Research shows that the effect of acute stress on intentional memory suppression could be modulated by individual differences in psychological traits. However, whether acute stress distinctly affects intentional memory suppression in high trait ruminators, a high at-risk group of stress-related disorders, and the neural correlations, remains unclear.
Method55 healthy college students were divided into high and low trait ruminators (HTR and LTR), Following stress manipulation, a Think/No Think task assessed the memory suppression performance. Functional near-infrared spectroscopy was applied to explore the neural correlates. Psychophysiological interaction analyses were used to assess how the functional connectivity between a seed region and another brain region was modulated by tasks during memory suppression, further mediating memory suppression performance and state rumination.
ResultsThe HTR exhibited poorer memory suppression performance than the LTR under the stress condition. Aberrant activation patterns and task-modulated functional connectivity in the dorsal prefrontal cortex (DLPFC) and superior temporal gyrus (STG) were observed only in the HTR during memory suppression under the stress condition. The effect of memory suppression performance on the state rumination of individuals was significantly mediated by the task-modulated functional connectivity between the DLPFC and STG.
ConclusionsThe findings could provide insights for prevention or early intervention in the development of stress-related disorders in HTR.
Rumination, a mode of thinking characterized by a compulsive and continuous focus on negative events or emotions, is a significant psychological process linked to the development of several stress-related disorders such as post-traumatic stress disorder (PTSD) (Catarino et al., 2015) and major depressive disorder (MDD) (Sacchet et al., 2017). Trait rumination reflects a tendency to engage in prolonged rumination (Nolen-Hoeksema, 1987). Duque et al. (2014) found that individuals with higher trait rumination showed more significant cognitive deficits such as negative attention bias. Clinical studies have also shown that high trait ruminators and patients with PTSD or MDD have similar clinical symptoms, suggesting that trait rumination may predict future symptoms and severity of these mental disorders (Ehring et al., 2008; Kleim et al., 2007; Spinhoven et al., 2015). Given that trait rumination can be considered as a “transdiagnostic factor”, gaining a better understanding of individuals with higher trait rumination could have significance for promoting the mental health of this subclinical group (Bomyea et al., 2012).
The process of responding to external threatening situations during acute stress events, has been found to easily induce short-term rumination which is referred to as state rumination (Smith & Alloy, 2009). In daily life, exposure to acute stress events can significantly impact an individual's cognitive behaviors. Studies have reported that high trait ruminators exhibit more control deficits such as impairment in attention control and inhibitory control after prolonged exposure to excessive stress (Qin et al., 2009; Sandi et al., 2005). Moreover, several clinical studies have shown that acute stress may increase levels of state rumination, thereby increasing the incidence of invasive memories (flashbacks) in patients with stress-related disorders (Ehlers et al., 2004; Hackmann et al., 2004). A similar phenomenon is exhibited in high trait ruminators, which may largely be due to acute stress impairing intentional memory suppression (Gagnepain et al., 2017; Hu et al., 2017).
The forgetting of memories has long been considered as an uncontrollable cognitive process (Ebbinghaus, 2013; Schacter, 1999). However, certain studies have shown that individuals can accurately recall memories and also intentionally suppress unwanted memories (Benoit et al., 2016; Levy & Anderson, 2008). This cognitive inhibition mechanism is highly adaptive, allowing individuals to retain valuable memories while facilitating the forgetting of memories that may threaten their well-being (Engen & Anderson, 2018; Nørby, 2018). Previous studies have explored the neural correlation between the brain regions associated with rumination and inhibitory control, reporting a negative correlation between trait rumination and state rumination with memory suppression performance (Dieler et al., 2014; Song et al., 2022). For example, people who have experienced acute stress events tend to exhibit significantly poorer performance in suppression-induced forgetting (SIF) (Deguire et al., 2019; Quaedflieg et al., 2020). This control deficit hinders the intentional suppression to unwanted memories, which may further strengthen the behavioral tendency of uncontrollably recalling unwanted memories and increase the levels of state rumination in individuals. More importantly, Ashton et al.'s (2020) findings hinted at how the impact of acute stress on memory suppression can be regulated by trait rumination. Hence, further research is needed to explore the distinct effects of acute stress on individuals with different levels of trait rumination in the process of memory suppression, as well as clarifying the potential underlying effects of such impaired memory suppression behavior on the levels of state rumination.
