In the last months great efforts have been developed to evaluate the more efficient therapeutic agents in the management of patients with COVID-19. Currently, no specific drug combination has consistently demonstrated an association with mortality. The aim of this study was to assess the pattern of associations observed between the different in-hospital treatments administered to a series of 238 patients admitted for COVID-19 and their relationship with mortality.
MethodsThe electronic medical records of patients that discharged or died from COVID-19 in the Hospital Universitario San Cecilio (Granada, Spain) between March 16 and April 10, 2020 were analysed. From these records, information was obtained on sex, age, comorbidities at admission, clinical information, analytical parameters, imaging tests and empirical treatments used. The outcome variable was the in-hospital mortality. To estimate the associations between the different therapeutic alternatives and the risk of mortality, Hazard Ratios adjusted for age, sex, previous pathologies and severity at discharge were estimated using Cox Regression models.
ResultsThe most frequently used combination of drugs was low molecular weight heparins, hydroxychloroquine, and ritonavir/lopinavir. None of the analysed treatments showed independent association with mortality. The drugs that showed a greater inverse association with mortality were tocilizumab and corticoids.
ConclusionsThe observed association patterns are consistent with previous literature. It seems necessary to design randomized controlled clinical trials that evaluate the possible protector effect of tocilizumab and corticoids in the risk of mortality for some subgroups of COVID-19 hospitalized patients.
En los últimos meses se han realizado grandes esfuerzos para evaluar las terapias más eficaces en el manejo de pacientes con COVID-19. Actualmente ninguna combinación ha demostrado de manera consistente una relación clara con la mortalidad. Nuestro objetivo fue valorar el patrón de asociaciones observado entre los distintos tratamientos intrahospitalarios administrados a 238 pacientes ingresados por COVID-19 y la mortalidad.
Materiales y métodosSe analizaron las historias clínicas electrónicas de aquellos pacientes dados de alta o que fallecieron por COVID-19 entre el 16 de marzo y el 10 de abril de 2020 en el Hospital Universitario San Cecilio (Granada, España). Se obtuvo información sobre sexo, edad, comorbilidades al ingreso, parámetros clínicos, analíticos, pruebas de imagen y tratamientos empíricos empleados. La variable de desenlace fue la mortalidad intrahospitalaria. Para estimar las asociaciones entre los diferentes tratamientos y el riesgo de mortalidad se estimaron, mediante modelos de regresión de Cox, hazard ratio ajustadas por edad, sexo, patologías previas y gravedad al ingreso.
ResultadosLa combinación de fármacos más frecuentemente empleada fue la formada por heparinade bajo peso molecular (HBPM), hidroxicloroquina y ritonavir/lopinavir. Ninguno de los tratamientos utilizados mostró una asociación independiente con la mortalidad. Los fármacos que mostraron una asociación inversa de mayor magnitud fueron el tocilizumab y los corticoides.
ConclusionesEl patrón se asociaciones obtenido es consistente con lo reportado en la bibliografía. Parece oportuno diseñar ensayos aleatorizados que valoren el posible efecto protector de los corticoides y el tocilizumab sobre el riesgo de muerte en algunos subgrupos de pacientes hospitalizados por COVID-19.
Over the last few months, the international scientific community has made great efforts to identify the effectiveness of the therapeutic agents used to treat the disease caused by SARS-CoV-2, namely COVID-19.1–3 Apart from supportive therapy such as administration of fluids and oxygen therapy,4 no specific treatments with proven efficacy have been identified. Thus a recent review5 which included 22 studies concluded that, despite the potential of some therapeutic alternatives, none can be recommended with the evidence that is currently available, and all are pending further drug development or the conclusion of clinical trials, the preliminary results of which are expected by the end of June 2020. This thesis coincides with that of the most recently published reviews.1,6,7 Despite the large number of studies carried out over the last few months, data is scarce from observational studies, as all medications are currently used based on their in vitro activity or on previous clinical experience with other coronavirus diseases,8 such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) or against the Ebola virus.
