Prior research indicates interdisciplinary pain rehabilitation program (IPRP) usual care (UC) does not sufficiently address sleep problems among individuals with comorbid chronic pain and clinical levels of insomnia. Cognitive behavioral therapy for insomnia (CBT-I) is an evidence-based insomnia intervention. The current study investigates the translation of CBT-I into an IPRP.
MethodIn this single-site, prospective, randomized controlled pilot study, insomnia and pain-related outcomes were examined for adults participating in a 10-week IPRP (N = 79) who were allocated to a 4-session group-based CBT-I (IPRP+CBT-I) or usual care (IPRP-UC) condition.
ResultsPatients in the IPRP+CBT-I group showed improvements in insomnia symptoms at the end compared to the beginning of the CBT-I group; however, there were no IPRP outcome differences relative to the IPRP-UC condition. Both groups reported statistically significant reductions in insomnia, pain severity, pain-related life interference, and depressed mood. Fewer than one-third of participants reported clinically meaningful reductions in insomnia symptoms following IPRP participation.
ConclusionsFurther efforts are needed to address sleep problems in pain rehabilitation settings.
Investigaciones indican que el nivel de cuidado habitual (UC, por siglas en inglés) de los programas interdisciplinarios de rehabilitación del dolor (IPRP, por siglas en inglés) no abordan suficientemente los problemas del sueño entre personas que padecen dolor crónico comórbido con niveles clínicos de insomnio. La terapia cognitivo-conductual para el insomnio (CBT-I, por siglas en inglés) es una intervención basada en la evidencia. Se investiga la translación de la CBT-I en un IPRP.
MétodoSe examinaron los resultados relacionados con insomnio y dolor en adultos que participaban en un IPRP de diez semanas (N = 79) asignados a CBT grupal de cuatro sesiones (IPRP + CBT-I) o nivel de cuidado habitual (IPRP-UC).
ResultadosLos pacientes IPRP + CBT-I mostraron mejoría en síntomas de insomnio al final del estudio en comparación con el comienzo del grupo CBT-I; no hubo diferencias significativas en los resultados de IPRP en relación con la condición de IPRP-UC. Ambos grupos informaron reducciones en insomnio, gravedad del dolor, nivel de interferencia en la vida relacionada con el dolor y estado de ánimo deprimido. Menos de un tercio de los participantes informaron reducciones clínicamente significativas en síntomas de insomnio después de participar en IPRP.
ConclusionesSe necesitan mayores esfuerzos para trabajar con problemas del sueño en el entorno de los programas de rehabilitación del dolor.
Cooccurring insomnia complicates the severity of and treatment outcomes for chronic pain. Estimates suggest that most patients seeking treatment for chronic pain also report moderate to severe clinical insomnia symptoms (Tang, 2008). Patients with comorbid insomnia and chronic pain are at higher risk of negative outcomes compared to individuals without insomnia (Tang, 2009). More specifically, studies have found that higher levels of insomnia are associated with increased difficulty in reducing opioid use, higher pain intensity, greater functional limitations, lower self-efficacy, higher pain catastrophizing, and more depressive symptoms compared to patients with less severe insomnia (Asih et al., 2014; Pigeon et al., 2012). Importantly, for patients with clinically significant insomnia symptoms, insomnia often does not improve to a meaningful extent as a result of chronic pain treatment alone (e.g., Pigeon et al., 2012). Accordingly, there is a significant need for dual treatment models that address both chronic pain and insomnia.
Cognitive-behavioral therapy for insomnia (CBT-I) is an evidence-based intervention for insomnia. In this treatment model, insomnia is viewed as originating from varying precipitating events (e.g., illness, life changes, pain symptoms), but maintained through sleep-related behaviors that increase sleep-related arousal, fragment sleep, and condition an association between bed/nighttime and wakefulness. Accordingly, CBT-I uses conditioning principles to decrease pre-sleep arousal and recondition a pattern of rapid, consolidated sleep. The key components of this intervention are sleep restriction therapy, which limits time in bed initially to the duration of actual sleep per night, and stimulus control, which involves modification of factors that associate bed/nighttime with wakefulness (e.g., time awake in bed, wakeful activities in bed; Williams et al., 2013). Several randomized controlled trials (RCTs) have been conducted to evaluate this treatment among patients with insomnia and a variety of comorbid chronic pain conditions. When compared to usual care or a waiting list control group, patients assigned to receive CBT-I have reported significantly better improvements in insomnia symptoms (Selvanathan, et al., 2021). In addition, results indicate that the effects of CBT-I delivered to patients with chronic pain have been maintained or improved over time (van der Zweerde et al., 2019). Further, outcomes demonstrate strong feasibility and acceptability of the treatment for individuals with chronic pain (Koffel et al., 2020). Studies support the efficacy of CBT-I in both individual and group therapy formats (Koffel et al., 2015). Finally, research supports the cost effectiveness of CBT-I compared to pharmacotherapy or no treatment (Natsky et al., 2020).
