Los tratamientos conductuales para la diabetes a menudo han tenido éxito, y se pueden beneficiar de una mejor comprensión de los efectos relativos del tratamiento de dos focos comunes -pérdida de peso y aumento de actividad física- sobre la glucosa en sangre. Adultos con sobrepeso y obesos (N = 59; M = 60 años), con la hemoglobina A1c (HbA1c) y con diabetes, participaron durante 6 meses en un tratamiento conductual basado en principios de la teoría de la auto-eficacia y la teoría cognitiva social. El tratamiento se asoció con el aumento de actividad física de forma significativa, la reducción del índice de masa corporal (IMC), y la reducción de los niveles de HbA1c (ps < 0,001). Los cambios en el IMC y la actividad física presentaban una parte significativa de la varianza en el cambio en la HbA1c, R2 = 0,13, p = 0,023. El cambio en la cantidad de actividad física b = -0,36, p = 0,007, pero sin cambiar el IMC, β = -0,03, p = 0,792, contribuyó significativamente a la varianza en el cambio de HbA1c encontrado. No hubo un efecto según el sexo de los participantes. La discusión se centró en cómo los resultados podrían afectar la eficacia, la eficiencia y la aplicación de tratamientos conductuales para el tratamiento de la diabetes.
Behavioral treatments for diabetes have often been unsuccessful and may benefit from a better understanding of the relative effects of two common treatment foci - decreased weight and increased volume of physical activity - on blood glucose. overweight and obese adults (N = 59; Mage = 60 years) with hemoglobin A1c (HbA1c) values consistent with diabetes participated in a 6-month community-based behavioral treatment based on tenets of self-efficacy theory and social cognitive theory. the treatment was associated with significantly increased physical activity, reduced body mass index (BMI), and reduced HbA1c levels (ps < .001). Changes in BMI and physical activity accounted for a significant portion of the variance in change in HbA1c, R2 = .13, p = .023. Change in volume of physical activity, β = -.36, p = .007, but not change in BMI, b = -.03, p = .792, significantly contributed to the variance in HbA1c change that was accounted for. there was no effect based on the sex of participants. Discussion focused on how findings might impact the efficacy, efficiency, and application of behavioral treatments for diabetes management.
Pagina nueva 2
The behavioral treatment of diabetes has been challenging and often unsuccessful. A reduction in excess weight through improved eating, and increased physical activity, are common foci of treatments. Nagrebetsky et al. (2012) and Yoshida et al. (2008), for example, demonstrated a link between obesity and elevated blood glucose levels. Other researchers (e.g., Snowling & Hopkins, 2006; Umpierre et al., 2011) identified physical activity to be a predictor of improved glucose levels in diabetics. In two studies, boulé, Haddad, Kenny, Wells, and Sigal (2001) and boulé, Kenny, Haddad, Wells, and Sigal (2003) showed an effect of physical activity on hemoglobin A1c (HbA1c) that was independent of weight loss, however, their analyses did not test effects of weight loss and increased physical activity relative to one another. This limited the applied value of their findings.
Both eating and physical activity behaviors have been extremely resistant to change (Annesi, 2010, 2012; burgos-Garrido, Gurpegui, & Jurado, 2011). This is evidenced by almost two-thirds of the U.S. population being overweight or obese (flegal, Carroll, ogden, & Curtin, 2006), and less than 4% completing even the minimum recommendation of weekly physical activity (troiano et al., 2009). This persists even with great amounts of physical activity and nutrition information present. Although concentrating on just one of these behaviors may, initially, be most beneficial (given the practical restraints of limited professional time and treatment resources), it is unclear whether increased physical activity or reduced weight is of greater importance for successfully managing diabetes.