The different treatments being used include chloroquine,9 hydroxychloroquine,10 lopinavir/ritonavir,11 favipiravir,12 remdesivir,13 darunavir,14 interferons,15 nitazoxanide,16 ivermectin17 and azithromycin.18 Reviews of these treatments indicate that no therapy currently has sufficient evidence of effectiveness supported by controlled clinical trials.19,20 As an example, hydroxychloroquine, which is widely included in COVID-19 treatment guidelines around the world, did not prove efficacy in a pilot clinical trial of 30 adults,21 nor did it have any significant association in an observational study of 1,376 patients in New York.22 This latter study used a Cox regression model adjusting for demographic factors, clinical factors, laboratory tests and other medications, and obtained a death with hydroxychloroquine hazard ratio (HR) of 1.04 (95% CI 0.82–1.32). Similar results were obtained in another trial that combined hydroxychloroquine with azithromycin.23
Alternatively, it has been suggested that the use of anti-inflammatory therapies could have a positive impact on the clinical progression of hospitalised COVID-19 patients.24 Thus, the use of corticosteroids,25 IL-6 inhibitors such as tocilizumab26 or sarilumab (NCT04315298), or other drugs with anti-inflammatory properties such as baricinitib,27 have been put forward as effective therapeutic alternatives, although the evidence of their usefulness in clinical practice is still limited.28 The use of corticosteroids especially, has been a topic of debate. While the World Health Organization does not recommend its use unless it is indicated for another reason, and some clinical studies do not support its potential for providing benefits and report its potential harm,29 other studies state that these drugs may be beneficial if utilised in the acute phase of the disease.30 In our setting, an association between the use of corticosteroid pulse therapy and a lower number of events (mortality and intubation) has been described in patients diagnosed with cytokine storm syndrome induced by COVID-19, obtaining a survival HR of 0.02 (95 CI % 0.0004–0.835; p = 0.04) in patients treated with glucocorticoid pulses and tocilizumab versus patients treated only with tocilizumab.31
In addition to antiviral and anti-inflammatory drugs, the use of antibodies, plasma transfusion and the current development of vaccines are considered as possible future strategies.32 The largest observational studies published, as well as the preliminary results of the two main clinical trials with remdesivir (mortality HR 0.70; 95% CI 0.47–1.0433 and clinical improvement HR 1.23; 95% CI 0.87–1.7534) do not provide conclusive results regarding any of the therapeutic strategies used against SARS-CoV-2.8,22 Furthermore, to date we have not found any published observational study that uses multivariate models to analyse the association of these treatments on morbidity and mortality due to COVID-19 in Spain.
However, despite the fact that there are multiple guidelines for the management and treatment of COVID-19 that incorporate the systematic use of many of the drugs described above in their recommendations, there are very few observational studies that analyse the association of such drugs with early mortality caused by SARS-CoV-2. And in the case of Spain, these studies are nonexistent to date. Such information would undoubtedly be very valuable for adapting and improving the said guidelines, based on the impact that the use of different drugs has in a clinical context like ours.
The objective of our study is, therefore, to assess the pattern of associations observed between the different in-hospital treatments administered to a series of 238 patients admitted for COVID-19, and the mortality rate.
Material and methodsDesignFor the design of this paper, we followed the recommendations of the STROBE (REF) guide. We have retrospectively analysed the case series of all 238 patients who, having been admitted for COVID-19 into the Hospital Universitario Clínico «San Cecilio» (HUSC) of Granada, died or were discharged between 16 March and 10 April 2020. There were no patients lost to follow-up. The HUSC is a tertiary level hospital, with 480 beds, which provides specialised healthcare coverage to a population of 291,797 inhabitants. The admission period for all patients included in the study was between 8 March and 5 April 2020.
VariablesInformation was obtained from the electronic medical records of each patient, including the following variables:
- •
General data on admission and from the emergency department report: date of admission, sex, age and presence of comorbidities (high blood pressure [HBP], diabetes mellitus [DM], previous lung disease, chronic kidney failure, cardiovascular disease [CVD], or active neoplasia).