Despite the documented evidence for the effectiveness of CBT-I, this treatment has not been widely adopted into interdisciplinary pain rehabilitation programs (IPRPs). These comprehensive, biopsychosocial programs emphasize functional restoration and typically involve physical and occupational therapy, medical visits, and mental health visits. A large body of research supports the claim that IPRPs improve pain, functioning, and quality of life among those with chronic pain (Craner et al., 2020; Gilliam et al., 2018; Huffman et al., 2017; Murphy et al., 2021). IPRPs have also demonstrated significant improvements in insomnia symptoms (Craner et al., 2020). However, IPRP participation alone appears inadequate for treating clinical insomnia (Asih et al., 2014; Davin et al., 2014). Results of a recent study suggested that approximately 80% of IPRP participants endorsed at least mild insomnia; however, only 33% of those reported a meaningful reduction in symptoms at discharge (Craner & Flegge, 2021). Furthermore, elevated levels of insomnia appeared to have a negative impact on treatment outcomes (Craner & Flegge, 2021). One possible explanation is that IPRPs typically emphasize sleep hygiene training without the active treatment components of CBT-I (i.e., stimulus control and sleep restriction therapy). Sleep hygiene consists of education on healthy sleep habits and is often used as a control treatment in CBT-I studies given evidence that it is an insufficient standalone treatment for clinical insomnia (e.g., Lami et al., 2018).
Hybrid CBT interventions for pain and insomnia have demonstrated successful outcomes for treating these comorbid conditions (e.g., Prados et al., 2020; Tang et al., 2012). However, the incorporation of CBT-I into and IPRP has not been previously studied. This gap in research has been previously documented (Wilson et al., 2016). The current study aims to address this gap by comparing treatment outcomes for IPRP participants randomized to a 4-session, group-based CBT-I treatment (i.e., IPRP + CBT-I group) or a waitlist control group receiving IPRP usual care (i.e., IPRP-UC group) in a real-world clinical setting. Specific hypotheses are as follows:
- 1)
Patients in the IPRP+CBT-I will report significant improvements in insomnia symptoms from the beginning to the end of the CBT-I group.
- 2)
Participants in the IPRP+CBT-I group will report greater improvement in insomnia symptoms compared to the IPRP-UC group from IPRP admission to discharge.
- 3)
Patients in the IPRP+CBT-I group will report greater improvements in pain-related outcomes compared to the IPRP-UC group from IPRP admission to discharge.
Adult participants with chronic pain were recruited for this study between June 2019 and January 2021 at the time of admission to a 10-week IPRP at a rehabilitation hospital in Michigan. Participants were provided with a consent form to agree to be contacted about the study and for their information to be used in the research study. Participants were told that they may receive additional treatment for insomnia as part of the IPRP, may not be offered any additional treatment, or may be invited to participate in the intervention after graduating from the IPRP.
Selection criteria were as follows: (1) enrolled in the IPRP, (2) chronic pain resulting in significant distress and/or functional impairment (assessed based on medical provider evaluation as part of admission to the IPRP), (3) mild, moderate, or severe insomnia based on Insomnia Severity Index (ISI) score ≥ 8, (4) English-speaking, (5) able to provide informed consent, and (6) 18 years of age or older. Exclusion criteria were: (1) non-English-speaking, (2) unable to provide informed consent (e.g., had a guardian), (3) < 18 years of age, (4) ISI score < 8, (5) not concurrent enrolled in the IPRP. Inclusion and exclusion criteria were intentionally broad to represent the patient population of interest. ISI cutoff score of 8 was based on prior research indicating that IPRP participants with mild or greater symptoms endorsed worse pain, distress, and IPRP treatment response compared to patients without insomnia symptoms (Craner & Flegge, 2021).