The aim of the present investigation was to estimate the relative benefits of reduced weight and increased physical activity on blood glucose levels in overweight/obese adults with diabetes. If, for example, weight loss is demonstrated to be the best predictor of reduced blood glucose, then a structured behavioral treatment focused on healthy eating may be a priority (Wing & Phelan, 2005). If increased physical activity is shown to be the better predictor, then facilitation of an evidence-based exercise support protocol may be most beneficial (Annesi, 2012). A community setting was used to enhance generalizability of findings so that they may readily benefit treatments (Glasgow, 2008).
Method
Participants
Adults from Canada were referred by medical professionals because of diagnosed overweight/obesity and diabetes.
Inclusion criteria were: (a) age ≥ 18 years, (b) no regular exercise (self-reported ≤ 20 min per week average over the previous year), (c) overweight or obesity (BMI ≥ 25 kg/m2), and (d) a HbA1c percentage consistent with the international standard for diabetes (≥ 6.5). A medical clearance to participate was required from a physician. Participants provided written informed consent and all study processes were in accord with the Declaration of Helsinki. The 38 women and 21 men (Mage = 59.8 years, SD = 3.4) had a racial/ethnic make-up of 90% Caucasian and 10% of other racial/ethnic groups. Most were in the lower to middle income ranges.
Measures
Volume of exercise. The Godin Leisure-time Exercise Questionnaire (Godin, 2011) measured volume of exercise. It requires reporting of weekly frequencies of strenuous ("heart beats rapidly") (e.g., running), moderate ("not exhausting") (e.g., fast walking), and light ("minimal effort") (e.g., easy walking) exercise for "more than 15 minutes" per session. Responses are then multiplied by 9, 5, and 3 standard metabolic equivalents (Jettè, Sidney, & blumchen, 1990), respectively, and summed for a final score. Test-retest reliability over 2 weeks was .74 (Godin, 2011), and construct validity was supported by significant correlations with scores of accelerometer and peak volume of oxygen uptake measurements (Jacobs, Ainsworth, Hartman, & Leon, 1993; Miller, freedson, & Kline, 1994).
Hemoglobin A1c (HbA1c). HbA1c was measured by high pressure liquid chromatography and reported in percentage of hemoglobin bound with glucose or glycohemoglobin (tankova, Chakarova, Dakovska, & Atanassova, 2012). This measure presents an individual's average blood glucose level over approximately 3 months.
Body mass index (BMI). BMI was assessed through measurement of height and weight, and expressed as kg/m2.
Procedure
Each participant was introduced to a certified wellness professional that administered a structured nutrition and exercise support protocol within 6 monthly meetings of 30 min each (Annesi, 2012). The setting was private offices within community centers that administer health promotion services. Participants were instructed in an array of self-regulation methods (e.g., cognitive restructuring, stimulus control, dissociation) to counter common barriers to exercise and healthy eating. These methods, based on social cognitive and self-efficacy theory (bandura, 1986, 1997), incorporated goal setting and progress feedback where even minor improvements were communicated and displayed as successes in order to improve feelings of self-efficacy and competence. the cognitive-behavioral treatment was supported by a computer application where behavioral contracts consistent with a participant's short-term goals were derived and tracked during each meeting. Tracking of daily exercise completion and food consumption was explained and encouraged, throughout. Methods of relapse prevention were also used to prepare for both situations that could prompt non-adherence, and indicate how to re-engage appropriate behaviors at times when compliance was not maintained.
Exercise plans were individually developed with modalities (e.g., walking, bicycling) chosen by participants. Each plan included at least three 20-30 min sessions of moderate cardiovascular activities per week. Eating plans were also individually tailored based on individual preferences. Uniformly, they included a reduction in fat and an increase in fruits and vegetables. recommendations on physical activity and/or nutrition by participants' physicians were accommodated. Personal identifiers were removed prior to data being transferred for analyses and reporting.
Data analysis
the treatment was completed by 71% of participants. Multiple imputation was used for the 14% of measure scores that were missing at Month 6. Statistical significance was set at α = .05 (two-tailed), with the bonferroni adjustment applied for multiple tests. Dependent t tests were first employed to assess within-group changes over the 6-month treatment. Changes in BMI and volume of physical activity were then simultaneously entered into a multiple regression equation as predictors of HbA1c change. Evaluation of change was based on recent suggestions (Glymor, Weuve, berkman, Kawachi, & robins, 2005), and change scores on all measures were calculated by subtracting the baseline score from the score at program end, unadjusted for baseline values.
