the SARS-CoV-2 infection ranges from asymptomatic to critical forms and several prognostic factors have been described. Atrial fibrillation (AF) is common in acute situations where it is linked with more complications and mortality. We aimed to evaluate the prognostic information of AF in this population.
Methodsretrospective analysis of a cohort of 517 patients consecutively admitted in a tertiary hospital due to SARS-CoV-2 infection. We divided the patients in two groups according the development of AF and compared the main features of both groups. An univariable and multivariable analysis of mortality were also performed.
Resultsamong 517 patients with SARS-CoV-2 infection admitted in a tertiary center, 54 (10.4%) developed AF. These patients are older (81.6 vs 66.5 years old, p<0.001) and present more hypertension (74% vs 47%, p<0.001), cardiomyopathy (9% vs 1%, p=0.002), previous heart failure admission (9% vs 0.4%, p<0.001), previous episodes of AF (83% vs 1%, p<0.001) and bigger left atrium (47.8 vs 39.9mm, p<0.001). AF COVID-19 patients present more acute respiratory failure (72% vs 40%, p<0.001) and higher in-hospital mortality (50% vs 22%, p<0.001). Predictors of AF development are age and previous AF. AF is not an independent predictor of in-hospital mortality. Predictors are age, creatinine>1.5mg/dL at admission, LDH>250UI/L at admission and acute respiratory failure.
ConclusionAtrial fibrillation appears in 10% of hospitalized patients with SARS-CoV-2 infection. These patients present more comorbidities and two-fold increase in hospital mortality. Atrial fibrillation is not an independent prognostic factor.
La infección por SARS-CoV-2 presenta un amplio espectro clínico, y varios factores pronósticos han sido descritos. La fibrilación auricular (FA) es frecuente en situaciones agudas, donde se ha relacionado con aumento de complicaciones y mortalidad. Nuestro objetivo ha sido evaluar el impacto pronóstico de la FA en esta población.
MétodosAnálisis retrospectivo de una cohorte de 517 pacientes con infección SARS-CoV-2 consecutivamente ingresados en un hospital terciario. Dividimos a los pacientes en dos grupos de acuerdo al desarrollo de FA durante el ingreso y comparamos las características de los grupos. Realizamos análisis univariado y multivariado de mortalidad.
ResultadosDe los 517 pacientes, 54 (10,4%) desarrollaron FA. Estos pacientes son mayores (81,6 vs. 66,5 años, p<0,001) y presentan más hipertensión (74% vs. 47%, p<0,001), miocardiopatía (9% vs. 1%, p=0,002), ingreso previo por insuficiencia cardiaca (9% vs. 0,4%, p<0,001), historia de FA (83% vs. 1%, p<0,001) y mayor aurícula izquierda (47,8 vs. 39,9mm, p<0,001). Los pacientes con FA presentan más fallo respiratorio agudo (72% vs. 40%, p<0,001) y mayor mortalidad hospitalaria (50% vs. 22%, p<0,001). Los predictores de FA son la edad y la historia de FA previa. La FA no es un predictor independiente de mortalidad hospitalaria. Los predictores son: edad, creatinina >1,5mg/dL al ingreso, LDH>250U/L al ingreso y el fallo respiratorio agudo.
ConclusiónLa FA aparece en el 10% de los pacientes hospitalizados por SARS-CoV-2. Estos presentan mayor comorbilidad y el doble de mortalidad hospitalaria, pero la FA no es un factor pronóstico independiente.
Coronaviruses are important human and animal pathogens. Although most human coronavirus infections are mild, severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV) presented mortality rates of 10% and 37% respectively.1–5
Coronavirus disease 2019 (COVID-19) emerged in early December 2019 in Wuhan (Hubei, China) as a viral infection with pneumonia and respiratory syndrome caused by a new coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).6 The spectrum of infection ranges from asymptomatic to critical forms.7–13 Among hospitalized patients, the proportion of critical disease is higher, with nearly 30% of patients requiring intensive care.14 Most of the fatal cases occurred in patients with advanced age or underlying medical comorbidities such us cardiovascular disease, arterial hypertension, diabetes mellitus, chronic lung disease, chronic kidney disease, obesity or cancer, etc.14–19 Other prognostic factors include acute respiratory failure or laboratory alterations as lymphopenia, elevated liver enzymes, elevated inflammatory markers or acute kidney injury.15,20,21
Atrial fibrillation (AF) is the most frequent arrythmia worldwide, and it is expected to increase its incidence due to population aging and comorbidities.22,23 This arrythmia is common in the context of acute situations such as myocardial infarction, cardiac surgery or infections, where it has linked with higher risk of complications and mortality.24
ObjectiveWe aimed to describe the clinical features and prognosis of COVID-19 patients with atrial fibrillation (AF) and evaluate the impact of this arrythmia on the short-term prognosis of the disease.
