A notable proportion of COVID outbreaks are generated by “super-spreading events”, where a few subjects transmit the pathogen to many secondary cases, increasing contact networks and the spread of the pathogen. We conducted a description of a COVID-19 superspreading event in Córdoba during July 2020, linked to a nightlife establishment.
Material and methodsRetrospective observational study describing characteristics of person, time, PCR result and contact network of confirmed cases. PCR results in Córdoba during July and August and information collected in surveillance systems were analyzed.
Results935 individuals associated with the outbreak were included; 120 (12.83%) became confirmed cases. July 17 was the day with the highest incidence, with 27 new cases (22.5% of the total). People under 25 years old represented 69.2% of the cases. The average number of close contacts per person was 10.7, with a decrease as age raised. During the outbreak, incidence increased at the provincial level compared to previous weeks; at the end, incidence did not return to initial values but remained high with a relevant percentage of cases having unknown epidemiological association.
ConclusionsA greater transmission capacity of SARS-CoV-2 was observed in a closed, crowded space, and among young people that tended to report a greater number of social contacts and may present little or no symptoms. Developing preventive measures in scenarios that combine these factors and early detection of cases are essential to avoid an increase in the spread of the virus.
Una proporción notable de brotes de COVID se genera por «eventos de superdifusión», donde unos pocos sujetos transmiten el patógeno a muchos casos secundarios, aumentando las redes de contacto y la propagación. Se realizó la descripción de un evento supercontagiador de COVID-19 en julio de 2020 relacionado con un local de ocio nocturno en Córdoba.
Material y métodosEstudio retrospectivo observacional describiendo las características de persona, tiempo, resultado de PCR y red de contactos de casos confirmados. Se analizaron los resultados de PCR de Córdoba durante julio y agosto y la información recogida en los sistemas de vigilancia.
ResultadosSe incluyeron 935 personas asociadas con el brote; 120 (12,83%) fueron casos confirmados. El 17 de julio fue el día de mayor incidencia, con 27 nuevos casos (22,5% del total). El 69,2% de los casos tenían menos de 25 años. La media de contactos estrechos por persona fue 10,7, que disminuía con el aumento de edad. Durante el brote aumentó la incidencia a nivel provincial respecto a semanas previas; tras su finalización no retornó a niveles iniciales y un porcentaje relevante de casos no tenía vínculo epidemiológico conocido.
ConclusionesSe observó una mayor capacidad de transmisión del SARS-CoV-2 en un espacio cerrado, con aglomeración de jóvenes, que tendían a referir más contactos sociales y además pueden presentar escasa o nula sintomatología. Desarrollar medidas preventivas en los escenarios que combinan estos factores y la detección temprana de casos son fundamentales para evitar un incremento de la diseminación del virus.
In December of 2019, China reported 27 cases of pneumonia of unknown aetiology in the City of Wuhan. After identifying the SARS-CoV-2 coronavirus as the causative agent, WHO declared the outbreak of the new coronavirus as a Public Health Emergency of International Concern in January of 2020.1 The worldwide increasing spread of SARS-CoV-2, together with its devastating impact, has highlighted the importance of expanding knowledge of its transmission mechanisms and natural history.
Since the confirmation of the first COVID-19 cases, a significant proportion of these has been linked to specific clusters. Outbreaks associated with social gatherings,2 work,3 recreational activities,4 religious5 and health centres6 have augmented the viral spread in the population by generating dozens of contacts, and thus enabling a faster pandemic expansion. ‘Superspreading events’ occur when a small number of subjects infect a large number of secondary cases, compared to an “average” infectious individual.7 They are punctual situations in time, frequently with high attendance in closed, crowded and/or poorly ventilated places, and therefore with a very high capacity to amplify contagion. Multiple studies have assigned critical importance to these events in the spread of COVID-19, pointing to 2% of cases being directly responsible for 20% of all infections.8 At the beginning of the pandemic, a modellization suggested that 10% of infected individuals infected 80% of secondary cases.9 In addition, a screening of asymptomatic population identified 2% of positive individuals who would carry 90% of the circulating pathogen (supercarriers), underlining the key role of this sector in dissemination.10
In response to the initial increases in cases and hospital occupancy, the Spanish government declared the first state of alarm on March 14, 2020. From this date, the lockdown ended on June 21, 2020, the transition to the “New Normality” was carried out in several phases, gradually resuming economic and social activities to minimize the risk to health and health systems.11 Nevertheless, the entry into the New Normal12 also implied the return of some social activities that involved less physical distancing and a greater concentration of people. In this new scenario, different alert levels that took into account health and epidemiological indicators were established, as well as several measures designed to respond to each of those levels.13 This strategic model has been maintained to date.