- •
Variables of the clinical examination and laboratory tests upon admission were: systolic and diastolic blood pressure, heart rate, temperature, baseline oxygen saturation (SatO2), required external oxygen supply (litres), fraction of inspired oxygen (FiO2), SatO2/FiO2 ratio, haemoglobin, lymphocytes, neutrophils, platelets, D-dimer, glycaemia, total bilirubin, lactate dehydrogenase (LDH), ferritin, C-reactive protein (CRP), procalcitonin, troponins, creatinine, kidney function (elevated urea and creatinine), partial pressure of arterial O2 and CO2 (PO2 and PCO2), bicarbonate, arterial pH, sequential organ failure assessment (SOFA index) and CURB65 index.
- •
The treatments administered during the patient’s stay in hospital were: low molecular weight heparins (bemiparin sodium at doses of 2,500 to 3,500, 5,000 to 7,000, or 7,500 to 10,000 were the doses used in our case series), hydroxychloroquine, ritonavir/lopinavir, azithromycin, corticosteroids (methylprednisolone < 61 mg, 80–160 mg, 200–250 mg, <500 mg, once again based on the observed data of the use in our case series), ACEI/ARA II and tocilizumab.
- •
Outcome variables: death and length of stay (in days).
A dependent variable was time to death, and Cox regression models were applied to quantify, by calculating the corresponding hazard ratios (HR), the magnitude of the associations between the hazard or instantaneous death rate, and each of the treatments administered to the patients in our case series. Two HR estimates were obtained for each treatment. The first (HR1) resulted from adjusting a Cox regression model separately for each treatment considered. Apart from the said treatment, it included variables that, in a previous modeling, behaved as predictors of the mortality risk (age, diabetes, SatO2/FiO2 and score on the SOFA and CURB65 scales), plus other variables ‘upon admission’ which, according to the reviewed bibliography,35,36 could also