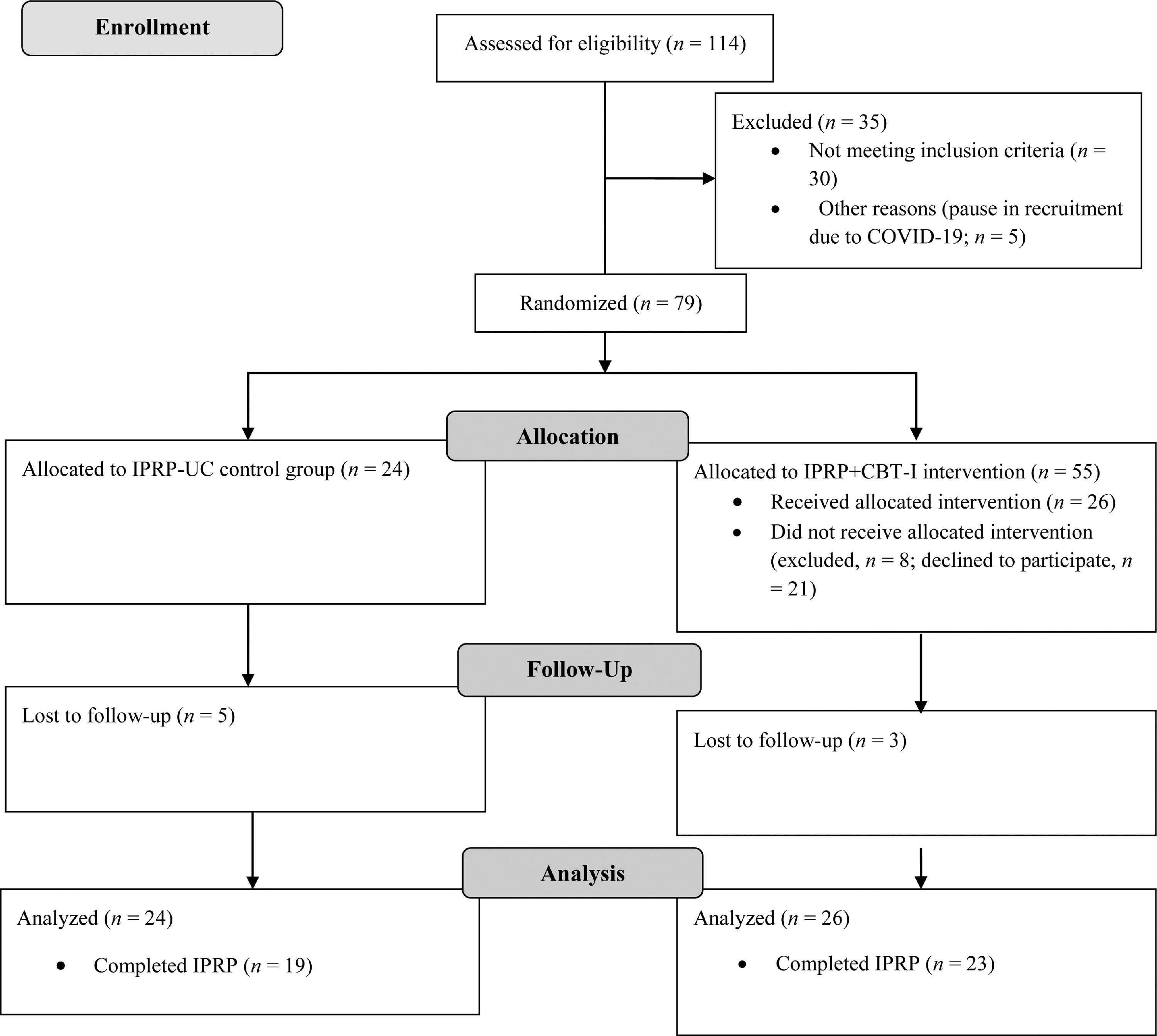
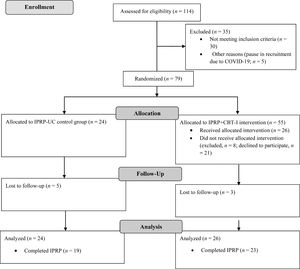
Figure 1 shows the study's participant flow. Table 1 displays demographic and clinical data for the sample. A total of 79 participants were randomized, including 24 allocated to the IPRP-UC group and 55 to the IPRP+CBT-I group. Of the 55 individuals who were recruited for the IPRP+CBT-I group, 26 agreed to participate and attended at least one group session. The final sample included 24 in the IPRP-UC group and 26 in the IPRP+CBT-I group. Recruitment ended due to sufficient sample size.
Participant characteristics at IPRP admission.
A total of 3 participants in the IPRP+CBT-I group and 5 in the IPRP-UC group were lost to follow up due to discontinuing the IPRP. Complete datasets for IPRP admission and discharge data were available for 22 participants in the IPRP+CBT-I group and 18 in the IPRP-UC group. Pre- and post- CBTI-I group intervention data were available for 15 participants in the IPRP+CBT-I group; however, only 8 participants completed sleep diaries at both time points.
DesignThis was a single-site, prospective, randomized controlled pilot study with a 2:1 allocation ratio for the IPRP+CBT-I treatment condition to account for expected attrition from this group. A random number generator was used for randomization. Participants were entered into a spreadsheet sequentially and assigned a study ID number by one of the investigators. A file matching study ID numbers to condition was used to allocate participants to each group. Participants and providers were not blinded to conditions given that there was only one treatment group with the control group receiving usual care. Participants’ treatment team members were not informed of treatment condition so as not to influence usual care; however, participants were able to share this information with their providers if they chose to do so.
ProcedureParticipants who enrolled in the IPRP were medically evaluated prior to admission. Eligibility criteria for this program are: (1) chronic non-malignant pain causing significant distress and/or functional impairment (e.g., fibromyalgia, chronic neck pain, headaches), and (2) medical and psychiatric stability not requiring a higher level of care (e.g., suicidality, severe shortness of breath).
All participants completed self-report questionnaires at program admission and approximately one week prior to the end of treatment as part of routine clinical practice. Participants who consented to be part of the study and met eligibility criteria were randomized to either the treatment or waitlist control condition as described above. Participants who were allocated to the IPRP+CBT-I group (n = 55) were then recruited to participate in the 4-session insomnia intervention through phone calls and mailed enrollment information. Groups were scheduled on a rolling basis so that participants could complete the entire 4-week intervention while enrolled in the 10-week IPRP. A subset of participants were excluded after randomization (n = 8) if there was not a group available that could be completed before the end of the IPRP. Participants who agreed to participate and met inclusion criteria (n = 26) provided informed consent for CBT-I treatment.
Participants in the IPRP-UC group were offered the opportunity to participate in the intervention following completion of the IPRP. Only 2 individuals in the IPRP-UC elected to participate. Recruitment for this group was put temporarily on hold from March to May 2020 due to the COVID-19 pandemic, resulting in 5 participants who were screened for eligibility but not randomized.
This study received funding from an internal grant at Mary Free Bed Rehabilitation Hospital and approved by the Rehabilitation Hospital's institutional review board.