In the case of Andalusia, the first major superspreading event was associated with an indoor nightclub in Córdoba. It occurred on 10th July 2020, soon after the entry to the New Normality. According to the media, attendance might have been around 400 people, although it was assured by owners that the seating capacity (200) was not exceeded at any moment. Official regional mandates for nightlife establishments at that time required a 40% of maximum indoor capacity and a maximum of 25 people per group of tables, always being seated and wearing masks if not consuming. Emphasis was placed on ventilation and differentiated entrance and exit circuits. Incidence rates in Córdoba City increased notably after the outbreak ceased, from 1.4 on 10th July to 39 on 10th August 2020.
Nowadays, incidence rates across the country are rising, and social factors and settings that favour the occurrence of superspreading events are increasingly common again. Also, Spanish people most frequently involved in these events present a lower full-vaccination rate than the general population (78% in 20–39 years old vs 90% in general population as of 28th August 2021). As superspreading events carry the greatest spreading capacity, focus should be placed on carrying out an in-depth study of the dynamics of these phenomena, to provide knowledge for conducting precise, well-targeted epidemiological actions.
Thus, the study of the outbreak associated with this venue was proposed. The research objectives included describing the profile of the associated cases, their respective contacts, and the areas of exposure; assessing the possible impact of the outbreak on the incidence trend in the province, and analyzing the changes in the dynamics of virus transmission in the province after the outbreak.
Material and methodsA retrospective observational study was conducted.
Inclusion and exclusion criteriaConfirmed COVID-19 cases linked to the outbreak and their close contacts were included as study subjects.
According to the current technical document of the Ministry of Health,14 confirmed cases were those with positive PCR (regardless of clinical manifestations) or those who met clinical criteria, with negative PCR and positive IgM serology results. Close contact was considered to be any person who had been at a distance of fewer than two metres from a case for more than 15min without adequate protective measures. An outbreak is defined as an episode in which three or more confirmed cases of COVID-19 are epidemiologically related.
Confirmed cases of COVID-19 who attended the nightclub on 10th July 2020 and those cases derived from their contact studies were associated with the outbreak. This association was recorded in RedAlerta, a tool of the Andalusian Epidemiological Surveillance System for the declaration of notifiable diseases, and based on the clinical history and information from epidemiological surveys carried out in Córdoba. The study of confirmed cases associated with the outbreak was performed by means of an anonymized data extraction from RedAlerta, assessing age (as a discrete quantitative variable and in three categories: under 25 years, between 25 and 50 years, and over 50 years), sex, date of onset of symptoms, date of the positive result, clinical evolution, hospitalization, number of close contacts, and number of close contacts converted into cases. For each case, the date of symptom onset was set as the reference date. In the absence of symptoms or a recorded date of symptom onset, we utilized the date of PCR as the reference date (as there were cases without symptoms at the time of microbiological diagnosis, due to early detection by contact study).
COVID-19 contacts analysisAnalysis of close contacts was performed employing the contact studies of the cases linked to the outbreak, which were elaborated by the Epidemiology team of Córdoba District. All close contacts were tested independently of the presence of symptomatology, following the epidemiological criteria for detection of any new cases. The variables studied were age, sex, PCR result and the exposure setting (household, indoor group vacation, work, school, social gathering, health centre, transportation, sports, unknown and others).
Analysis of the outbreak impact on the viral incidence in the province of Córdoba was performed by compiling the microbiological results of SARS-CoV-2 PCRs conducted at the Clinical Microbiology laboratory of the Reina Sofia University Hospital (RSUH) from 22/06/2020 to 30/08/2020, evaluating their trend and their association with any COVID-19 outbreak during that period.
This study was approved by the Córdoba Provincial Research Ethics Committee on 27th April, 2021.
Statistical analysisAbsolute and relative frequencies were calculated for qualitative variables. Shapiro–Wilk test was performed on quantitative variables. According to the result of this test, the arithmetic mean and standard deviation (SD), or the median and interquartile range (IQR), were computed. Data were processed with the statistical programme R, version 4.1.
ResultsA total of 935 persons with confirmed epidemiological link to the outbreak were studied, of whom 120 were confirmed cases of COVID-19.