MethodsStudy designWe compare the main features of patients with SARS-CoV-2 infection who develop AF during admission with the rest of patients and look for independent predictors of AF development. To evaluate the prognosis impact, we performed an univariable and multivariable analysis of in-hospital mortality, including the most important risk factors.
SettingWe included patients older than 15 years old, admitted at a tertiary hospital between March 10 2020 and April 15, 2020, with definitive diagnosis of SARS-CoV-2 infection. Only patients with a positive real time-polymerase chain reaction of nasal or pharyngeal swabs and lung damage demonstrated by chest imaging were admitted to the hospital (n=517). Otherwise they were given oral medical treatment and advised to come back to the hospital if symptoms worsen.
Participants and variablesObservational study with prospective recruitment of patients and retrospective data analysis including all patients with a final diagnosis of SARS-CoV-2 infection.
A total of 210 variables per patient were gathered in a specific database from consecutive patients with, which were retrospectively collected from the electronic medical records. Data encompassed a clinical history, temperature, blood pressure, symptoms, laboratory findings, ECG findings, chest imaging results, and events. All patients underwent at least one electrocardiogram at admission, and during hospitalization electrocardiograms were performed at medical discretion using either conventional machine or remote mobile transmission Alivecor Kardia monitor.25
Laboratory parameters consisted of a complete blood count, coagulation testing including D-dimer, iron metabolism including ferritine, electrolytes, assessment of liver and renal function, C-reactive protein, lactate dehydrogenase, procalcitonin and creatine kinase. Troponin and interleukin-6 were obtained at the discretion of the physician. Fever was considered when temperature was over 37.5°C. Respiratory failure was defined as pO2 less than 60mmHg in arterial gasometry or O2 saturation measured with pulse oximetry of less than 90% breathing room air. Imaging always included a chest-X-ray and a CT scan when required. Interpretation was performed by an expert radiologist.
To verify reliability of the gathered data 50 patients’ records were reviewed by an independent observer and only 0.04% variables were found to be incorrect. Moreover, all quantitative outliers (more than mean±2SD) were checked by an independent observer. The study was approved by our local ethics committee.
Statistical methodsCategorical variables are reported as frequency (n) and percentages and continuous variables as mean value and standard deviation. Normal distribution of quantitative variables was verified with the Kolmogorov–Smirnov test and visually through Q-Q plot graphics. Qualitative variables were compared with the chi-squared test and Fisher's exact test. Continuous variables were compared with Student's t test.
Multivariable analysis were performed by a logistic regression model with the maximum likelihood method using forward stepwise selection. We included the most important known risk factors. The ratio variable/event was controlled to avoid overfitting. For the final models, odds ratios (OR) adjusted for each of the variables included along with their 95% confidence intervals (95%CI) were calculated. Non-collinearity was checked among the variables included in the model. The area under the receiver operating characteristic curve (ROC curve) was used to measure how well the models discriminated between patients with high and low risk of in-hospital mortality. Calibration was evaluated with the Hosmer–Lemeshow test and with plots comparing predicted and observed mortality for different levels of risk.
The statistical analyses were performed with the use of R software, version 3.6.1 (R Project for Statistical Computing) and IBM SPSS Statistics, Version 25.0. Armonk, NY: IBM Corp. Differences were considered statistically significant when p value was<0.05.