InstrumentsInsomniaThe Insomnia Severity Index (ISI; Bastien et al., 2001) is a short self-report instrument that measures insomnia symptoms. The ISI is composed of 7 items that evaluate: the severity of sleep-onset (initial), sleep maintenance (middle), early morning awakening (terminal) problems, satisfaction with current sleep pattern, interference with daily functioning, noticeability of impairment attributed to the sleep problem, and level of distress caused by the sleep problem. Each of these items is rated on a five-point Likert scale ranging from 0 (not at all) to 4 (extremely) and assesses insomnia symptoms over the past week. Total scores range from 0 to 28. Higher scores represent greater insomnia severity (Bastien et al., 2001). ISI has been shown to meaningfully measure treatment response (Morin et al., 2011; Yang et al., 2009). For the purposes of this study, we used a minimally important difference (MID) cutoff of 8 to represent treatment response (Morin et al., 2011) and a posttreatment score of less than 7 to represent remission (Wilson et al., 2016). The ISI was administered to participants in both groups at admission and discharge from the IPRP. The IPRP+CBT-I group also completed the ISI at sessions 1 and 4 of the CBT-I group.
Sleep DiarySleep outcomes were measured using self-report sleep diaries prior to start of CBT-I treatment and each subsequent week of treatment. These diaries ask patients to answer specific questions such as “What time did you get into bed?”, “What time did you try to go to sleep?”, and “How long did it take you to fall asleep?” Patients’ sleep diary entries were then used to calculate total sleep time (TST), time in bed (TIB), sleep efficiency (SE), sleep latency (SL) and sleep quality (SQ).
Pain ratingsPatients were asked to rate their best, worst, and average pain across the previous month on a scale of 0-10 (no pain – worst pain imaginable).
Pain interferenceThe Patient Reported Outcomes Measurement Information System (PROMIS) Pain Interference – Short Form 8a (Amtmann et al., 2010) is an 8-item self-report questionnaire that measures pain-related intrusions in social, emotional, recreational, and work-related domains. The measure assesses impact on functioning through questions (e.g., “how much did pain interfere with your day-to-day activities?”) rated on a scale between 1-5 (never – always). Scaled scores range from T = 40.7 – 77.0 with higher scores indicating more interference in daily life due to pain.
DepressionThe PROMIS Depression – Short Form 8a (Pilkonis et al., 2011) is an 8-item self-report questionnaire that measures depressive symptoms over the past week. Items (e.g., “I felt worthless”) are rated on a scale of 1-5 (never – always). Scaled scores range from T = 38.2 – 81.3 with higher scores representing greater level of depressed mood.
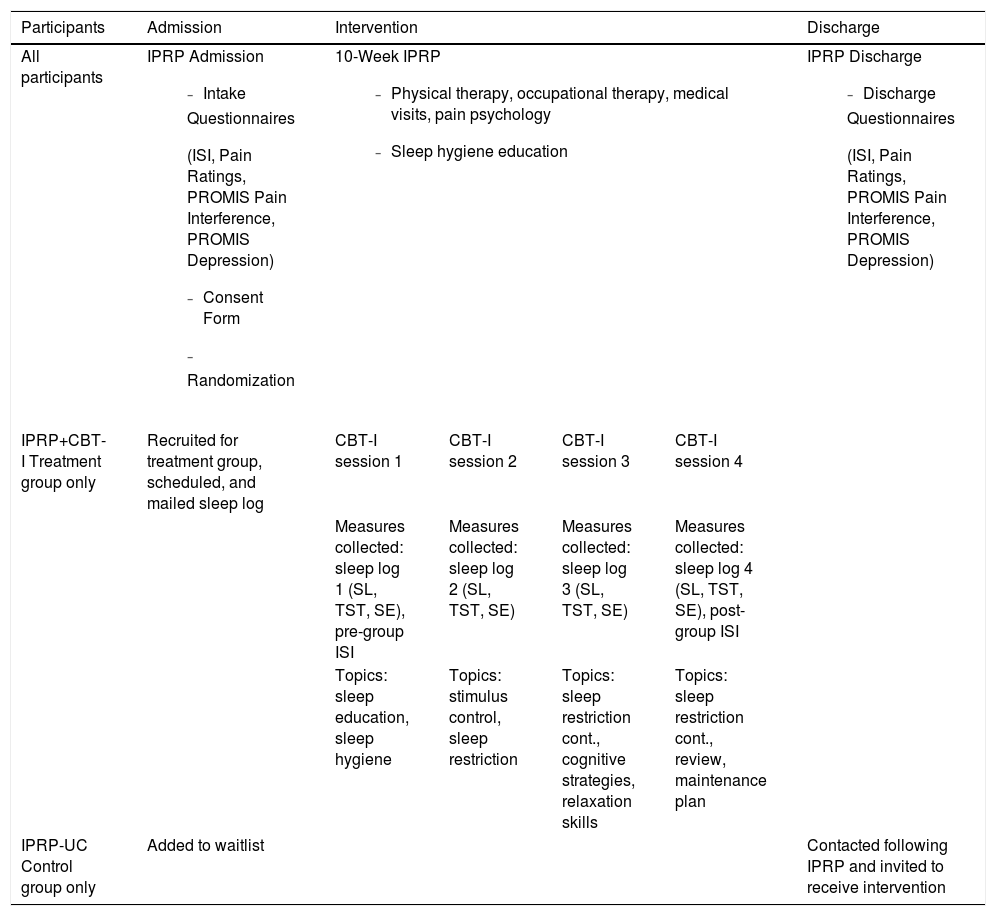
InterventionTable 2 displays intervention conditions and measure completion timeline for both groups.