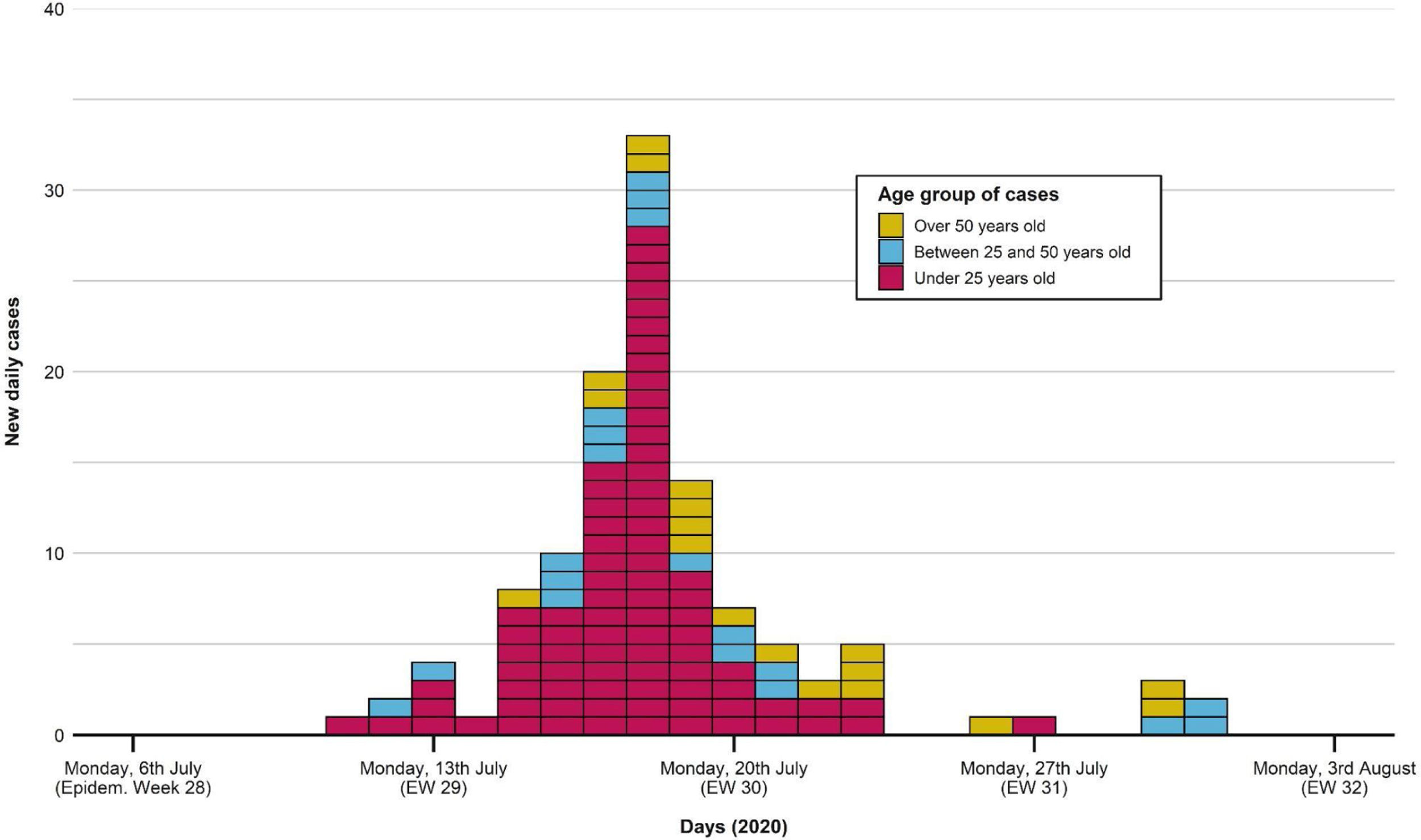
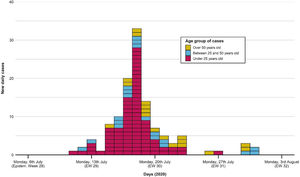
The first case occurred on 11th July, as shown in the epidemic curve of the outbreak (Fig. 1). The highest incidence day (18th July) sets in week 29, with 33 confirmed cases (22.5% of the total). The last associated case occurred on 31st July.
Portraying the epidemic curve of cases that were epidemiologically associated with the outbreak. For those with a recorded date of symptom onset, the latter was established as the case date. If no symptom onset was registered, the date of PCR swabbing was utilized instead. The first case appeared on 11th July; the last one was registered on 31st July. Peak incidence was reached on 18th July. Distribution of cases tends to normal, with most cases being under 25 years old.
We found a similar gender distribution among confirmed cases, with 58 males (48.3%) and 62 females (51.7%). Their median age was 22 years (IQR 19–27). 69.2% of the subjects were under 25 years old, 15.8% were between 25 and 50 years old, and 15% were over 50.
39.1% of the total number of cases were detected by surveillance based on the appearance of symptoms. Of those affected, 99.2% were cured without sequelae, with only one death occurring in a 92-year-old patient with chronic pathologies. The hospitalization was necessary in other 4 cases (3.3%); the mean age of these was 70 years, and all had comorbidities.