behave as confounding factors (sex, history of CVD, HBP, CKF and days since the admission of the first patient in the case series). Secondly, and being as this is an observational study the administration of the different treatments applied was not, in principle, independent of each other (the strength of association of each one with death could be affected by the effect of the rest of the treatments administered), the HRs were obtained for each treatment in a single model in which the set of administered treatments (HR2) was added to the previous variables. The corresponding 95% confidence intervals (95% CI) were obtained for all HR estimations. The software used for data analysis was the statistical package STATA® (version 15.0) (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC.).
EthicsThe present study complied with the necessary suitability requirements in relation to the proposed endpoint and was in line with the ethical principles applicable to this type of design. It was approved by the Research Ethics Committee of the Province of Granada (CEI) on 13 April 2020.
ResultsPatient distribution according to the variables ‘upon admission’ has been described in a previous study (currently in the publication phase). This study analysed the risk factors ‘upon admission’ and they can be consulted in the appendix (Appendix B Table A).
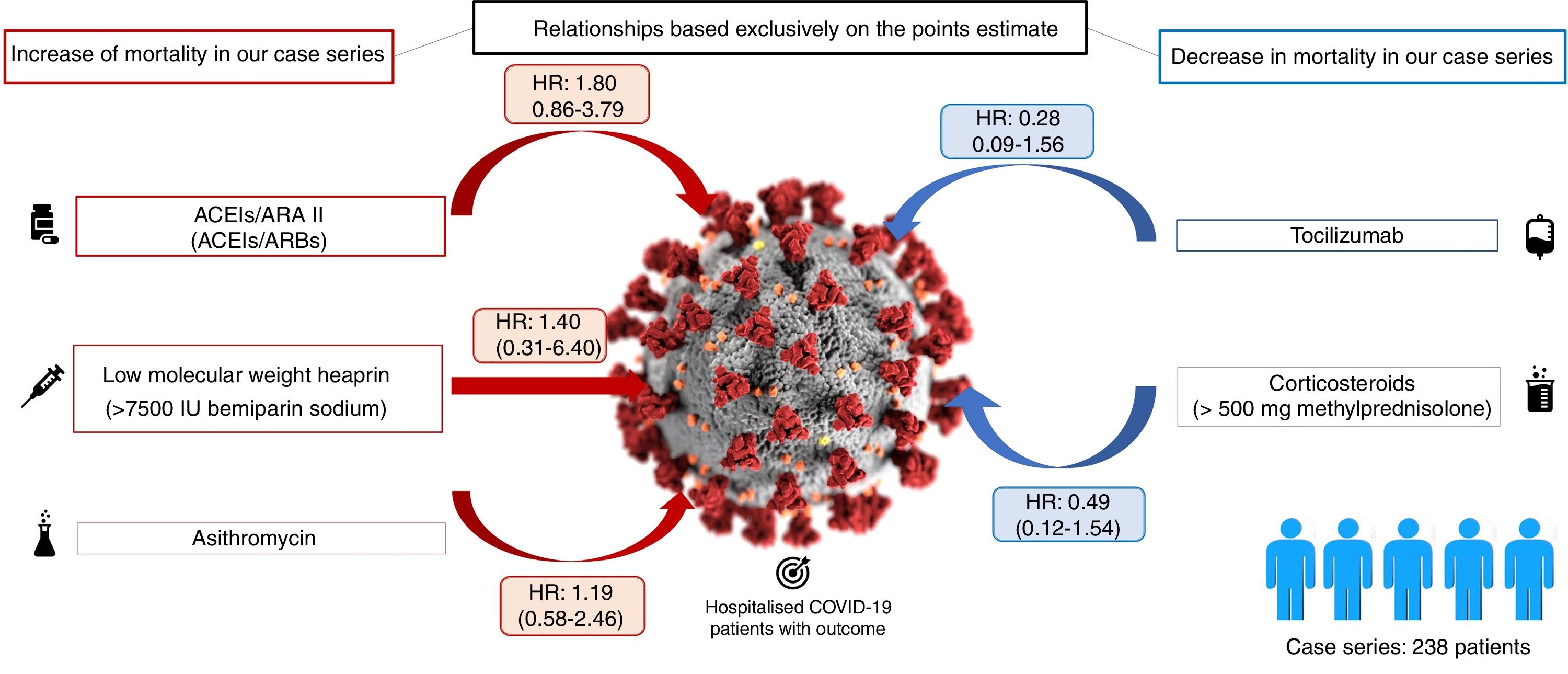
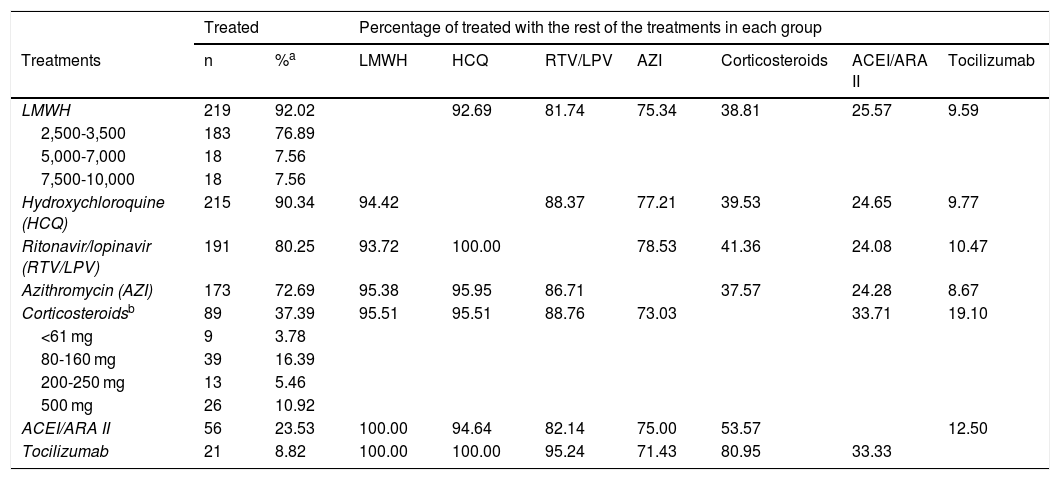
Table 1 shows the distribution of the 238 patients according to the treatment administered. It can be observed that almost all the patients (more than 90%) received LMWH at some point during their admission, most at low doses, and hydroxychloroquine. Also, a high percentage of patients received ritonavir/lopinavir and azithromycin. 38% of hospitalised patients received corticosteroids (at variable doses), 23% received ACEI/ARB II and, finally, only 9% of patients received tocilizumab Fig. 1.