Description of IPRP + CBT-I and IPRP-UC conditions.
Participants in both conditions engaged in a 10-week IPRP, which involved physical therapy, occupational therapy, medical visits, and Acceptance and Commitment Therapy (ACT) based pain psychology sessions. Participants engaged in approximately 6-7 hours of treatment occurring over 2-3 days each week on average. The program typically included twice weekly physical therapy, occupational therapy, and individual pain psychology visits; however, frequency may have been lower based on cancellations/no shows or clinical recommendation. Medical visits occurred every 1-2 weeks or based on clinical recommendation. The treatment focus of each component of the program is described below:
- 1)
Physical therapy: pain neuroscience education (PNE), mechanical assessment and treatment, stretching and strengthening exercises to manage pain and improve function, flare-up management.
- 2)
Occupational therapy: spinal anatomy and posture, body mechanics training, moderation/modification, structured movement program, focus on return to valued activities.
- 3)
Pain psychology: education about the relationship between stress, emotions, and pain, relaxation training, biofeedback, mindfulness, pain acceptance, values-based living, cognitive defusion, committed action.
- 4)
Nursing: a nurse or medical assistant takes vitals, obtains a summary of symptoms and progress, and helps manage care.
- 5)
Medical visits: a physician, nurse practitioner, or physician's assistant provides education about symptoms and medical recommendations. May change or reduce medications including tapering opioid pain medications.
Treatment team members met weekly to review each case and collaborate on care. Participants in this program may graduate early or be extended on a case-by-case basis due to clinically evaluated medical necessity. Standard care included sleep hygiene instructions provided by multiple disciplines during the program and a sleep hygiene handout as part of the patient education folder. Details about this program and overall treatment outcomes are published elsewhere (Craner & Flegge, 2021; Craner et al., 2020).
CBT-I GroupThe CBT-I intervention was led by one of two psychologists or postdoctoral psychology fellow with backgrounds in cognitive-behavioral therapy and health psychology. They were trained in administration of each group session by the first author. Group sizes were small with between 2-4 participants in each group cohort. Additional information on CBT-I is available elsewhere (Edinger & Carney, 2008; Ernstrom, 2016). The specific treatment protocol was based on a previously established 4-session group treatment delivered in primary care settings and modified for the current study (Mack et al., 2019). Treatment components reflected in this study are explained below.
Sleep educationSleep education began with an overview of how insomnia develops and how behavioral factors contribute to the development and continuation of insomnia symptoms.
Sleep hygieneParticipants were provided with instructions on healthy sleep habits. Instructions provided included maintaining a consistent schedule, limiting screen time before bed, avoiding caffeine and nicotine prior to going to bed, and establishing a pre-bedtime routine. Participants were encouraged to identify items that may be contributing to their insomnia and create goals and action steps to address those items.
Sleep monitoringParticipants were instructed to monitor their sleep while participating in the intervention using a sleep diary. Sleep diary entries were used to calculate total sleep time (TST), time in bed (TIB), sleep efficiency (SE), and sleep latency (SL).
Stimulus controlParticipants were provided with education on stimulus control, which is aimed to decrease pre-bedtime arousal and increase rapid, consolidated sleep through conditioning principles. Participants were given directions that included eliminating extra time in bed, associating bed/nighttime with sleep, and avoiding “awake” behaviors in bed.
Sleep restrictionSleep restriction focuses on sleep efficiency and limits the amount of time that participants spend in bed to an amount that matches their ability to fill this time mostly with sleep. Instructions included calculating sleep efficiency based on sleep monitoring diaries. If sleep efficiency was ≥ 90%, participants were told to move their bedtime to 15 minutes earlier, if sleep efficiency was between 85-89%, participants were told to keep their sleep schedule the same, and if sleep efficiency was < 85%, participants were told to go to bed 15 minutes later. Participants were encouraged to recheck their sleep efficiency after 4 nights and adjust their sleep schedule again, if needed.
Cognitive strategiesCognitive strategies aim to identify, challenge, and replace dysfunctional beliefs and attitudes about sleep and insomnia (e.g., unrealistic expectations of sleep, fear of missing sleep, overestimation of consequences of poor sleep). Participants were given specific strategies to work on including challenging their thoughts, distancing—or unhooking—from their thoughts, schedule working/planning/problem-solving time, and acceptance.
Relaxation skillsRelaxation skills target hyperarousal and work to reduce tension to facilitate sleep. Specific techniques reviewed included diaphragmatic breathing, progressive muscle relaxation, guided imagery, and mindfulness meditation. Only rationale and brief overview of strategies was covered in the CBT-I group as these were covered in-depth in the IPRP.
Data analytic strategyGroup characteristics were compared using t-tests, chi-square analyses, and fisher's exact tests, as appropriate. In order to evaluate treatment outcomes for the CBT-I group intervention, insomnia severity (ISI) and sleep diary measures (i.e., TST, TIB, SE, SL, and SQ) were compared for the IPRP+CBT-I group at session 1 and session 4 for using paired samples t-tests.