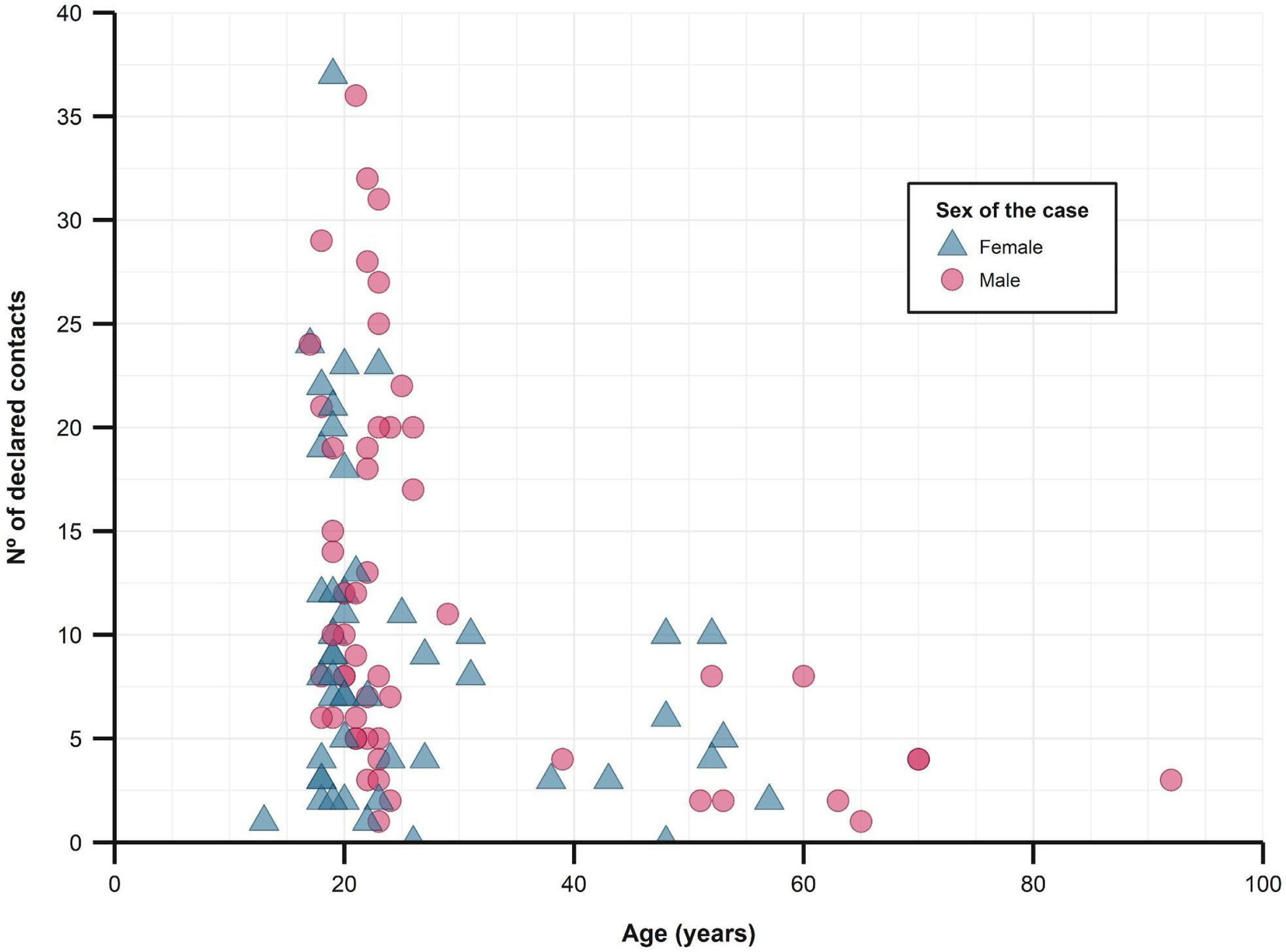
The median number of close contacts referred per case was 8 (IQR 4–16), of which a median of 1.4 (SD 1.1) became a confirmed case. The overall secondary attack rate was 16.9%.
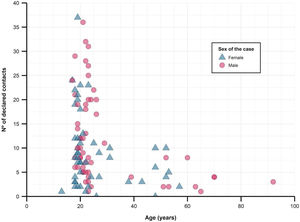
When studying close contacts stratified by age group of the cases, we found a decrease in the number of referred close contacts as age increased (Fig. 2 and Table 1), although similar medians of contacts converted to cases were found. The secondary attack rate for each stratum did rise with increasing age (from 16.6% in those under 25 years old to 30.9% in those over 50). The study found no differences between sexes, regarding the number of contacts or secondary attack rates.