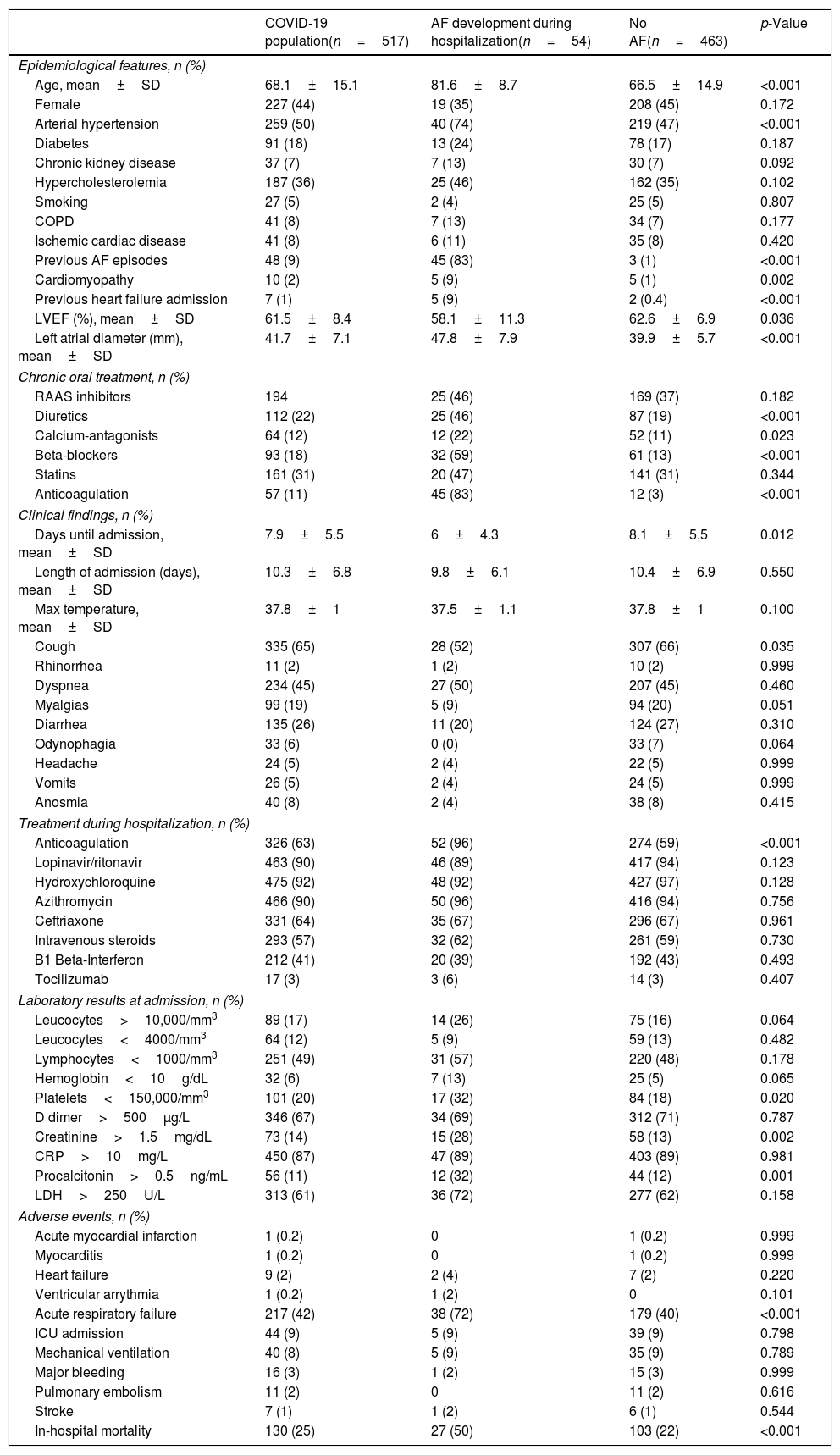
ResultsCharacteristics of the populationTable 1 presents the main characteristics of patients with SARS-CoV-2 infection. Mean age was 68.1±15.1 years, with a high incidence of cardiovascular risk factors (arterial hypertension 50%, hypercholesterolemia 36% and diabetes 18%). More frequent symptoms were cough (65%) and dyspnea (45%) followed by myalgias (19%) and diarrhea (26%). Most frequent laboratory abnormalities were the increase of CRP (87%), D Dimer (67%) and LDH (61%) as long as lymphopenia (49%). Acute respiratory failure appeared in 42% of patients, with 9% of ICU admission and 8% of mechanical ventilation. In-hospital mortality was 25%. During hospitalization, almost 90% of patients underwent the combination of lopinavir/ritonavir, hydroxychloroquine and azithromycin, with 57% of intravenous steroids and 63% of anticoagulation use.
