To characterize the rate of change of ocular surface temperature (OST) under lid manipulation after cooling the intact cornea with balanced salt solution (BSS).
MethodsIn a patient for refractive surgery, prior to the ablation, the temperature of the cornea was continuously recorded with a high speed infrared (350Hz) camera. Two millilitre of chilled BSS with a temperature of 8.6°Celsius (°C) was instilled for about 3s. Using exponential functions, the three contributions have been determined, subjacent corneal layers, environment, and chilled BSS.
ResultsThe mean temperature of the cornea preoperatively was 34.5°C. After applying the chilled BSS the temperature decreased about 14°C down to an OST of 20°C and the time needed afterwards to get the normal (OST) temperature of about 30°C was 40s. Due to the inserted speculum and missing blink, OST did not reach the original OST of 34.5°C and faded at about 32.5°C. According to our best fitted model, absolute value of each contributing component was 31.4°C (subjacent corneal layers), 26.8°C (environment) and 8.6°C (BSS).
ConclusionsApplying chilled BSS to the cornea quickly reduces the temperature of the cornea with a thermal relaxation time of 3s and a amplitude decrease of 8.6°C. This together with a relaxation time of 7s for subjacent corneal layers, and 184s for environment after instillation of BSS combined with a well-controlled environment provides a period of 40s of corneal temperature below baseline, which may be of clinical benefit when applying chilled BSS immediately before or immediately after ablation.
Describir el índice de cambio de la temperatura de la superficie ocular (OST) con manipulación del párpado tras el enfriamiento de la córnea intacta con solución salina balanceada (BSS).
MétodosEn un paciente sometido a cirugía refractiva, con anterioridad a la ablación, registramos continuamente la temperatura de la córnea con una cámara de infrarrojos de alta velocidad (350Hz). Instilamos durante alrededor de 3s dos mililitros de BSS a una temperatura de 8,6° Celsius (°C). Utilizando funciones exponenciales, se determinaron los valores de las tres contribuciones: capas corneales subyacentes, ambiente, y BSS fría).
ResultadosPreoperatoriamente, la temperatura media de la córnea fue de 34,5°C. Tras aplicar la BSS fría, la temperatura descendió alrededor de 14°C hasta alcanzar una OST de 20°C, precisándose un tiempo posterior de 40 segundos para alcanzar la OST normal de unos 30°C. Debido a la inserción del espéculo y a la ausencia de parpadeo, la OST no alcanzó el valor original de 34,5°C, permaneciendo en unos 32,5°C. De acuerdo a nuestro modelo de mejor ajuste, el valor absoluto de cada componente participante fue de 31,4°C (capas corneales subyacentes), 26,8°C (ambiente) y 8,6°C (BSS).
ConclusionesLa aplicación de BSS fría a la córnea reduce rápidamente la temperatura de la misma, con un tiempo de relajación térmica de 3s y un descenso de amplitud de 8,6°C. Estos hallazgos, junto con tiempos de relajación de 7s para las capas corneales subyacentes, y de 184s para el ambiente tras la instilación de BSS, junto con un entorno bien controlado, proporciona unos 40s de temperatura corneal inferior a la basal, lo que puede suponer un beneficio clínico cuando se aplica BSS fría inmediatamente antes o inmediatamente después de la ablación.
Temperature of the cornea is a function of the equilibrium of heat transfer between the cornea and the surrounding tissues or atmosphere. Therefore, any change that affects the heat loss or gain may affect the corneal temperature.1,3,4 The normal corneal surface temperature has been reported to range from 32.9 to 36°C.5 Under LASIK settings, when lid speculum has been applied, the dynamic heat balance shifts towards heat loss from the cornea to the surrounding cooler air and baseline corneal temperature (prior to initiating surgery) decreases to approximately 31°C. When excimer laser is initiated, every single pulse adds heat to the cornea and contributes to the marginal increase in the local corneal temperature.6 Laser refractive surgery is based on the sequential delivery of multiple laser pulses, with each pulse ablating a small amount of corneal tissue and in the process causing a marginal increase in the local corneal temperature around the laser spot. In general, Excimer laser treatments may cause a significant increase in corneal temperature mainly due to the heat generation exceeding the heat dissipation during the laser treatment.6
These thermal effects may cause tissue damage and potentially reduce the predictability of the refractive outcomes.1 Usually cold balanced salt solution (BSS) is applied after the ablation procedure to reduce the postoperative increment of the ocular surface temperature (OST) which leads to several problems which by definition can be later introduced as haze and scattering. It is really important to use either a laser technology like the flying spot and spot size,4,5 or other ways to reduce the temperature with cold fluid prior or after the ablation. Our motivation is to characterize the response in OST with the use of BSS, and explore a potential method to reduce OST below the baseline temperature prior to ablation, to compensate the heat generation during the laser treatment.