Description of close contacts and secondary attack rates, stratified by age group of the cases.
| Age group of cases | % | Age (median, IQRa) | Declared close contacts (median, IQR) | Contacts subsequently infected (median, IQR) | Secondary attack rate | Secondary attack rate, by setting |
|---|---|---|---|---|---|---|
| Under 25 years old | 69.2% | 20 (19–22) | 9 (5–19) | 1 (0–2) | 16.6% | Other: 33.5%Household: 27.1%Social gathering: 18.4% |
| Between 25 and 50 years old | 15.8% | 29 (26.5–41) | 8.6 (6.6)b | 1 (0–2) | 13% | Household: 30.9%Other: 24.2%Social gathering: 12.1% |
| Over 50 years old | 15% | 59 (53–69) | 4 (2–5) | 1 (1–2) | 30.9% | Household: 66.4%Other: 11%Social gathering: 7.3% |
| Total | – | 22 (19–27) | 8 (4–16) | 1.4 (1.1)b | 16.9% | Household: 31.9%Other: 22.9%Social gathering: 15% |
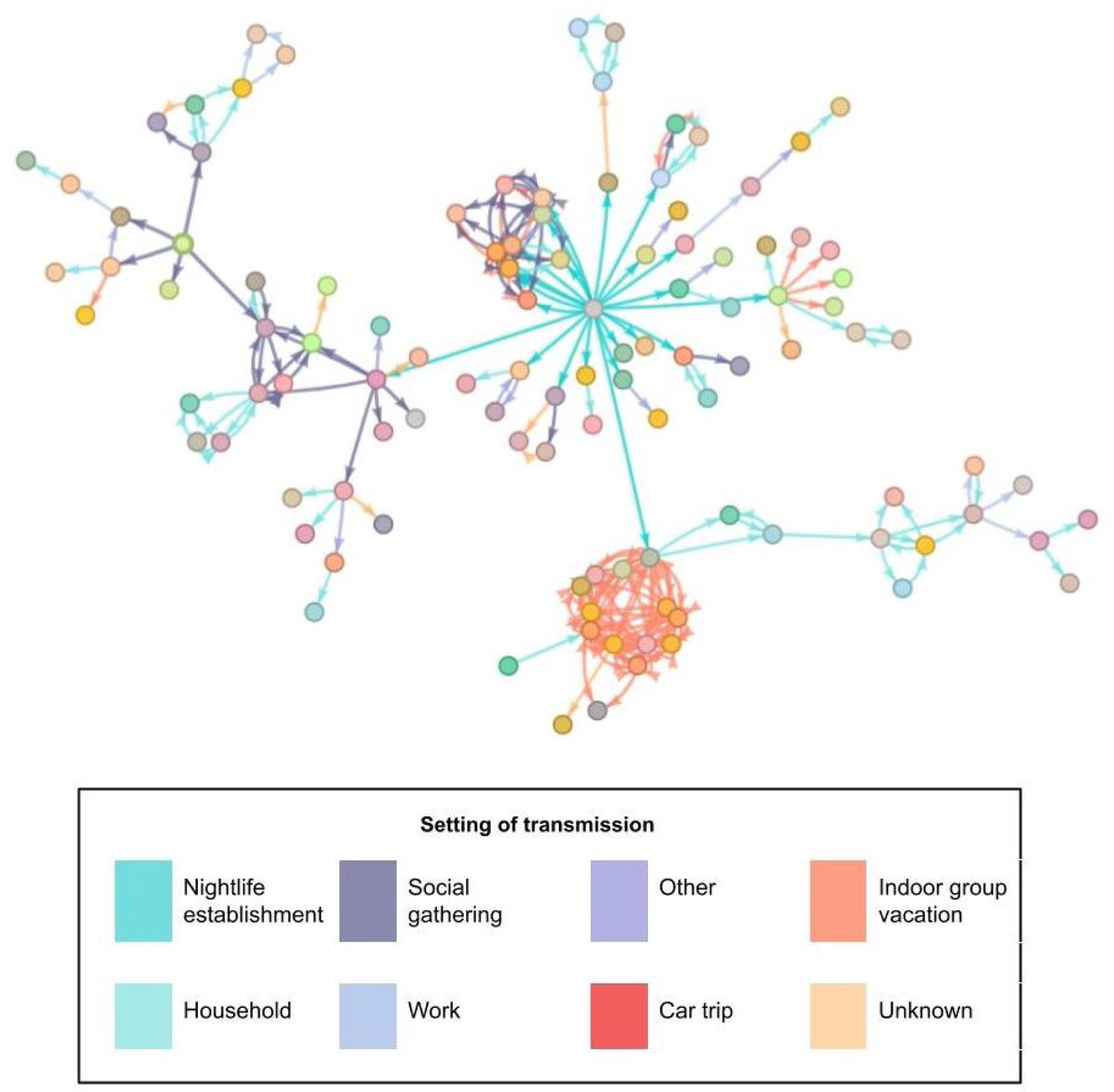
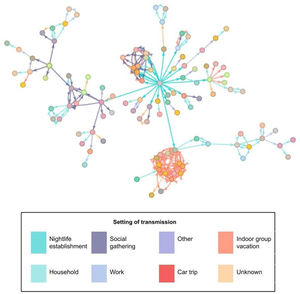
The most frequently described exposure setting was ‘household’ (32.1%). In the analysis by age group, we observed this setting to be the most frequent for contacts in cases over 50 years of age (66.4%). However, in cases under 25 years, the most frequent setting was under the category “other” (33.5%). Of the total number of people studied, 2.8% were directly related to the nightlife venue. Fig. 3 shows the relationships between the confirmed cases in the outbreak; the central grey dot at the centre represents attendance at the event.