Distribution of the treatments in the sample of patients (n = 238).
| Treated | Percentage of treated with the rest of the treatments in each group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatments | n | %a | LMWH | HCQ | RTV/LPV | AZI | Corticosteroids | ACEI/ARA II | Tocilizumab |
| LMWH | 219 | 92.02 | 92.69 | 81.74 | 75.34 | 38.81 | 25.57 | 9.59 | |
| 2,500-3,500 | 183 | 76.89 | |||||||
| 5,000-7,000 | 18 | 7.56 | |||||||
| 7,500-10,000 | 18 | 7.56 | |||||||
| Hydroxychloroquine (HCQ) | 215 | 90.34 | 94.42 | 88.37 | 77.21 | 39.53 | 24.65 | 9.77 | |
| Ritonavir/lopinavir (RTV/LPV) | 191 | 80.25 | 93.72 | 100.00 | 78.53 | 41.36 | 24.08 | 10.47 | |
| Azithromycin (AZI) | 173 | 72.69 | 95.38 | 95.95 | 86.71 | 37.57 | 24.28 | 8.67 | |
| Corticosteroidsb | 89 | 37.39 | 95.51 | 95.51 | 88.76 | 73.03 | 33.71 | 19.10 | |
| <61 mg | 9 | 3.78 | |||||||
| 80-160 mg | 39 | 16.39 | |||||||
| 200-250 mg | 13 | 5.46 | |||||||
| 500 mg | 26 | 10.92 | |||||||
| ACEI/ARA II | 56 | 23.53 | 100.00 | 94.64 | 82.14 | 75.00 | 53.57 | 12.50 | |
| Tocilizumab | 21 | 8.82 | 100.00 | 100.00 | 95.24 | 71.43 | 80.95 | 33.33 | |
Table 1 also shows how the administration of each drug was distributed in comparison to the other treatments used. In general, it is found that the percentage of patients who received each treatment does not vary substantially regardless of having received other treatments. Only the association between the administration of tocilizumab and corticosteroids is noteworthy.
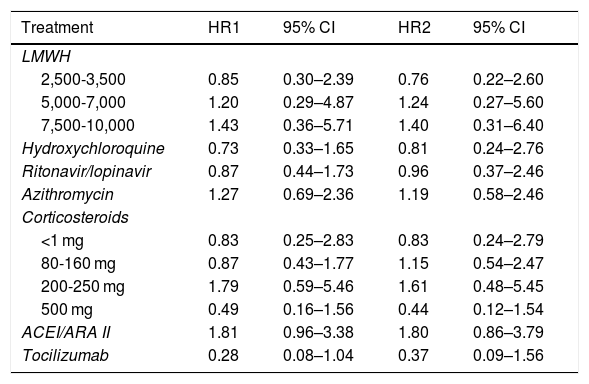
The duration of patient follow-up included in our case series (equivalent to the length of their stay), ranged from 0 (three patients died the same day of admission) to 27 days. 25.6% of the patients died. There were no deaths under 50 years of age. However, above that age, the proportion of deceased grew exponentially: 5.8% (95% CI 1.2–15.9%) mortality in patients aged 50–59 years, 12.8% (95% CI 4.8–25.7%) in patients aged 60–69 years, 28.6% (95% CI 16.6–43.3%) in patients aged 70–79 years and 79.2% (95% CI: 65.0–89.5%) in patients aged 80 and over. Table 2 presents the HR estimates for each treatment and, in the case of LMWH and corticosteroids, for each subgroup of administered doses, obtained with the two models designed (the one adjusted for each treatment by the main confounding variables [days of stay, sex, age, comorbidities, etc.] and the one that includes these variables plus the set of treatments received by each patient). It can be seen how the pattern of associations obtained for the different treatments is similar in both models, although in the second the width of the confidence intervals is somewhat greater. Overall, no obvious associations were found for any of the estimated associations, neither in the model adjusted by the principle confounding variables (days of stay, sex, age, comorbidities, etc.), nor in the model adjusted by these variables plus the rest of the treatments received by each patient. The drugs that were most consistently associated with a lower risk of mortality among hospitalised patients were tocilizumab (HR2 0.37; 95% CI 0.09–1.56), and corticosteroids at doses of 500 mg or more (HR2 0.44; 95% CI 0.12–1.54).