Features of COVID-19 patients and comparative analysis according to AF development.
| COVID-19 population(n=517) | AF development during hospitalization(n=54) | No AF(n=463) | p-Value | |
|---|---|---|---|---|
| Epidemiological features, n (%) | ||||
| Age, mean±SD | 68.1±15.1 | 81.6±8.7 | 66.5±14.9 | <0.001 |
| Female | 227 (44) | 19 (35) | 208 (45) | 0.172 |
| Arterial hypertension | 259 (50) | 40 (74) | 219 (47) | <0.001 |
| Diabetes | 91 (18) | 13 (24) | 78 (17) | 0.187 |
| Chronic kidney disease | 37 (7) | 7 (13) | 30 (7) | 0.092 |
| Hypercholesterolemia | 187 (36) | 25 (46) | 162 (35) | 0.102 |
| Smoking | 27 (5) | 2 (4) | 25 (5) | 0.807 |
| COPD | 41 (8) | 7 (13) | 34 (7) | 0.177 |
| Ischemic cardiac disease | 41 (8) | 6 (11) | 35 (8) | 0.420 |
| Previous AF episodes | 48 (9) | 45 (83) | 3 (1) | <0.001 |
| Cardiomyopathy | 10 (2) | 5 (9) | 5 (1) | 0.002 |
| Previous heart failure admission | 7 (1) | 5 (9) | 2 (0.4) | <0.001 |
| LVEF (%), mean±SD | 61.5±8.4 | 58.1±11.3 | 62.6±6.9 | 0.036 |
| Left atrial diameter (mm), mean±SD | 41.7±7.1 | 47.8±7.9 | 39.9±5.7 | <0.001 |
| Chronic oral treatment, n (%) | ||||
| RAAS inhibitors | 194 | 25 (46) | 169 (37) | 0.182 |
| Diuretics | 112 (22) | 25 (46) | 87 (19) | <0.001 |
| Calcium-antagonists | 64 (12) | 12 (22) | 52 (11) | 0.023 |
| Beta-blockers | 93 (18) | 32 (59) | 61 (13) | <0.001 |
| Statins | 161 (31) | 20 (47) | 141 (31) | 0.344 |
| Anticoagulation | 57 (11) | 45 (83) | 12 (3) | <0.001 |
| Clinical findings, n (%) | ||||
| Days until admission, mean±SD | 7.9±5.5 | 6±4.3 | 8.1±5.5 | 0.012 |
| Length of admission (days), mean±SD | 10.3±6.8 | 9.8±6.1 | 10.4±6.9 | 0.550 |
| Max temperature, mean±SD | 37.8±1 | 37.5±1.1 | 37.8±1 | 0.100 |
| Cough | 335 (65) | 28 (52) | 307 (66) | 0.035 |
| Rhinorrhea | 11 (2) | 1 (2) | 10 (2) | 0.999 |
| Dyspnea | 234 (45) | 27 (50) | 207 (45) | 0.460 |
| Myalgias | 99 (19) | 5 (9) | 94 (20) | 0.051 |
| Diarrhea | 135 (26) | 11 (20) | 124 (27) | 0.310 |
| Odynophagia | 33 (6) | 0 (0) | 33 (7) | 0.064 |
| Headache | 24 (5) | 2 (4) | 22 (5) | 0.999 |
| Vomits | 26 (5) | 2 (4) | 24 (5) | 0.999 |
| Anosmia | 40 (8) | 2 (4) | 38 (8) | 0.415 |
| Treatment during hospitalization, n (%) | ||||
| Anticoagulation | 326 (63) | 52 (96) | 274 (59) | <0.001 |
| Lopinavir/ritonavir | 463 (90) | 46 (89) | 417 (94) | 0.123 |
| Hydroxychloroquine | 475 (92) | 48 (92) | 427 (97) | 0.128 |
| Azithromycin | 466 (90) | 50 (96) | 416 (94) | 0.756 |
| Ceftriaxone | 331 (64) | 35 (67) | 296 (67) | 0.961 |
| Intravenous steroids | 293 (57) | 32 (62) | 261 (59) | 0.730 |
| B1 Beta-Interferon | 212 (41) | 20 (39) | 192 (43) | 0.493 |
| Tocilizumab | 17 (3) | 3 (6) | 14 (3) | 0.407 |
| Laboratory results at admission, n (%) | ||||
| Leucocytes>10,000/mm3 | 89 (17) | 14 (26) | 75 (16) | 0.064 |
| Leucocytes<4000/mm3 | 64 (12) | 5 (9) | 59 (13) | 0.482 |