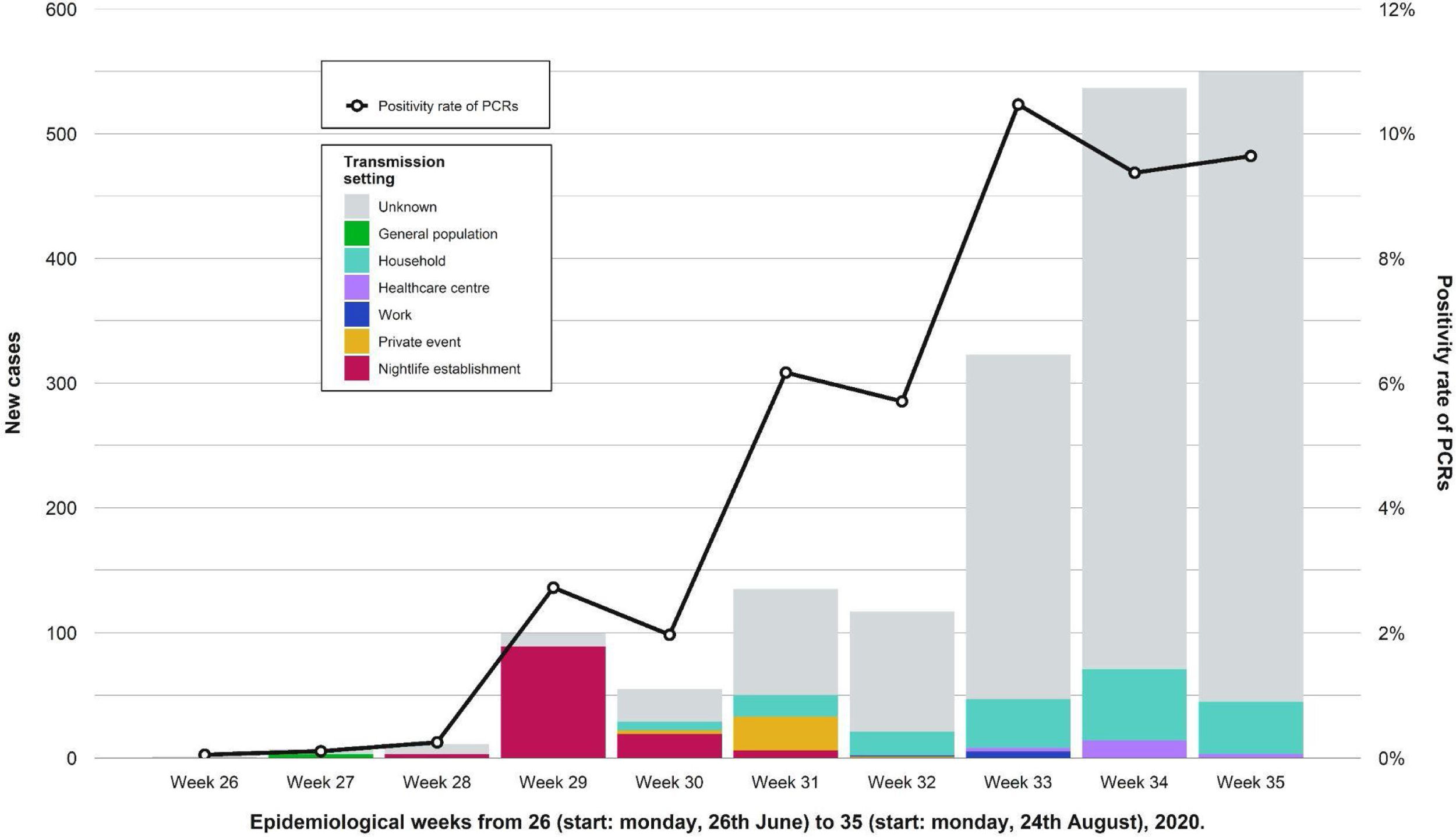
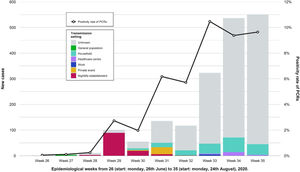
During the weeks following the outbreak, we found an increase in the number of cases in the province and the positivity rate of PCRs performed in the Clinical Microbiology laboratory of RSUH. A decrease in the percentage of known infection setting was also registered (Fig. 4). Table 2 shows a comparison of the number of reported cases and incidence rates between the province and the city of Córdoba in epidemiological weeks twenty-seventh to thirty-third. Other outbreaks of smaller magnitude and scope were identified that occurred during that period and overlapped with the outbreak under study.