Results
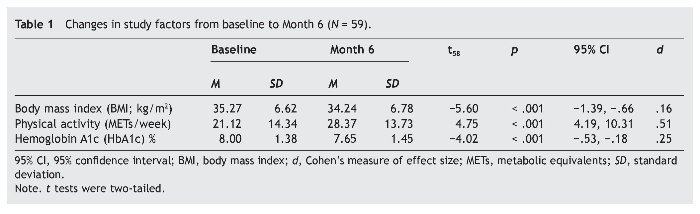
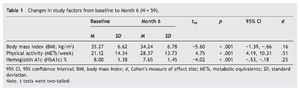
BMI, volume of physical activity, and HbA1c each significantly improved (table 1). The effect sizes were small for BMI and HbA1c change, and moderate for change in volume of physical activity. Changes in BMI and volume of physical activity accounted for a significant portion of the variance in change in HbA1c, R2 = .13, F2, 58 = 4.05, p = .023. Change in volume of physical activity, β = -.36, SE = .01, p = .007, but not change in BMI, β = -.03, SE = .06, p = .792, significantly contributed to the explained variance in HbA1c change. These results did not appreciably differ when baseline scores on BMI and HbA1c were controlled (thus, corresponding findings are not reported). Entering participants' sex into the regression equation did not add to the variance explained, ΔR2 = .00, F1, 55 = 0.001, p = .981. Age of participants was homogenous so it was not entered into the analysis. The relationships between increased physical activity and reduced BMI, both not controlling and controlling for baseline values, were significant and marginally significant (rs = -.26, p = .050 and -.25, p = .060, respectively).
Discussion
Results suggested a superior effect of increased physical activity on blood glucose in contrast with reduced weight. This has clinical significance because, while each measure is an independent predictor of improvements in health risks, findings supported an emphasis on increasing physical activity and exercise over dieting for weight loss in the behavioral treatment of diabetes. However, because the treatment utilized was associated with only small changes in weight (a mean of 3%), and a greater effect on weekly volume of physical activity, this may have affected the findings somewhat. The small effect of HbA1c may indicate that sustained behavioral change may be required before clinically important improvements in the management of diabetes are realized. Although research from toledo et al. (2007) supported the present findings by demonstrating that increased energy expenditure through physical activity, but not weight reduction, was associated with skeletal muscle cell mitochondria improvement (proposed to be a strong predictor of glucose control), it is clear that considerable replication is required to increase confidence in findings. For example, larger and more diverse samples, evaluated over longer periods, will improve generalizability of the results. Also, enhanced experimental control by better accounting for adherence to exercise and diet, is required. It is hoped that related research will continue to examine components of behavioral interventions in applied settings so that their increased effectiveness and efficiency may benefit the growing, but treatable, health pathology of diabetes
*Corresponding author at:
YMCA of Metropolitan Atlanta, 100,
Edgewood Avenue NE, Suite 1100, Atlanta, Georgia 30303, USA.
E-mail address:jamesa@ymcaatlanta.org (J.J. Annesi).
Received October 22, 2012;
Accepted January 25, 2013
References
Annesi, J J. (2010). Relationship of physical activity and weight loss in women with Class II and Class III obesity: Mediation of exercise-induced changes in tension and depression. International Journal of Clinical and Health Psychology, 10, 435-444.
Annesi, J. J. (2012). Supported exercise improves controlled eating and weight through its effects on psychosocial factors: Extending a systematic research program toward treatment development. Permanente Journal, 16, 7-18.
Bandura, A. (1986). Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice Hall.
