
| Sole-Violan, 2010 [b] | Adult | Spain | Caucasian | 162/817 | 68/76/18 | 392/341/84 | 0.441 | 8 |
| Sole-Violan, 2010 [c] | Adult | Spain | Caucasian | 137/1001 | 59/62/16 | 474/423/104 | 0.504 | 8 |
Bold values indicate the results were deviated from HWE (Hardy–Weinberg equilibrium); mt, mutant type; wt, wild type.
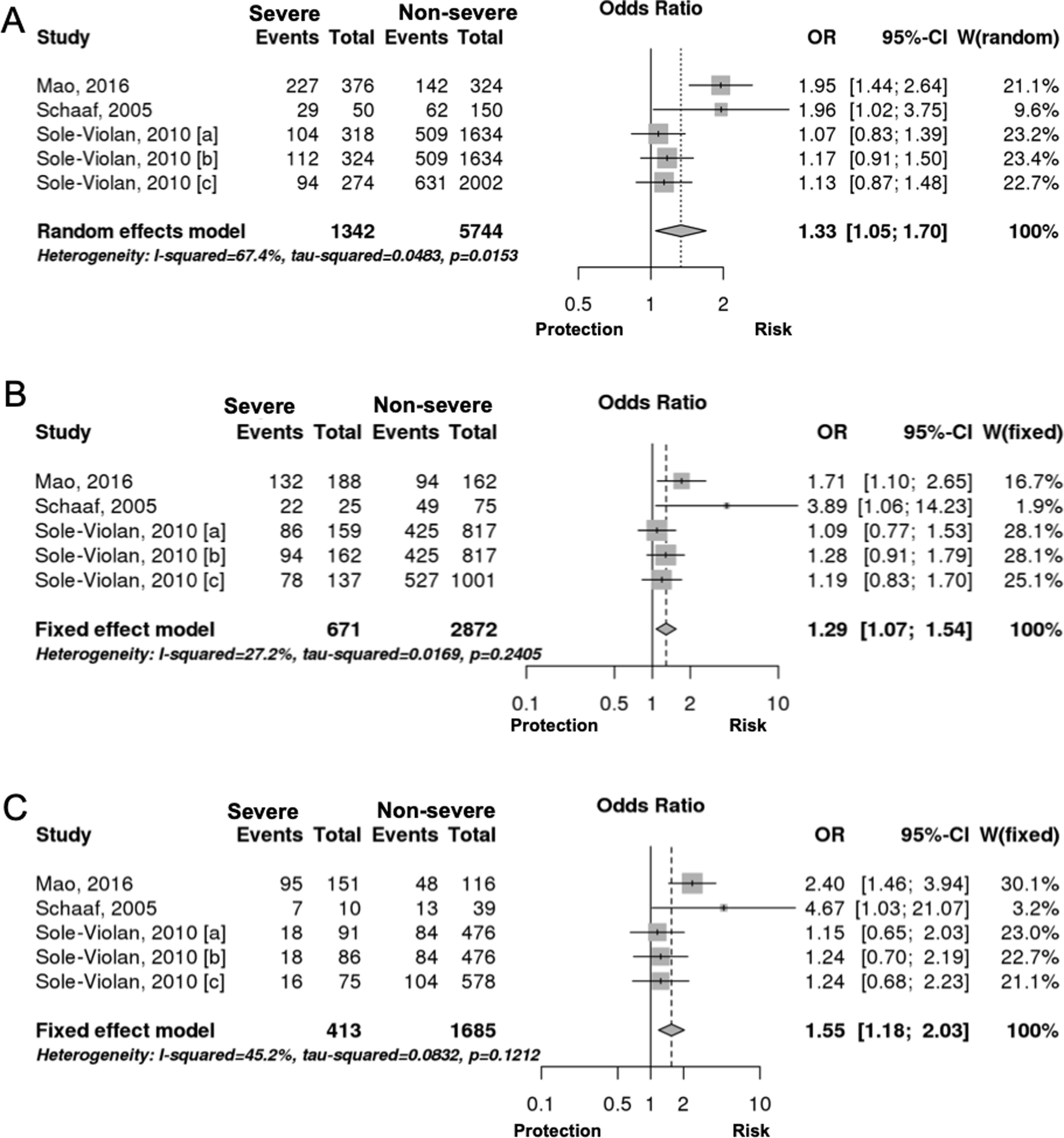
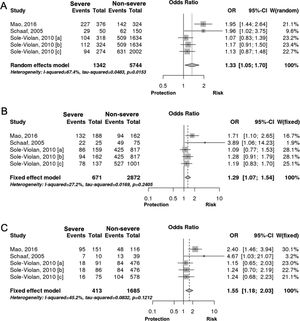
Interestingly however, we found that IL-6 −174G/C polymorphism was significantly associated with the severity of pneumonia (C vs. G, OR: 1.33, 95%CI 1.04–1.69, p=0.019, Fig. 3A; particularly in the Caucasian population, OR: 1.15, 95%CI 1.00–1.33, p=0.049; CC+GC vs. GG; OR: 1.20, 95%CI 1.07–1.53, p=0.006, Fig. 3B; CC vs. GG; OR: 1.55, 95%CI 1.18–2.03, p=0.001, Fig. 3C, Table 3). In line with our results, Feng et al. [Supp. Ref. 8] observed that carriers of the IL-6 −174G/C had a 2.42-fold higher risk for pneumonia-induced septic shock, thereby implying a higher tendency of severe pneumonia in patients harboring the IL-6 −174C. Indeed, the CC genotype has been correlated with significantly higher IL-6 levels [Supp. Ref. 3,9]. Moreover, it has been shown that the haplotype spanning from −1363 to +4835 from the transcription start site of IL-6 conferred susceptibility to acute lung injury (ALI) [Supp. Ref. 10] (Table 4).
Meta-analysis results of IL-6 gene polymorphism and pneumonia.
| Genetic model | Group | No. of studies | Test of association | Test of heterogeneity | p Egger's test | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | Model | p (Q test) | I2 (%) | ||||
| A. Case - Control | |||||||||
| −174G/C [rs1800795] | |||||||||
| C vs. G | Overall | 10 | 1.02 | [0.88; 1.18] | 0.776 | Random | 0.006 | 60.71 | 0.477 |
| Overall* | 8 | 1.02 | [0.94; 1.10] | 0.591 | Fixed | 0.260 | 21.23 | 0.502 | |
| CC vs. GC+GG | Overall | 8 | 0.92 | [0.69; 1.18] | 0.462 | Random | 0.015 | 59.41 | 0.443 |