Showing weekly new cases and the trend for the positivity rate of PCRs (black line) in the province of Córdoba. Epidemiological weeks from 26 (start: Monday, 26th June) to 32 (start: Monday, 24th August), 2020, were included. After the nightlife establishment outbreak ended, the amount of weekly new cases and the proportion of those with unknown transmission setting both increased. We observed other outbreaks of less magnitude and different transmission settings.
Comparison of the number of new cases and incidence rates between the city of Córdoba and its province (with and without Córdoba capital city).
| Epidemiolog. week | New cases | Incidence rate (new cases*100,000 inhabitants) | ||||
|---|---|---|---|---|---|---|
| Córdoba city | Province (Córdoba City included) | Province without Córdoba City | Córdoba City | Province (Córdoba City included) | Province without Córdoba City | |
| 27 | 4 | 6 | 2 | 0.76 | 1.23 | 0.43 |
| 28 | 6 | 9 | 3 | 1.15 | 1.84 | 0.66 |
| 29 | 92 | 100 | 8 | 12.79 | 28.22 | 1.76 |
| 30 | 42 | 55 | 13 | 7.04 | 12.88 | 2.85 |
| 31 | 91 | 134 | 43 | 17.15 | 27.91 | 9.44 |
| 32 | 56 | 117 | 61 | 14.97 | 17.18 | 13.39 |
| 33 | 77 | 309 | 232 | 39.54 | 23.62 | 50.94 |
Our study analyzed an outbreak of COVID-19 that occurred between 11 and 30 July 2020 in Córdoba, in association with a nightlife establishment. In total, 120 confirmed cases and 815 close contacts were identified; 4 hospitalizations and a single death occurred among the confirmed cases in patients with comorbidities.
Results obtained in this study differ in some respects from those published in an official nationwide report from Spain, including cases diagnosed between 10th May 2020 and 30th September 2020. The median number of identified close contacts was 3 (as opposed to 8 in our study). The median age was 39 years old, higher than the obtained in our assessment,15 as was the percentage of hospitalization (5% versus 3.3% in our study). Both studies meet in the household setting as the most frequent type of exposure.
We sought to identify any possible index cases following epidemiological criteria; thus, we found only three possible index cases that presented symptomatology within 48h of the event, all aged between 20 and 25 years old. The delay in diagnosis results in a significant latency time between SARS-CoV-2 infection and isolation of cases, favouring high spread rates and making it possible for such an event to generate dozens of new cases in a single day.10,16,17
Surveillance of COVID-19 based solely on symptomatology is less effective in detecting and controlling superspreading events, as there is a significant percentage of pre-symptomatic cases or cases that present symptoms late when the disease is already widely disseminated.6,18–20 A study conducted in Shenzhen (China),21 aimed at evaluating the effect of epidemiological surveillance for the early detection of potential sources of spread. They concluded that monitoring transmission chains through a study of close contacts reduces the time an infected individual continues to transmit the virus to the community by two days. In most scenarios, effective contact investigation and case isolation are sufficient to control a new COVID-19 outbreak within three months.22 Due to the high media impact and the increasing percentage of positive results for SARS-CoV-2 caused by this outbreak, an announcement was made by the Córdoba City Council inviting all attendees of the event to take a PCR test in order to detect infected persons and to try controlling the outbreak.
For the design of effective prevention and early response measures, more information is needed on the heterogeneity and factors associated with virus transmission. Since the beginning of the pandemic, there is accumulating evidence about the influence of age on the viral spread.8,23 According to Lau et al.,8 people under 60 years of age can be up to 2.8 times more infective than those over 60, thus constituting a relevant vector for spread. Also, the number of cases and risk of disease severity increases with age, while cases reported in children are sharply lower.15,16,24 This fact may relate to a milder symptom presentation in children, lower susceptibility to infection, or a combination of both.
When it comes to indoor spaces, providing adequate ventilation is one of the essential tools for preventing superspreading events. In a Chinese study that included 318 outbreaks, an indoor virus spread happened in all but one, involving two people25; it is clear that an enclosed space makes airborne transmission more likely. Nevertheless, diverse outdoor outbreaks have now been reported. Many of these have also been associated with crowded venues, which further underlines the need to maintain a reasonable capacity limit, also considering the possibility of added direct transmission through contact or droplets as fomites in very physically close people.17,26–28 In particular, there is compelling evidence of the role of indoor restaurants and venues in generating superspreading events.29
This gives rise to the first limitation of the study. We cannot assume that the lists of close contacts included every exposed individual. It is possible that due to the crowding in the facility, there were more close contacts than reported. As a second limitation, only positive PCRs and close contact studies within the Córdoba District have been subjected to analysis; however, there are some individuals related to the outbreak from other provinces for whom these results are not known. Furthermore, clinical information is sometimes unavailable or incomplete, probably due to the overload of the health system and the lack of resources in the summer period in the context of an outbreak.