Next, to compare group differences in IPRP treatment outcomes, we a series of 2 (Group: IPRP+CBT-I, IPRP-UC) x 2 (Time: admission, discharge) mixed model ANOVAs were conducted. Individual-level data were analyzed for the frequency of participants in each group reporting meaningful change in insomnia symptoms. Clinically significant improvement in insomnia was defined as an improvement of ≥ 8 points on the ISI (Bastien, et al., 2001). Treatment remission was examined using a posttreatment score in the normal range (i.e., ≤ 7 points) as criteria for symptom remission (Wilson et al., 2016). Sample size was determined based on a priori power calculations for within-subjects ANOVA using G*Power (Faul et al., 2009) with the following parameter estimates: f = .33, α = .05, 1-β = .80. This resulted in a sample size estimation of at least N = 22 to detect a small effect. Analyses were performed with IBM SPSS version 26.0.
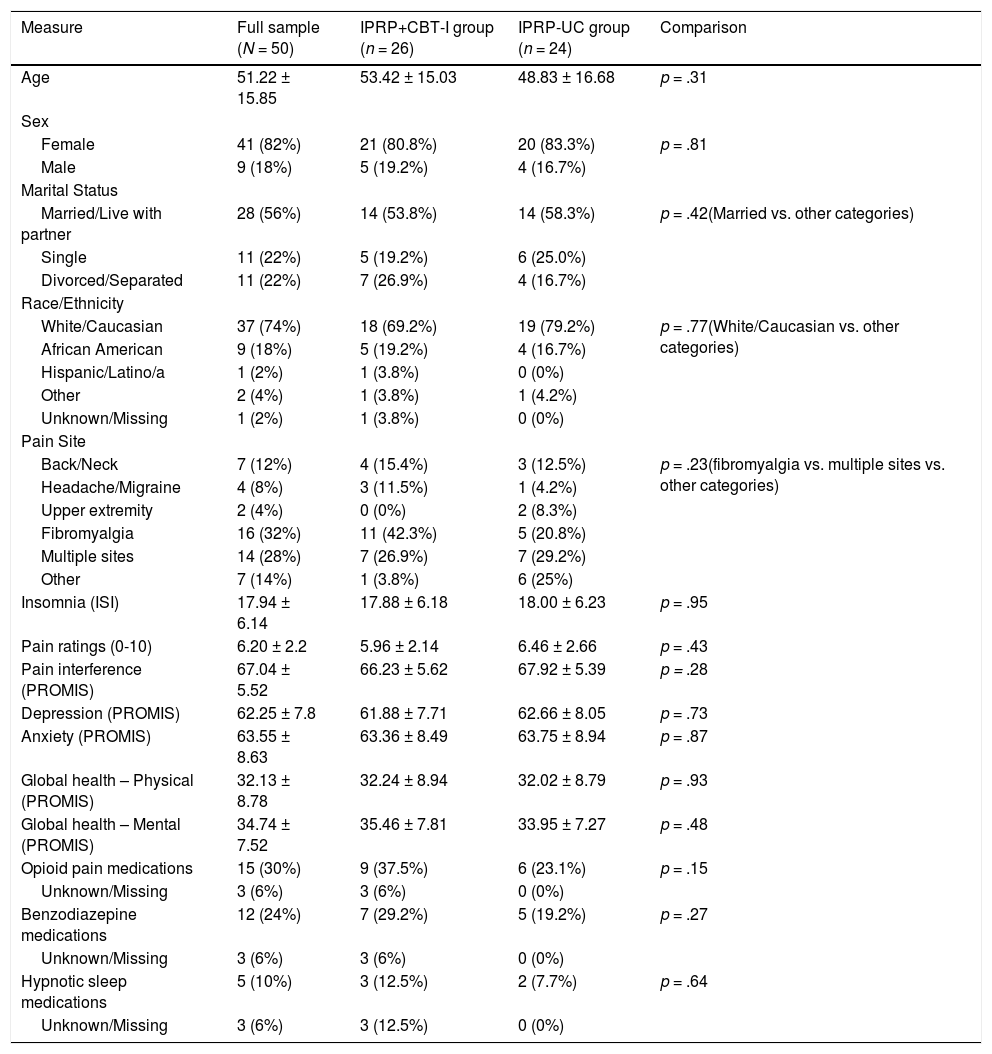
ResultsParticipant characteristicsParticipants mean age was 51.22 years (SD = 15.85) and the majority were female (82%), married (56%), and identified as White/Caucasian (74%). Participants most commonly presented with fibromyalgia (32%) or multiple pain sites (28%). Table 1 shows the baseline participant characteristics for the full sample and by group. There were no significant differences between groups in demographic or clinical characteristics including: age, sex, marital status, race/ethnicity, pain site, insomnia severity, pain ratings, pain interference, depressed mood, anxiety, global health, or opioid, benzodiazepine, or hypnotic sleep medication use.
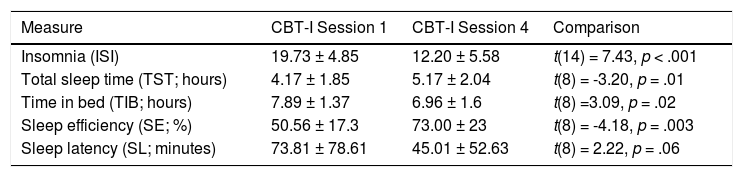
CBT-I intervention resultsParticipants in the IPRP+CBT-I condition completed measures of sleep at sessions 1 and 4 to evaluate the effectiveness of the CBT-I intervention. As expected, results indicated improvement in insomnia severity on the ISI (ΔM = 7.53, SD = 3.93). Data extracted from weekly sleep diaries also indicated an increase in total sleep time (hours; ΔM = 1, SD = 0.93), decrease in time in bed (hours; ΔM = 0.93, SD = 0.9), and increase in sleep efficiency (ΔM = 22.42, SD = 16.09). There was a non-significant trend (p = .06) in sleep latency (minutes; ΔM = 28.74, SD = 38.99). However, only 9 out of 17 participants who completed treatment returned sleep diaries at session 4. Overall, the results indicate that participants reported improvement in the areas targeted in the CBI-I group intervention. See Table 3.