MethodsOur objective was to characterize the rate of change of the temperature of the corneal surface after cooling the cornea with balanced salt solution (BSS). We used cooled balanced salt solution (BSS) from the refrigerator with a temperature of 8.6°C and 2ml of this solution was applied on an eye prior to excimer laser refractive surgery. For measuring the cornea temperature, we used a high frequency infrared camera, VarioCAM® HR (Jena, Germany), which takes 350 measurements per second. The camera provides thermal images with a resolution of 640×480 pixel and measures the ocular surface temperature (OST) within the spectral range of 7.5–14μm with a resolution of ±0.08K using a micro bolometer-FPA detector. The typical error of a similar set up used in one of our previous study was approximately ±0.5°,2 but we did not measure the typical error with this set up although this error was expected to be lesser than ±0.5°. An elliptical region of interest of size ∼9mm was positioned manually such that it covered the cornea completely and tightly. The maximum temperature observed within this region of interest was recorded.
After applying two drops (at room temperature) of anaesthesia Conjucain EDO (Oxybuprocain HCl, Bausch Lomb), a lid speculum was positioned on the eye. We started to record the thermal response of the cornea at this point. After lid speculum insertion, cooled BSS was applied for about 3s. All our measurements were taken after speculum insertion and before epithelium removal. Subsequently, the epithelium removal was performed in a single step by means of a transepithelial photo refractive keratectomy (PRK). The method of epithelial removal shall not affect our results due to the design of our experiment.
The temperature of the room was maintained at 23.4°C with a humidity of slightly less than 40%. The central ocular surface temperature [OST] was 34.5°C at the beginning of the temperature measurement. Betney et al.3 reported ocular surface temperatures of 32.05°C measured in normal eyes that had not undergone any procedure and had an intact epithelium.
We quantify in this report, the time needed for the cornea to reach back to its original OST after instillation of chilled BSS (relaxation time). Using exponential functions, the three contributing components associated with the thermodynamic response of the OST have been determined as subjacent corneal layers, environment, and chilled BSS, the latter through an impulse exponential limited in time. Based on these three exponential functions corresponding to the contributing factors, we design a model that fits our experimental data using least square fitting with the following equation:
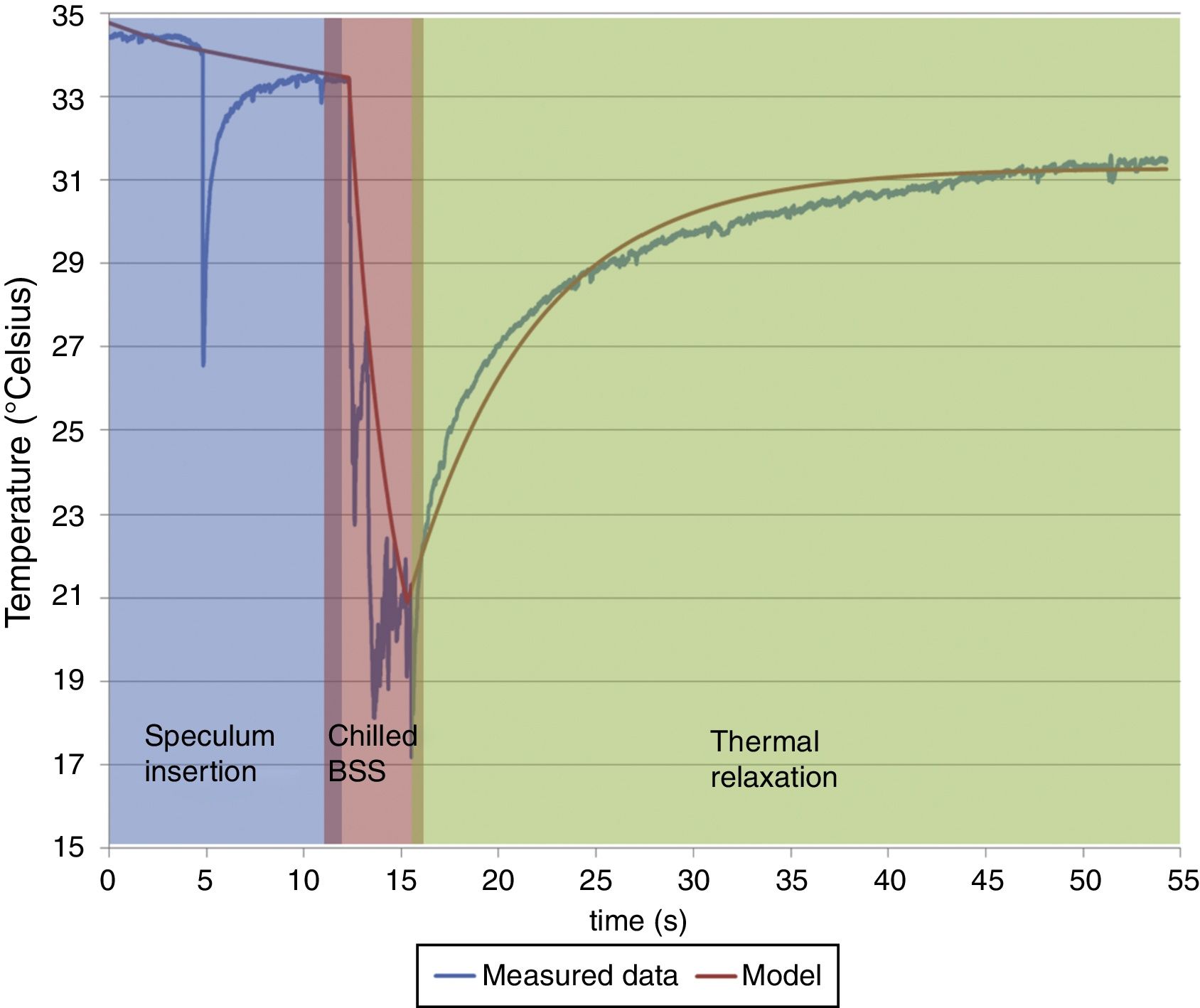