At the neural level, neuroimaging studies have found that memory suppression relies on the dorsal lateral prefrontal cortex (DLPFC) guided by the executive control network. This network down-regulates the brain activation of the prefrontal cortex, enabling suppression of unwanted memories (Ashton et al., 2020; Benoit et al., 2016; Quaedflieg et al., 2015). However, exposure to acute stress decreases brain activation in the regions of the DLPFC (McEwen & Morrison, 2013). Moreover, research has shown dynamic interaction between the prefrontal cortex and the temporal gyrus during the process of memory suppression (Depue et al., 2007). Specifically, an increase in brain activation in the DLPFC was found to be accompanied by decreased activity in the hippocampus during memory suppression (Costanzi et al., 2021). These findings indicate that brain regions including the DLPFC, the temporal gyrus and hippocampus play an important roles during memory suppression. However, it remains unclear whether distinct activation patterns and dynamic interactions in the DLPFC and the temporal gyrus are modulated by acute stress and the level of trait rumination. It is also not known whether the dynamic interactions within the DLPFC and the temporal gyrus mediate the effects of acute stress on memory suppression performance and further affect the levels of state rumination of individuals.
Previous studies have shown that the impact of acute stress on the ability to actively suppress memories is regulated by individual differences (Ashton et al., 2020). However, to date there has been limited research investigating whether acute stress impairs the process of active memory suppression in individuals with high trait rumination, as well as the changes in neural activation patterns in potentially related brain regions so far. The current study utilized functional near-infrared spectroscopy (fNIRS), a relatively new functional imaging method known to provide excellent temporal resolution in measurement results and highly compatible with natural contexts, to explore the impact of acute stress on the process of intentional memory suppression in individuals with high trait rumination, along with its underlying neural correlates and connectivity. We hypothesized that (i) individuals with high trait rumination or low trait rumination will both exhibit poorer behavioral performance in the memory suppression task after experiencing the stress-induced task compared to those in the control condition; (ii) individuals with high trait rumination will exhibit poorer suppression performance during memory suppression task compared to those with low trait rumination under stress condition; (iii) there will be a significant difference in neural activities and functional connectivity in the prefrontal cortex and temporal lobules between individuals with high and low trait rumination under the stress conditions, while such difference will not be significant under the control condition; (iv) the neural activities and functional connectivity in the prefrontal cortex and temporal lobules might be involved in the process of acute stress impairing memory suppression performance in high trait ruminators, further mediating the changes in the levels of state rumination.
MethodsParticipantsParticipants were recruited by convenience sampling to complete online questionnaires, including the Chinese version of the Ruminative Response Scale (RRS) (Han & Yang, 2009) and the Chinese version of the Patient Health Questionnaire-9 (PHQ-9). Exclusion criteria comprised depression, subjects who suffered from acute physical diseases, neural system diseases, drug abuse, chronic or acutes diseases affecting brain function. Meanwhile, female participants who were pregnant, menstruating or using hormonal contraceptives were also excluded as these factors are known to affect the cortisol response (Strahler et al., 2017). A total of 296 Chinese college students from the Southern Medical University completed the online questionnaires. Subjects whose RRS scores were in the top 30 % and exceeded 50 were categorized into the high trait ruminators (HTR), while participants whose RRS scores were in bottom 30 % and lower than 30 were categorized into the low trait ruminators (LTR) (Huang et al., 2019). This study was reviewed and approved by the Ethics Committee of the Southern Medical University. After being briefed on the experimental procedure, all participants provided their written informed consent and received appropriate monetary compensation after the experiments.