| Lymphocytes<1000/mm3 | 251 (49) | 31 (57) | 220 (48) | 0.178 |
| Hemoglobin<10g/dL | 32 (6) | 7 (13) | 25 (5) | 0.065 |
| Platelets<150,000/mm3 | 101 (20) | 17 (32) | 84 (18) | 0.020 |
| D dimer>500μg/L | 346 (67) | 34 (69) | 312 (71) | 0.787 |
| Creatinine>1.5mg/dL | 73 (14) | 15 (28) | 58 (13) | 0.002 |
| CRP>10mg/L | 450 (87) | 47 (89) | 403 (89) | 0.981 |
| Procalcitonin>0.5ng/mL | 56 (11) | 12 (32) | 44 (12) | 0.001 |
| LDH>250U/L | 313 (61) | 36 (72) | 277 (62) | 0.158 |
| Adverse events, n (%) | ||||
| Acute myocardial infarction | 1 (0.2) | 0 | 1 (0.2) | 0.999 |
| Myocarditis | 1 (0.2) | 0 | 1 (0.2) | 0.999 |
| Heart failure | 9 (2) | 2 (4) | 7 (2) | 0.220 |
| Ventricular arrythmia | 1 (0.2) | 1 (2) | 0 | 0.101 |
| Acute respiratory failure | 217 (42) | 38 (72) | 179 (40) | <0.001 |
| ICU admission | 44 (9) | 5 (9) | 39 (9) | 0.798 |
| Mechanical ventilation | 40 (8) | 5 (9) | 35 (9) | 0.789 |
| Major bleeding | 16 (3) | 1 (2) | 15 (3) | 0.999 |
| Pulmonary embolism | 11 (2) | 0 | 11 (2) | 0.616 |
| Stroke | 7 (1) | 1 (2) | 6 (1) | 0.544 |
| In-hospital mortality | 130 (25) | 27 (50) | 103 (22) | <0.001 |
AF: atrial fibrillation. SD: standard deviation. COPD: Chronic obstructive pulmonary disease. LVEF: left ventricle ejection fraction. RAAS: renin-angiotensin-aldosterone system. CRP: C-reactive protein. LDH: lactate dehydrogenase. ICU: intensive care unit.
Among 517 patients with SARS-CoV-2 infection, 54 (10.4%) developed AF during admission: 83% (n=45) have had previous AF episodes before and only 17% (n=9) presented “de novo” AF during hospitalization. Among 463 patients who did not develop AF during admission, only 3 (1%) have had previous AF episodes before.
Table 1 presents the comparative analysis of patients with SARS-CoV-2 infection who developed AF with patients who did not. Patients with AF were significantly older (81.6 vs 66.5 years old) and present more arterial hypertension (74% vs 47%), cardiomyopathy (9% vs 1%), previous heart failure admission (9% vs 0.4%) and previous AF episodes (83% vs 1%). Left atrial size was higher in AF patients (47.8 vs 39.9mm). Clinically, they present a shorter time to admission (6 vs 8.1 days), but not different symptomatic profile. Procalcitonin (32% vs 12%) and creatinine increase (28% vs 13%) and platelets decrease (32% vs 18%) were more frequent. Acute respiratory failure (72% vs 40%) and in-hospital mortality (50% vs 22%) were higher among AF COVID-19 patients despite no differences in ICU admission, mechanical ventilation or standard treatments. Anticoagulation was more utilized in AF patients (96% vs 59%).
Independent predictors of AF development during admission were age (Log OR 0.078, 95% CI 0.025-0.13, p=0.003) and previous history of AF (Log OR 6.21, 95% CI 4.82–7.59, p<0.001) with an area under ROC curve of 0.79 (95% CI 0.75–0.84) and a pvalue in the Hosmer–Lemeshow test of 0.93.