| Overall* | 7 | 0.97 | [0.75; 1.24] | 0.833 | Random | 0.051 | 51.99 | 0.694 | |
| CC+GC vs. GG | Overall | 10 | 1.08 | [0.90; 1.30] | 0.394 | Random | 0.025 | 52.56 | 0.304 |
| Overall* | 8 | 1.04 | [0.94; 1.15] | 0.432 | Fixed | 0.400 | 3.84 | 0.211 | |
| CC vs. GG | Overall | 8 | 0.94 | [0.72; 1.24] | 0.690 | Random | 0.033 | 53.86 | 0.514 |
| Overall* | 7 | 1.03 | [0.87; 1.21] | 0.711 | Fixed | 0.226 | 26.52 | 0.949 | |
| GC vs. GG | Overall | 10 | 1.10 | [0.91; 1.33] | 0.312 | Random | 0.028 | 51.82 | 0.229 |
| Overall* | 8 | 1.04 | [0.93; 1.16] | 0.447 | Fixed | 0.243 | 23.34 | 0.252 | |
| −572C/G [rs1800797] | |||||||||
| G vs. C | Overall | 2 | 2.06 | [0.57; 7.45] | 0.268 | Random | 0.000 | 97.25 | NA |
| GG vs. CG+CC | Overall | 2 | 1.70 | [0.62; 4.65] | 0.293 | Random | 0.022 | 80.90 | NA |
| GG+CG vs. CC | Overall | 2 | 2.46 | [0.50; 11.97] | 0.262 | Random | 0.000 | 97.26 | NA |
| GG vs. CC | Overall | 2 | 2.23 | [0.51; 9.75] | 0.284 | Random | 0.000 | 90.90 | NA |
| CG vs. CC | Overall | 2 | 2.54 | [0.51; 12.49] | 0.251 | Random | 0.000 | 96.50 | NA |
| B. Severe - Non-severe | |||||||||
| −174G/C [rs1800795] | |||||||||
| C vs. G | Overall | 5 | 1.33 | [1.04; 1.69] | 0.019 | Random | 0.015 | 67.44 | 0.320 |
| Caucasian | 4 | 1.15 | [1.00; 1.33] | 0.049 | Fixed | 0.409 | 0 | 0.043 | |
| CC vs. GC+GG | Overall | 5 | 1.42 | [0.98; 2.06] | 0.058 | Random | 0.088 | 50.60 | 0.743 |
| Caucasian | 4 | 1.16 | [0.85; 1.57] | 0.331 | Fixed | 0.842 | 0 | 0.002 | |
| CC+GC vs. GG | Overall | 5 | 1.20 | [1.07; 1.53] | 0.006 | Fixed | 0.240 | 27.16 | 0.059 |
| Caucasian | 4 | 1.21 | [0.99; 1.47] | 0.054 | Fixed | 0.308 | 16.64 | 0.061 | |
| CC vs. GG | Overall | 5 | 1.55 | [1.18; 2.03] | 0.001 | Fixed | 0.121 | 45.15 | 0.561 |
| Caucasian | 4 | 1.28 | [0.92; 1.77] | 0.131 | Fixed | 0.392 | 0 | 0.004 | |
| GC vs. GG | Overall | 5 | 1.17 | [0.96; 1.43] | 0.103 | Fixed | 0.460 | 0 | 0.229 |