Here, T represents the temperature; t represents the time with t1, t2 and t3 representing the effective contribution time for each contributing component; B, C, D represents the absolute value of each contributing component; Γ represents the thermal relaxation time of each contributing component. It must be noted that our experiment set up measured the OST and not the temperature at the deeper layers of the cornea. Since the BSS is applied on the outermost corneal layer, the deeper layers shall be affected lesser by the BSS comparatively. The subjacent layers shall eventually contribute in increasing the temperature of the outer layers to maintain a thermodynamic equilibrium. Therefore, the subjacent corneal layer component in our analysis is taken as a potential contributing component that can affect the temperature change in the OST after the application of BSS.ResultsThe OST decreased by 14°C (down to 20°C) due to the application of chilled BSS for 3s (Fig. 1 red background, from about 12 to about 16s). After this time, the temperature increased again by 8°C (28°C, 57% of original OST) after 10s (Fig. 1 green background, at about 25s), by 10°C (30°C, 71% of original OST) at 20s (Fig. 1 green background, at about 35s) and by 12°C after 40s (32°C, 86% of the original OST) (Fig. 1 green background, at about 55s). The decreased temperature of at least 2°C from the initial OST baseline can be ascribed to the effect of room temperature (23.4°C), the effect of the lid speculum and the missing blink frequency (Fig. 1 blue background, from 0 to about 12s).
The central ocular surface temperature [OST] was of 34.5°C before beginning the procedure of epithelial removal. The OST decreased by 14°C due to the application of chilled BSS for 3s. After this time the temperature increases again by 8°C, 10°C, and 12°C after 10, 20, and 40s respectively. The decreased temperature of at least 2°C from the initial OST baseline can be ascribed to the effect of room temperature (23.4°C), the effect of the lid speculum and the missing blink frequency. According to our best fitted model, the absolute value of each contributing component was 31.4°C (subjacent corneal layers), 26.8°C (environment) and 8.6°C (BSS). The corresponding relaxation time relevant to these exponential functions was, for subjacent corneal layer (48s and 7s before and after the application of BSS), for environment (80s and 184s before and after the application of BSS) and for BSS (3s). Please notice that the inverted spike observed at ∼4s is due to the manipulation in measurement during the instillation of chilled BSS.
According to our best fitted model, the absolute value of each contributing component was 31.4°C (subjacent corneal layers), 26.8°C (environment) and 8.6°C (BSS). The corresponding relaxation time relevant to these exponential functions was for subjacent corneal layer 48s and 7s before and after the application of BSS, for environment 80s and 184s before and after the application of BSS and for BSS 3s. The relaxation time represents here the time required for the OST to reach the baseline temperature recorded at the beginning of the experiment. These exponential functions have been regarded here as representative of human corneas.