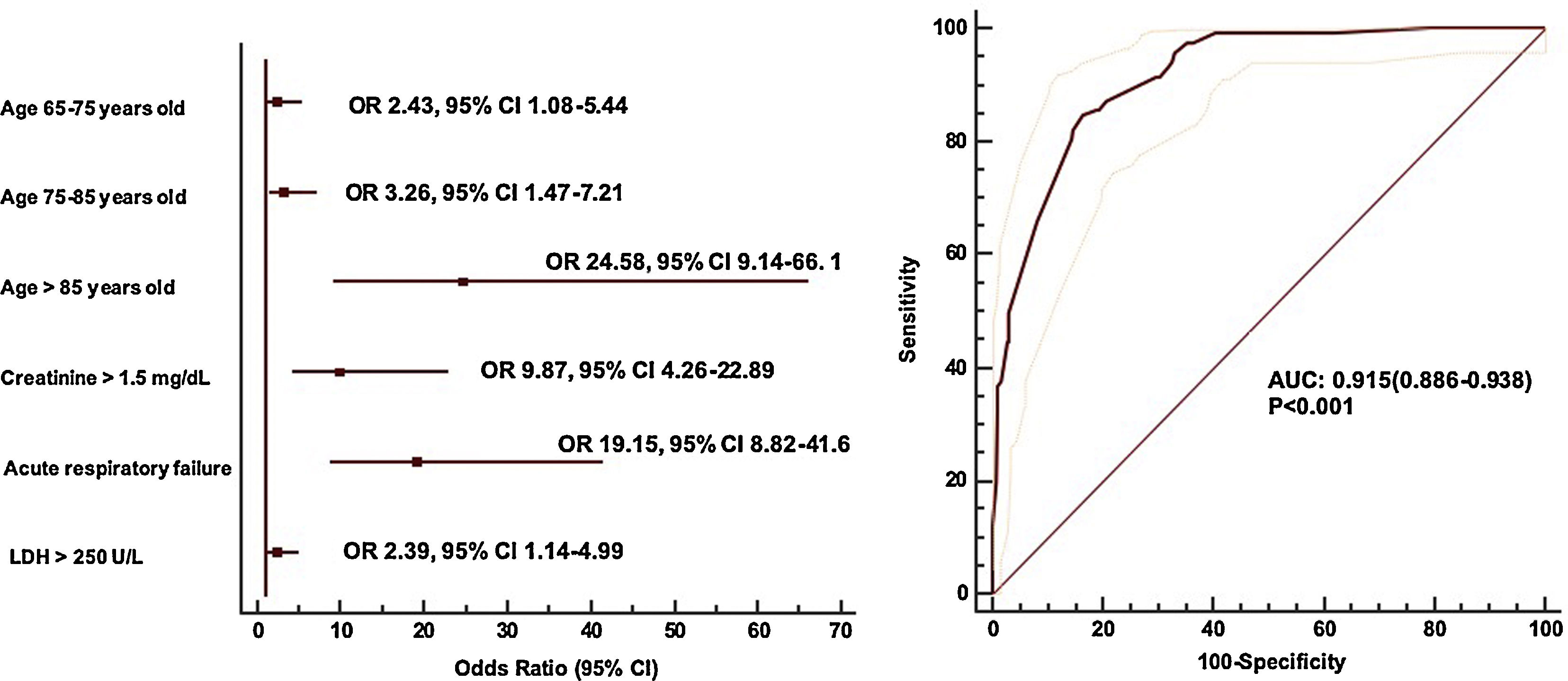
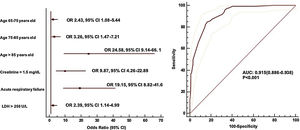
Prognostic impact of atrial fibrillation on SARS-CoV-2 infectionUnivariable analysis of in-hospital mortality of SARS-CoV-2 infection shows a linear relation between AF development and mortality (mortality on patients with AF 21% vs mortality on patients without AF 7%, p<0.001) (Supplementary Table 1). However, the multivariable analysis showed as independent predictors of mortality, age, creatinine at admission>1.5mg/dL, LDH at admission>250UI/L and acute respiratory failure (area under ROC curve 0.915, 95% CI 0.886–0.938) (Fig. 1).
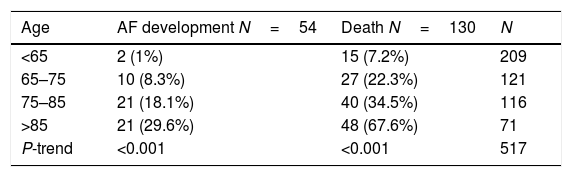
AF development is not an independent predictor of mortality, its prognostic impact is totally influenced by its interaction with age, as presented in Table 2.