Bandura, A. (1997). Self-efficacy: The exercise of control. New York, NY: Freeman. Boulé, N. G., Haddad, E., Kenny, G. P., Wells, G. A., & Sigal, R. J. (2001). Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: A meta-analysis of controlled clinical trials. Journal of the American Medical Association, 286, 1218-1227.
Boulé, N. G., Kenny, G. P., Haddad, E., Wells, G. A., & Sigal, R. J. (2003). Meta-analysis of the effect of structured exercise training on cardiorespiratory firness in type 2 diabetes mellitus. Diabetología, 46, 1071-1081.
Burgos-Garrido, E., Gurpegui, M., & Jurado, D. (2011). Personality traits and adherence to physical activity in patients attending a primary health centre. International Journal of Clinical and Health Psychology, 11, 539-547.
Flegal, K. M., Carroll, M. D., ogden, C. L., & Curtin, L. r. (2006). Prevalence and trends in obesity among US adults, 1999-2008. Journal of the American Medical Association, 303, 235-241.
Glasgow, R. E. (2008). What types of evidence are most needed to advance behavioral medicine? Annals of Behavioral Medicine, 35, 19-25.
Glymor, M. M., Weuve, J., Berkman, L. f., Kawachi, I., & Robins, J. M. (2005). When is baseline adjustment useful in analyses of change? An example with education and cognitive change. American Journal of Epidemiology, 162, 267-278.
Godin, G. (2011). the Godin-Shephard leisure-time physical activity questionnaire. Health and Fitness Journal of Canada,4, 18-22.
Jacobs, D. R., Ainsworth, B. E., Hartman, T. J., & Leon, A. S. (1993). A simultaneous evaluation of 10 commonly used physical activity questionnaires. Medicine andScience in Sports and Exercise, 25, 81-91.
Jettè, M., Sidney, K., & Blumchen, G. (1990). Metabolic equivalents (MEts) in exercise testing, exercise prescription and evaluation in functional capacity. Clinical Cardiology, 13, 555-565.
Miller, D. J., Freedson, P. S., & Kline, G. M. (1994). Comparison of activity levels using Caltrac accelerometer and five questionnaires. Medicine and Science in Sports andExercise, 26, 376-382.
Nagrebetsky, A., Griffin, S., Kinmonth, A. L., Sutton, S., Craven, A., & Farmer, A. (2012). Predictors of suboptimal glycaemic control in type 2 diabetes patients: the role of medication adherence and body mass index in the relationship between glycaemia and age. Diabetes Research and Clinical Practice, 96, 119-128.
Snowling, N. J., & Hopkins, W. G. (2006). Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients. DiabetesCare, 29, 2518-2527.
Tankova, T., Chakarova, N., Dakovska, L., & Atanassova, I. (2012). Assessment of HbA1c as a diagnostic tool in diabetes and prediabetes. Acta Diabetology, 49, 371-378.
Toledo, F. G. S., Menshikova, E. V., Ritov, V. B., Azuma, K., Radikova, Z., DeLany, J., & Kelley, D. E. (2007). Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes, 56, 2142-2147.
Troiano, R. P., Berrigan, D., Dodd, K. W., Mâsse, L. C., Tilert, T., & McDowell, M. (2009). Physical activity in the United States measured by accelerometer. Medicine and Science in Sports and Exercise, 40, 181-188.
Umpierre, D., Ribeiro, P. A., Kramer, C. K., Leitao, C. B., Zucatti, A. T. N., Azevedo, M. J., Gross, J. L., & Ribeiro, J. P. (2011). Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes. Journal of the American Medical Association,305, 1790-1799.
Wing, R. R., & Phelan, S. (2005). Long-term weight loss maintenance. American Journal of Clinical Nutrition, 82, S222-S225.
Yoshida, D., Toyomura, K., Fukumoto, J., Ueda, N., Ohnaka, K., Adachf, M., Takayanagf, R., & Kono, S. (2008). Waist circumference, body mass index and glycated hemoglobin in Japanese men and women. Diabetes and Metabolic Syndrome: Clinical Research and Reviews, 3, 7-11.