CBT-I group intervention outcomes.
Note. 15 participants had complete ISI data and 9 participants had complete sleep diary data used to calculate TST, TIB, SE, and SL.
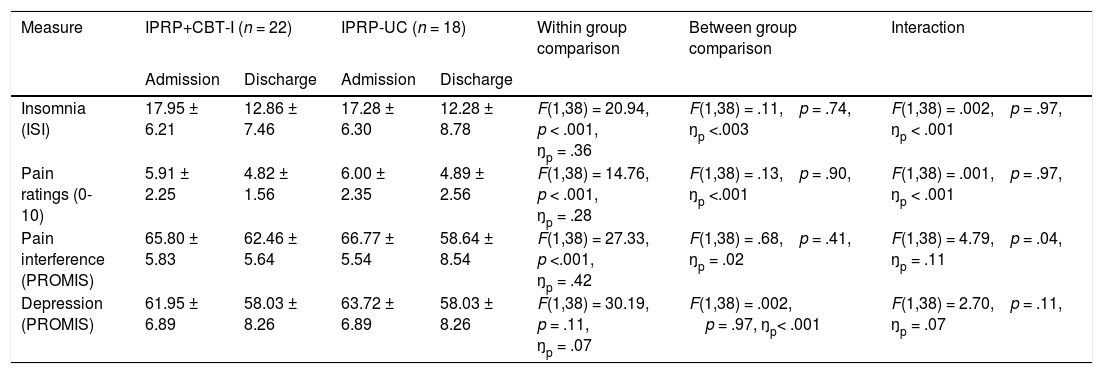
See table 4 for IPRP treatment outcomes and group comparisons.
IPRP Treatment outcomes for IPRP + CBT-I and IPRP-UC conditions.
Note: Analyses represent 2 (Group) x 2 (Time) mixed-model ANOVAs.
Results identified a main effect for Time, F(1, 38) = 20.94, p < .001, ŋp = .36. There was no main effect for Group, F(1, 38) = 0.11, p = .74, ŋp <.003 or Group by Time interaction, F(1,38) = .002. p = .97, ŋp < .001. These findings indicate that both groups reported improvement in insomnia symptoms at the end of the IPRP. Contrary to hypotheses, there were no differences between the IPRP+CBT-I and IPRP-UC groups.
Next, the frequency of participants meeting criteria for insomnia treatment response and remission were compared between groups. Results indicated that 7 (26.9%) participants in the IPRP+CBT-I group and 8 (33.3%) participants in the IPRP-UC group had clinically significant reductions in insomnia symptom scores at IPRP discharge. This difference was not statistically significant, Χ2 = .67, p = .41. Four (15.4%) participants in the IPRP+CBT-I group and 7 (29.2%) participants in the IPRP-UC group were in remission at the end of the IPRP, which was not a statistically significant difference, Χ2 = 2.13, p = .15.
Pain ratingsThere was a significant effect for Time, F(1, 38) = 14.76, p < .001, ŋp = .28. As before, there was no main effect for Group, F(1,38) = .13, p = .90, ŋp <.001, or Group by Time interaction, F(1, 38) = .001. p = .97, ŋp < .001. These results suggest decrease in pain ratings in both groups with no differences in treatment response between groups.
Pain-related life interferenceResults indicated a Group x Time interaction, F(1, 38) = 4.79, p = .04, ŋp = .11 for pain interference; however, follow up tests revealed no simple effects for Group at either intake, F(1,38) = .29, p = .59, ŋp = .01, or discharge, F(1, 38) = 2.88, p = .10, ŋp = .07, suggesting no significant differences between the groups at either time point. There was also a simple effect for Time within each group suggesting that both the IPRP+CBT-I (p = .03) and IPRP-UC group (p < .001) reported decreased pain interference at discharge compared to admission.
Depressed moodAnalyses indicated a main effect for Time, F(1, 38) = 30.19, p = .11, ŋp = .07. There was no main effect for Group, F(1,38) = .002, p = .97, ŋp = <.001, or Group by Time interaction, F(1,38) = 2.70, p = .11, ŋp = .07. These results suggest that both the IPRP+CBT-I and IPRP-UC groups reported lower depressive symptoms at discharge compared to program admission, with no differences between the two groups.
DiscussionThe purpose of this study was to evaluate the added benefit of incorporating a group-based CBT-I intervention into a 10-week IPRP. Participants in this pilot RCT were randomized to receive IPRP usual care (IPRP-UC) or IPRP treatment with the addition of a 4-session CBT-I group intervention (IPRP+CBT-I). Results of this study indicated that patients in the IPRP+CBT-I group reported improvements in insomnia symptoms comparing session 1 to session 4. However, contrary to hypotheses, participants in the IPRP+CBT-I group did not have superior insomnia or pain-related outcomes compared to the IPRP-UC control group. Both the IPRP-UC and IPRP+CBT-I groups endorsed reductions in insomnia severity, pain severity, pain-related life interference, and depressed mood, with no significant differences between the two groups.