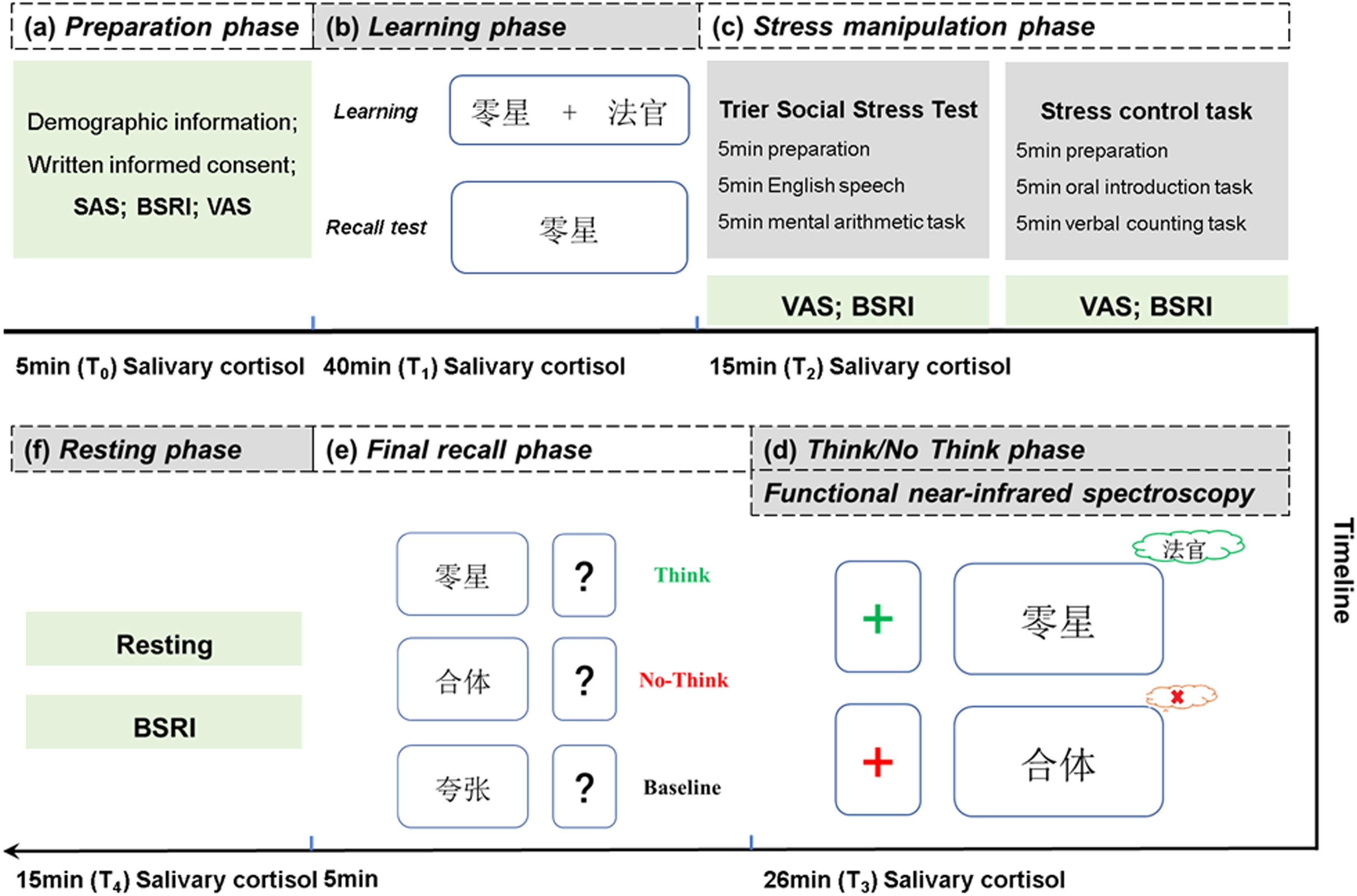
Experimental design and paradigmsA long-arm cross-experimental design was applied in the current study (Figure S1 of the Supplementary materials). Specifically, participants in each group were assigned to a stress-induction task as the stress condition and a control task as the control condition. To avoid order and practice effects, half of the subjects in each group were randomly selected to participate in the stress condition experiment prior to the control condition experiment 2 weeks later, with the other half of the subjects following the reverse order. In addition, all subjects were required not to do any physical exercise or to drink or eat within 2 h prior to the start of the experiment. All experiments were conducted between 12:30 and 18:00 in the afternoon and the female participants were asked not to attend the experiment during their menstruating period to control for the rhythm of cortisol. The overall experimental procedure is illustrated in Fig. 1.