Hazard ratio adjusted to quantify the association between each treatment and the hazard of death.
| Treatment | HR1 | 95% CI | HR2 | 95% CI |
|---|---|---|---|---|
| LMWH | ||||
| 2,500-3,500 | 0.85 | 0.30–2.39 | 0.76 | 0.22–2.60 |
| 5,000-7,000 | 1.20 | 0.29–4.87 | 1.24 | 0.27–5.60 |
| 7,500-10,000 | 1.43 | 0.36–5.71 | 1.40 | 0.31–6.40 |
| Hydroxychloroquine | 0.73 | 0.33–1.65 | 0.81 | 0.24–2.76 |
| Ritonavir/lopinavir | 0.87 | 0.44–1.73 | 0.96 | 0.37–2.46 |
| Azithromycin | 1.27 | 0.69–2.36 | 1.19 | 0.58–2.46 |
| Corticosteroids | ||||
| <1 mg | 0.83 | 0.25–2.83 | 0.83 | 0.24–2.79 |
| 80-160 mg | 0.87 | 0.43–1.77 | 1.15 | 0.54–2.47 |
| 200-250 mg | 1.79 | 0.59–5.46 | 1.61 | 0.48–5.45 |
| 500 mg | 0.49 | 0.16–1.56 | 0.44 | 0.12–1.54 |
| ACEI/ARA II | 1.81 | 0.96–3.38 | 1.80 | 0.86–3.79 |
| Tocilizumab | 0.28 | 0.08–1.04 | 0.37 | 0.09–1.56 |
HR1 adjusted by days since the admission of the first patient in the case series, sex, age, diabetes, CVD, HBP, CKF, SatO2FiO2, SOFA and CURB65 index.
HR2 adjusted by the previous variables plus the rest of the treatments administered (n = 232).
This study reveals that the therapeutic options most commonly used in hospitalised COVID-19 patients in our case series, aside from baseline treatment with oxygen therapy, fluid therapy, etc., were LMWH, mainly at doses equal to or less than 3,500 IU (92.4%), hydroxychloroquine (90.7%), and ritonavir/lopinavir (80.6%). The prescription of these three drugs combined was identified on average in more than 83.3% of the patients. The prescriptions of azithromycin, corticosteroids or tocilizumab appear to a lesser degree. This finding is consistent with the management carried out with the patients who made up the first published series of COVID-19 in China.11,21,36 This is completely understandable if we take into account that both our outcome (discharge home or death) and the period under study (16 March to 10 April 2020) define a very specific study subpopulation: one that was made up of those patients who suffered their disease during the first weeks of the pandemic in our country.37
It has been difficult for us to compare our results with those of other studies published to date, given the limited number of studies that analyse the adjusted relationship between treatments and mortality through observational studies with multivariate analyses.
Regarding the increased mortality found in the use of low molecular weight heparin at high doses (7,500–10,000 IU of bemiparin sodium), the interpretation of these results can be twofold. On the one hand, it may be due to the fact that the use of this drug at high doses increases mortality due to adverse effects, or the cause that justifies the indication: the need for anticoagulation (for example, heart disease). On the other hand, given that this drug is dosed according to weight, it is possible that this variable is acting as a confounding factor and it is actually the heavy weight that is associated with mortality (the same bias is applicable to the corticosteroids analysed in our case series). In any case, this study highlights the need to routinely include, as far as possible, the weight and height of patients during hospitalisation. This is data we have not been able to access and which makes the choice of correct dosage very difficult in situations of therapeutic uncertainty such as the one we are currently experiencing with the COVID-19 pandemic. However, the dose-response pattern found for LMWH which may be partially explained by weight, was not observed in the increasing doses of corticosteroids. In our sample the use of corticosteroids was not applied extensively, but to a subgroup of patients with, presumably, special characteristics (we assume that they developed an acute inflammatory response to the virus). It would be especially interesting to compare corticosteroid-treated and non-corticosteroid-treated patients in this subgroup, but our data prevent us from performing this type of analysis in our study.
There is no doubt that the increase in mortality found with ACEI/ARB II could be due to an indication bias. Patients who were prescribed these treatments are probably patients who were already under them at home due to chronic pathologies (for example, hypertension or kidney disease). It is possible that the said pathologies are responsible for the increase in mortality. Either way, after adjusting for them, a tendency towards increased mortality continues to appear in our study. However, we do not know the consequences of stopping the administration of these drugs in these patients, so a cautious interpretation of these results must be made.