There are several possible interpretations for these results. First, less than half (47.3%) of participants who were allocated to the IPRP+CBT-I group agreed to participate in the intervention. This calls into question the acceptability and feasibility of adding this treatment component to the IPRP studied and may have impacted our findings. Group-based interventions are widely used in pain rehabilitation programs (Stanos, 2012) and have demonstrated efficacy in CBT-I studies (Koffel et al., 2015). However, our intervention represented an additional treatment component outside of the typical 6-7 hours of treatment per week. Many potential participants were either not willing or able to come to the clinic for additional appointments. The CBT-I group was only offered one day and time per week, meaning that it was not possible to coordinate the group with all participant schedules. In addition, a portion of this study took place during the COVID-19 pandemic. As a result, an in-person group-based intervention may have been less acceptable to potential participants. More research would be needed to clarify these potential barriers.
It is also possible that the IPRP providers, who were knowledgeable about sleep hygiene and stimulus control strategies, may have provided aspects of CBT-I informally within the usual course of treatment. Similarly, the IPRP included relaxation skills, ACT-based cognitive strategies, and physical reconditioning. Even though these were directed toward pain versus sleep problems, these treatment components overlap with CBT-I and may have positively impacted insomnia symptoms. Given that this study took place in a true clinical setting (versus a research setting), it was not possible to control what occurred in usual care. Specific information on usual care providers (e.g., number of occupational and physical therapists, psychologists, and medical providers) or number of visits for each component of the IPRP were not collected. Sleep diary data was not collected in the IPRP-UC condition because it was thought that self-monitoring of sleep (a component of CBT-I) could alter the results. In addition, the participants in the IPRP+CBT-I condition attended four sessions than the IPRP-UC group; therefore, the number of sessions was not equivalent between groups. Further, both groups concurrently engaged in a high intensity pain treatment program. These factors suggest there could be potential biases related to treatment fidelity and overlapping treatment components could have confounded the results. These issues may have resulted in both groups reporting a similar level of improvement in insomnia symptoms.
Another possible explanation is that a 4-session group-based CBT-I intervention is not a high enough level of care for this population. Individuals with high impact chronic pain may warrant longer, more intensive, individualized treatment specifically for sleep problems. For example, calculating sleep efficiency and providing detailed feedback and adjustments to participants was more challenging in a group setting and required some level of independent participation from group members. It may be the case that more individualized care is more beneficial in this population. Due to the dearth of existing literature examining CBT-I treatment within the context of IPRPs, more research is needed to explore these explanations.
Although both groups reported improvement in insomnia symptoms, it is important to note that only a minority of patients in either group reported clinically significant improvement (27-33%) or remission (15-29%). These findings are similar to those reported in a prior sample of participants in this treatment program, which indicated 36% of patients with mild or greater insomnia symptoms had clinically significant improvement and 26% were in remission at IPRP discharge (Craner & Flegge, 2021). These results suggest that IPRP treatment alone is insufficient for treating clinical insomnia; however, the findings of the current study suggest that a 4-session add-on group CBT-I intervention does not appear to address this gap. This finding is valuable in and of itself – it is important to understand unsuccessful intervention attempts to figure out what will be effective in this setting. These findings provide a helpful starting point for this and other IPRPs to develop solutions for sleep problems among IPRP participants.
Future research is needed to understand how to best address insomnia among IPRP participants. It is possible that modifications could improve outcomes. For example, CBT-I treatment components could be more formally integrated into IPRP programming. Alternately, individual treatment, or telehealth options could be pursued. These options could increase CBT-I participation with less time burden on IPRP participants. It may also be possible to provide CBT-I prior to IPRP participation while patients are waiting for treatment or as a post-IPRP treatment option for patients continuing to experience insomnia after IPRP completion. These options warrant future research exploration.
Additional limitations of this study include relatively small sample size, single geographic site, reliance on self-report data, and low return rate for follow-up sleep diary data. Participants and researchers were not blinded to condition given that there was only one treatment condition while the comparison group received usual care. We retained only those who agreed to participate in the CBT-I intervention in the IPRP+CBT-I group, rather than all who were randomized to this condition (i.e., intent-to-treat analysis). As a result, there is the possibility of selection bias. It is unknown how these limitations could have impacted the findings of the study. It is also unknown how overlap with the COVID-19 pandemic (e.g., reductions in in-person treatment participation, increased psychosocial stress, illness) could have influenced our results.
Overall, the results of this study highlight difficulties with conducting research in a clinical versus a research setting and illustrate the challenges in translating evidence-based care into a real-world setting. Translation science helps test out the implementation of interventions to improve uptake and both patient outcomes and population health. Research studies like this one, can clarify which implementation strategies work best for different populations and setting, while promoting use of evidence-based care and discovering the processes by which they work (Titler, 2018). Despite its limitations, the current study addresses a gap in the literature, serves as a reference point for other IPRPs, and provides a springboard for future research.
FundingThis work was supported by an internal grant from the Mary Free Bed Rehabilitation Hospital John F. Butzer Center for Research and Innovation.
We would like to thank Mariceli O'Neill, Psy.D. for her assistance with this project.