DiscussionApplying chilled BSS to the cornea quickly reduces the temperature of the cornea with a thermal relaxation time of 3s and a amplitude decrease of 8.6°C. This together with the relaxation time of 7s for subjacent corneal layers, and 184s for environment after instillation of BSS combined with a well-controlled environment provides a period of 40s of corneal temperature below baseline, which may be of clinical benefit when applying chilled BSS immediately before or immediately after ablation. However, the application of cold BSS after the ablation leads to several problems which by definition can be later introduced as haze and scattering.
We know that surface ablation causes haze in some cases whereas the causes of this are not well known, but a temperature increase over 40°C during the ablation is one potential cause of denaturation4 and haze. There are several ways to minimize the temperature increase of the cornea during the surgery, one is the development of flying-spot ablation pattern that controls the local repetition rates to minimize the thermal load of the treatment for a smooth ablation with minimized risk of thermal damage.4.5 In addition to such methods, our results suggest that using chilled BSS preoperatively could help maintain OST below the baseline temperature and eventually help compensate the increase in OST due to laser ablation. The overall relaxation time we observed under the effect of chilled BSS (about 40s) are long enough to provide a time window that can be used to design safer refractive procedures over and above the existing methods (described above) used to minimize the temperature increase.
Studies of the use of cold fluids postoperatively have presented the action of the reduced temperature as postop results or postop terms like reduced pain or reduced haze.6–13 However, these studies have not looked for a quantified method like the one we describe. Furthermore, our method essentially quantifies the result of preoperative BSS application and its potential benefits on thermal effects postoperatively.
We acknowledge the strong limitation that we have not measured more than one patient in this comprehensive manner. Furthermore, the temperature before and after the instillation of anaesthesia and the temperature of the speculum and anaesthetic drops were not analysed in our study. The temperature was measured continuously throughout the procedure and the manipulations in the measurements during the instillation of anaesthesia were avoided. More patients and a strong statistical analysis would also help elucidate the implications of our work. In this sense, our study can be regarded as a qualitative study. This study is meant more to engender thoughts, and we think it brings up some interesting points. To truly know the quantitative changes a more detailed study will need to be performed in a prospective fashion. Typical variation in surface temperature over the cornea, inter-subject variations and how these factors affect the procedure needs to be explored in details. Hopefully, this case study will be a good precursor for a formal prospective study to clarify some of the issues raised in this paper.
Conflict of interestThe authors have no proprietary interest in the materials presented herein.



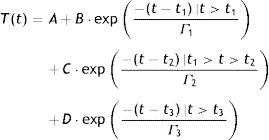
![The central ocular surface temperature [OST] was of 34.5°C before beginning the procedure of epithelial removal. The OST decreased by 14°C due to the application of chilled BSS for 3s. After this time the temperature increases again by 8°C, 10°C, and 12°C after 10, 20, and 40s respectively. The decreased temperature of at least 2°C from the initial OST baseline can be ascribed to the effect of room temperature (23.4°C), the effect of the lid speculum and the missing blink frequency. According to our best fitted model, the absolute value of each contributing component was 31.4°C (subjacent corneal layers), 26.8°C (environment) and 8.6°C (BSS). The corresponding relaxation time relevant to these exponential functions was, for subjacent corneal layer (48s and 7s before and after the application of BSS), for environment (80s and 184s before and after the application of BSS) and for BSS (3s). Please notice that the inverted spike observed at ∼4s is due to the manipulation in measurement during the instillation of chilled BSS. The central ocular surface temperature [OST] was of 34.5°C before beginning the procedure of epithelial removal. The OST decreased by 14°C due to the application of chilled BSS for 3s. After this time the temperature increases again by 8°C, 10°C, and 12°C after 10, 20, and 40s respectively. The decreased temperature of at least 2°C from the initial OST baseline can be ascribed to the effect of room temperature (23.4°C), the effect of the lid speculum and the missing blink frequency. According to our best fitted model, the absolute value of each contributing component was 31.4°C (subjacent corneal layers), 26.8°C (environment) and 8.6°C (BSS). The corresponding relaxation time relevant to these exponential functions was, for subjacent corneal layer (48s and 7s before and after the application of BSS), for environment (80s and 184s before and after the application of BSS) and for BSS (3s). Please notice that the inverted spike observed at ∼4s is due to the manipulation in measurement during the instillation of chilled BSS.](https://static.elsevier.es/multimedia/18884296/0000000800000003/v2_202107280542/S1888429615000126/v2_202107280542/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w90elkTtpMHXMkN9jatH7+0Y=)