Our results do not show a conclusive association between azithromycin, ritonavir/lopinavir, hydroxychloroquine, and mortality. But these treatments were prescribed in a systematic way, making it very difficult to make a comparison with patients who did not receive these treatments.
Apart from that, the fact that the drugs most strongly associated with a lower risk of mortality are, above all, tocilizumab and high doses of corticosteroids among the patients in our case series, is consistent with various recently published reviews. Their findings point out that immunosuppression, as a defense mechanism against the exaggerated inflammatory response generated by the virus in the body, has been shown to be an effective therapeutic strategy, especially in younger patients with adult respiratory distress syndrome (ARDS).38,39
LimitationsThe main limitation of the present study is its observational nature, which makes it impossible to causally interpret the HRs estimated for each treatment. Secondly, as already mentioned in the methods section, our study is not a true cohort of admitted patients, as we excluded those who were still in hospital at the close of the enrollment period. Since the length of stay tends to be inversely associated with mortality, this leads to a selection bias that, on the one hand, will tend to overestimate the proportion of deaths in our sample and, on the other, the variables could bias the magnitude of the associations between the treatments administered and death. There may also be a sampling bias, similar to that already described in a review on studies of prognostic factors in COVID-19 patients.40 In summary, this bias is due to the fact that, although our reference population was the cohort of all patients admitted to the hospital for COVID-19 over a certain period, our study sample was only able to include admitted patients that had either recovered and had been discharged or had died during the enrollment period. Thus, it excluded patients who, having been admitted to our hospital, were still in hospital when the recruitment period closed (that is, they had not died nor had they been discharged). This would be the censored data in a conventional cohort study, whereas in our study it has been necessary to exclude it. The extent in which the risk of death of those excluded patients differs from the rest, would be the sampling bias which would affect the estimation of the risk of death (survival): if the length of stay of the excluded patients is, as can be assumed, greater than that of those that were included, the estimated survival in our sample would be lower than that of the complete cohort of admitted patients. The sampling bias may also affect the magnitude of the estimates of association between treatments and death, although in this case its direction is difficult to predict. Anyhow, to try to minimise it, we chose to consider the time to death as a dependent variable and, consequently, apply Cox regression models. Third, the small sample size not only limited the power of the study (which is reflected in the width of the confidence intervals), but it prevented the application of other causal analysis methods for most of the treatments evaluated. All the same, we have not been able to include in the models the corresponding terms of interaction between treatments, nor evaluate secondary endpoints such as the need for intubation or admission to the ICU, or possible specific adverse effects of each drug, or to perform exploratory analyses according to patient subgroups. Finally, we are aware of the more than probable residual confusion in our estimates as we were unable to incorporate variables such as the patients’ weight. In light of all these limitations, we wish it to be clear that we present a descriptive study of an exploratory nature, whose sole objective is to identify patterns of associations that could be the object of further studies.
ConclusionsOur results show that the therapeutic combination most frequently used among the patients in our case series was that of LMWH, hydroxychloroquine, and ritonavir/lopinavir. None of the applied treatments have been clearly associated with in-hospital mortality. The drugs associated with lower mortality in our case series were tocilizumab and high-dose corticosteroids. Analytical observational studies with adjusted estimates using multivariate models and pure experimental studies are still needed to analyse the relationship between treatments and mortality from COVID-19.
FundingNo source of funding has been used.
Conflict of interestsThe authors declare that there is no conflict of interest.
The authors thank the Cátedra de Docencia e Investigación SEMERGEN-Medicina de Familia at the University of Granada for their support in carrying out this study. Similarly, the authors thank all the professionals who have worked at the San Cecilio University Hospital during the pandemic, for their tireless work in caring for the patients.
Please cite this article as: Rivera-Izquierdo M, Valero-Ubierna MdC, R-delAmo JL, Fernández-García MÁ, Martínez-Diz S, Tahery-Mahmoud A, et al. Agentes terapéuticos utilizados en 238 pacientes hospitalizados por COVID-19 y su relación con la mortalidad. Med Clin (Barc). 2020;155:375–381.