The Experiment Pipeline. The Think/No Think (TNT) task applied in current study includes the (a) preparation phase for participants to sign up the informed written consent and complete the basic demographic information; (b) learning phase of TNT task; (c) stress manipulation phase with a Trier Social Stress Test (TSST) under the stress condition, and a control task under the control condition; (d) TNT phase for participants to intentionally retrieve or suppress the target stimuli; (e) final recall phase to assess the effect of the TNT manipulation, and (f) resting phase to alleviate the TSST-induced stress of participants. SAS, Self-rating Anxiety Scale; BSRI, Brief state rumination inventory; VAS, Visual analogue scale.
On the day of the experiment, all subjects were required to sign an informed consent form and provide the basic demographic information. The Self-rating Anxiety Scale (SAS), the Chinese version of the Brief State Rumination Inventory (BSRI) (Wang et al., 2022), and a visual analogue scale (VAS) were used to measure the baseline level of anxiety, state rumination and subjective stress. The Think/No Think (TNT) paradigm was used to investigate the process of memory suppression. In the learning phase, participants were instructed to memorize 40 Chinese word pairs across four runs. Each run was followed by a cued recall test to record the rate of correct responses. Considering the prevalent negative attention bias observed in individuals with cognitive deficits (Gao et al., 2022), the stimuli employed in the TNT task were categorized into neutral and negative valences (20 each). The valence of stimuli was included in subsequent analysis to explore whether attention bias exists in HTR under the stress condition. At the end of the learning phase, an additional recall test was conducted with the correct recall rates recorded and included in the subsequent data analysis. After the learning phase, participants in the stress condition were instructed to perform the Trier Social Stress Test (TSST) (Kirschbaum et al., 1993), containing a preparation period, a free speech and a mental arithmetic task. All TSSTs were completed in front of a review panel consisting of two experimenters in order to successfully induce social psychological stress. Participants also completed a control task without the review panel in the control condition, and this task had the same duration as the TSST. In the subsequent TNT phase, participants were instructed to intentionally suppress or retrieve the target words based on whether they were presented with a green (Think) or red (No Think) fixation displayed on a computer screen with a total of 192 trials conducted. After the TNT phase, the same recall test was performed to assess the effect of the TNT manipulation. The correct recall rates were recorded and included in the subsequent data analysis. More details can be found in the Supplementary materials.
Assessment of acute stress and state ruminationTo evaluate the effectiveness of acute stress induction, measurements of subjects’ subjective stress, state rumination and salivary cortisol were taken at several time points during the experiment. Subjective stress was measured using five 100 mm VAS. Before the experiment began (T0), participants were asked to rate how stressful, unpleasant, difficult, annoyed and fearful they had perceived the past month (0=”not at all”, 100=”extremely”) as the baseline level. Subjective stress was also measured by VASs on which participants scored the same five feelings they had felt immediately after the stress manipulation (T2). The difference between the two scores represented the change in the subjective stress level through the experiment, with the positive values representing an increase of the subjective stress and negative values representing a decrease. State rumination, referring to a temporary rumination process induced by a specific situation (Martin & Tesser, 1996), was measured at three timepoints: T0, T2, and 15 min after the end of the final recall phase (T4) by the BSRI. The BSRI consists of eight forward-scoring items, each of which is scored on a 100 mm VAS ranging from 0 (“strongly disagree”) to 100 (“strongly agree”), and the total score is obtained by summing up all items. Furthermore, salivary cortisol samples were obtained at five time points: T0, after the learning phase (T1), T2, after the end of the Think/No Think phase (T3), and T4.
Behavioral data analysisData analyses of the behavioral data were performed using SPSS Statistics 25. First, changes in the subjective stress level were measured by subtracting the VAS scores collected at T2 from the scores collected at T0; the scores were then analyzed by an ANOVA test, with groups (HTR and LTR) as the between-subject variable and conditions (control and stress) as the within-subject variables. Moreover, to investigate the differences between levels of state rumination, another ANOVA test on the BSRI scores was conducted, with conditions and time points (T0, T2 and T4) as the within-subject variables, and the groups as the between-subject variable. Finally, the changes in salivary cortisol levels collected at the five time points were assessed by an ANOVA test, with groups as the between-subject variable, time points (T0∼T4) and conditions as the within-subject variables.