DiscussionWe present a work specifically addressing the influence of atrial fibrillation on SARS-CoV-2 infection. Main findings are: (1) 10% of patients with SARS-CoV-2 infection develop atrial fibrillation during hospitalization. (2) Patients with SARS-CoV-2 infection and atrial fibrillation present higher in-hospital mortality. (3) Atrial fibrillation is not an independent predictor of mortality.
Atrial fibrillation is the most common arrhythmia worldwide, and it is known its greater prevalence among older people and patients with conditions such as hypertension, diabetes mellitus, chronic kidney disease or heart disease.22,26–31 In our work, approximately 10% of patients developed atrial fibrillation during admission, with only 2% of incident atrial fibrillation. Bhatia et al recently reported 25 episodes of incident atrial fibrillation among 700 episodes of SARS-CoV-2 infection but excluded patients with previous history of atrial fibrillation.32 We included these patients, and demonstrated that previous AF is the most important predictor of AF development during admission. We could have found higher incidence of atrial fibrillation if continuous monitorization had been systemically performed, however, it was not contemplated in our protocol. AliveCor Kardia monitorization has demonstrated useful for AF screening in ambulatory patients and was acquired to improve arrythmia detection minimizing the risk of contagious.25 Patients with SARS-CoV-2 infection and atrial fibrillation are older, and present higher incidence of arterial hypertension, cardiomyopathy and heart failure, and of course, higher left atrium size.
In our work, AF COVID-19 patients presented twice in-hospital mortality (50 vs 22%, p<0.001), however, AF is not an independent predictor of mortality, similarly to previous reports.32 AF population has a two-fold increase in all-cause mortality, especially due to cardiovascular mortality.33–36 Several risk factors have been described, such as older age as long as underlying medical comorbidities such us cardiovascular disease, arterial hypertension, diabetes mellitus, chronic lung disease, chronic kidney disease, obesity or cancer, etc.14–19 These risk factors are also important risk factors for AF development and are more frequent in our AF COVID-19 population. Other prognostic factors include acute respiratory failure or laboratory alterations as lymphopenia, elevated liver enzymes, elevated inflammatory markers or acute kidney injury.15,20,21
In our work we found age, creatinine>1.5mg/dL at admission, LDH>250UI/L at admission and acute respiratory failure as independent predictors of mortality, but not atrial fibrillation. In this context, AF development could be only a marker of a higher risk population which is older and presents more comorbidities, but also a marker of a more aggressive SARS-CoV-2 infection clinical course with higher acute respiratory failure, acute renal failure or acute heart failure or myocarditis. It is known that AF is common in acute situations such as myocardial infarction, cardiac surgery or infections where is associated with increased length of hospital admission and higher rates of complications and mortality.24 Nonetheless, it is not the unique arrythmia in this context, where sinus tachycardia is the most common one even in the absence of fever. It has been described persistent sinus tachycardia after hospital discharge in approximately 40% survivors of SARS coronavirus disease.37
We are aware of some limitations in our work. We only include hospitalized patients, so our conclusions cannot be assumed for outpatients. Echocardiogram was not included in the protocol and we only have information of a selected population with echo performed prior to admission. As already mentioned, it is possible that due to extremely high contagious rate of disease, electrocardiograms have been minimized to avoid contacts, conducting to a reduction in AF detection. We tried to use AliveCor Kardia monitor to improve arrythmia detection but continuous monitorization was not routinely performed in our centre, and to be honest, the retrospective character of our work prevented having the information.25 It is probable that some patients could have developed asymptomatic paroxysmal AF, only detected with monitorization.
In conclusion, atrial fibrillation appears in 10% of hospitalized patients with SARS-CoV-2 infection. These patients present more comorbidities and a two-fold increase in hospital mortality. Atrial fibrillation is not an independent prognostic factor.
FundingGerencia Regional Salud. Consejería Sanidad. Junta Castilla y León. GRS COVID 114/A/20 CARDIOVID: Estudio de la incidencia de eventos cardiovasculares adversos tras el alta en pacientes que han superado la COVID-19.
Sociedad Española de Cardiología: SEC/FEC-INVCLI 20/030 «Incidencia de eventos cardiovasculares adversos a medio plazo en pacientes que han superado la COVID-19. Caracterización del daño miocárdico sufrido durante la COVID19 mediante técnicas de imagen cardíaca avanzada».
Conflicts of interestNone.