| Caucasian | 4 | 1.20 | [0.98; 1.48] | 0.076 | Fixed | 0.371 | 4.21 | 0.086 | |
Bold values indicate statistically significant differences between severe and non-severe cases. Asterisk (*) indicates that studies deviated from HWE (Hardy–Weinberg equilibrium) were excluded.
Tocilizumab, Sarilumab, or Siltuximab are humanized recombinant monoclonal antibodies that inhibit IL-6 signal transduction of IL-6 by binding with the soluble and membrane IL-6R, sIL-6R and mIL-6R, respectively. So far, anti-IL-6R antibody is mainly used to treat rheumatoid arthritis patients with favorable safety profile.11 Since these agents are immunosuppressive, their administrations are normally contraindicated in patients with active infection, thrombocytopenia, and an elevated liver function, which is also observed in COVID-19-infected patients2 (Supp. Ref. 11). Interestingly, however, pooled results collected from nine studies indicated that anti-IL-6R antibody treatment could effectively treat severe COVID-19-infected patients, marked by suppression of CRP and improvement of clinical symptoms. This may be due to transcriptional induction of the CRP gene was inhibited by TCZ, which then further suppressed inflammatory responses during SARS-CoV-2 infection. Although IL-6 gene polymorphism results may not directly correlate with novel coronavirus pneumonia (NCP), this analysis demonstrated that IL-6 −174C allele carrier status is associated with higher level of IL-6 production and more severe forms of pneumonia in general. This analysis strengthens the notion that IL-6 plays a pivotal role in novel coronavirus pneumonia (NCP) progression.
At present, 32 clinical trials have been registered (clinicaltrials.gov) to evaluate the efficacy and safety of anti-IL-6R antibodies. Despite the limited number of participants so far, suppression of IL-6 signaling cascade shows a promising therapy in the ARDS induced by SARS-CoV-2 infection.
Conflict of interestNone to declare.