Today, there is a stable increase in social contact with a still uncontrolled transmission of the virus, while consistent rises in incidence rates are concerning. In addition, younger population show a lower vaccination rate when compared to older age groups, and gather frequently at places with idoneous characteristics for the occurrence of superspreading events. Although immunization campaigns have proven to decrease contagions and severity of disease, risk is still present. Therefore, to reduce infections and control the pandemic with the best cost-effective measures, actions should keep focusing on situations with the greatest contagion capacity. After a review of the decisions and actions taken to control this and other outbreaks as reflected in literature, we propose several key points for improvement.
We insist on the point that establishments with large attendance capacity, hosting indoor, crowded gatherings and/or limited ventilation must ensure the implementation of preventive measures: good compliance with face masking, dispensing points for hydroalcoholic solution, limitation of attendance capacity in accordance with national guidelines at the moment, and visible self-explanatory posters portraying and reminding measures to the public. We first and foremost recommend recording attendance on a continuous basis, for a faster and improved contact tracing strategy. Although public calls for mass testing become an useful tool when there is an incomplete register of attendance, studying and tracing contacts should be prioritized as the most effective measure.22 Either way, we should struggle to facilitate a simpler, more universal access to diagnostic tests. In summary, actions should be encouraged to reduce social contacts in indoor establishments, for it is well documented it highly helps reduce virus spread.29
It is worth highlighting the contact and traceability study model that has been applied during recent months by the Cantabrian autonomous community to seek hidden primary cases of COVID-19. This strategy aims to identify close contacts up to 7 days before the onset of symptoms if there has been any high-risk exposure, intending to find asymptomatic individuals that have gone unnoticed. The effectiveness obtained by this model was recognized both by several Spanish autonomous communities and the World Health Organisation (WHO) and we propose its implementation in our current situation (middle-to-low transmission and variable, high vaccination rates).
Other proposals for improvement can be found in the literature, like monitoring the flow of masses through geolocation and contact tracing applications, or exhaustively studying the circulation patterns of the population at key points and times (public transport, schools, shopping centres). In any case, any additional measures to prevent, control and mitigate the effects of this type of events should be further investigated, given that the evidence pointing to their role in increasing the incidence rate is compelling.30 In this sense, our study also suggests the possible potentiating role of the superspreading outbreak studied in the arousal of the incidence rate in the city and later the region.
Finally, we propose the implementation of outbreak response indicators, such as time from event to detection of the outbreak, the average time of contact with the person related to the event or the average time from event to testing or quarantine of contacts. These indicators will better guide public health systems and improve the quality of outbreak response, leading to a decrease in cases and potential hospital admissions, and better control of the pandemic.
This study supports the hypothesis of a greater and more rapid viral spread in enclosed, overcrowded, poorly ventilated spaces. We found that transmission capacity seems to be influenced by age, either through a higher proportion of asymptomatic cases in younger subjects or by a higher number of interpersonal relationships when compared to older individuals. Anyhow, virus dissemination by asymptomatic or pre-symptomatic cases hinders the identification of new ones and also plays a determining role in the progress of superspreading events. Therefore, understanding the factors and scenarios most associated with increased interpersonal contact and thus increased risk of transmission is critical for the development of effective early detection and early response measures. Nevertheless, it is essential to reinforce and provide control strategies with stable, dynamic resources, also including and promoting the role and understanding of policy-makers. The impact of a superspreading event in a municipality with low incidence can lead to uncontrolled local virus transmission.
Institutional review board statementThe study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Córdoba Provincial Research Ethics Committee on 27 April 2021 (protocol code “TFG-COVID-2020”).
Informed consent statementPatient consent was waived due to data anonymization, which was performed by an external person before datasets were delivered to the authors. Patient consent was also waived due to the retrospective observational nature of this study. The ethics committee exempted the need to seek written informed consent.
Data availability statementRestrictions apply to the availability of these data, which were provided by the General Office for Public Health and Pharmaceutical Organisation of the Andalusian Regional Ministry of Health and Families. This information is stored in the Epidemiological Surveillance System of Andalusia (SVEA).
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare no conflict of interest.
To epidemiologists and the team of COVID-19 nurses and trackers of the Córdoba District for their tireless work and the data collection that made this study possible. To the physicians of the Preventive Medicine and Public Health service of the Reina Sofía University Hospital of Córdoba, for their support, recommendations and advice received for the completion of this final degree thesis. To all healthcare professionals for their work during the COVID-19 pandemic.