Subjects’ behavioral performance in the TNT task was measured by the SIF index. The SIF of each subject was calculated by the percentage of word pairs correctly recalled by subjects in the No-Think trials minus those in the baseline trials. The resulting scores were analyzed using a three-factor ANOVA to assess the effect of the experimental procedure on the SIF of subjects, with groups as the between-subjects variable and the conditions and valence of materials (neutral and negative) as the within-subject variables. Subjects’ gender, age, and the scores of SAS, BSRI, and PHQ-9 were included in the analysis as covariates.
fNIRS acquisition and analysisThe principle of fNIRS is described in the Supplementary materials. In the current study, the NIRScout system (NIRx, USA) was used with a time resolution of 4.64 Hz to measure the relative changes in the concentration of HbO2 and HHb as indicators of brain activity (Obrig et al., 2000). Immediately after the learning phase (T1), a 26-channel array consisting of 14 optical emitters and 14 photo-detectors with an interoptode distance of 3 cm was fastened on the subjects’ head and was removed after the end of the Think/No Think phase (T3) (Figure S2 and Table S1 of Supplementary materials), lasting for approximately 40 min. Based on previous research (Benoit et al., 2016; Costanzi et al., 2021), the regions of interest (ROIs) in the current study were located in the inferior frontal gyrus (IFG), the middle frontal gyrus (MFG), the superior temporal gyrus (STG) and the superior parietal lobule (SPL) of both hemispheres. Furthermore, considering that previous research has suggested the higher sensitivity and signal-to-noise ratio of the HbO2 signal in capturing cortical blood flow changes, current study primarily concentrated on analyzing HbO2 signals (Kinder et al., 2022).
The nirsLAB software was used to process the changes in the blood oxygen signal. First, the fNIRS data were preprocessed to remove the fluctuations and spikes, and parts of the physiological noise were removed using the band-pass filter. The first ten frames preceding the first stimulation was defined as the baseline period. Then a GLM model estimated the signal changes of HbO2 in eight ROIs under the No Think > Think conditions during the TNT task which were represented by β to obtain the neural activation of the cerebral cortex in the process of memory suppression. Finally, a three-factor ANOVA test of β was performed with the groups as the between-subjects variable and the conditions and valence of stimulus materials as the within-subject variables.
Psychophysiological interactions (PPI) analysisPPI analysis is used to assess the correlation between activation within a seed ROI and task-related activation in another brain region (Hirsch et al., 2017). In the current research, PPI analyses were independently performed through the codes based on MATLAB R2015b in eight ROIs as seeds. This process obtained an 8 × 8 vector composed of the PPI coefficient βi, which represents the altered functional connectivity between activation within each seed ROI and task-modulated activation in other non-seed ROIs (Supplementary materials). Three-factor ANOVAs in each seed ROI were then performed with groups as the between-subject variable and the conditions and valence of stimulus materials as the within-subject variables, to estimate the contribution of the experimental procedure to the task-dependent functional connectivity in the ROIs during the TNT task.
Correlation analyses and mediation effect modelTo explore the association between the neural signals during memory suppression and the behavioral performance in the TNT task, a Pearson correlation coefficient was calculated between the mean value of activation in eight ROIs and the SIF index of each subject during the TNT task. Moreover, Pearson correlation coefficients for the PPI values between the MFG and STG, the SIF index, and BSRI scores at T4 were calculated to find out whether significant correlations among these variables existed. Then a simple mediation analysis was performed to further investigate to what extent memory suppression performance predicts changing levels of state rumination, and whether the relationship between memory suppression performance and state rumination was mediated by the task-modulated functional connectivity in the MFG in the HTR. The mediation analysis was performed via the PROCESS macro for SPSS (Hayes, 2013). A 95 % confidence interval for the indirect effect was calculated by a bootstrapping methodology with 5000 samples (Preacher & Hayes, 2008).
ResultsSample characteristics55 subjects meeting the inclusion criteria for the experiments were recruited. Data from five subjects who failed to complete the two experiments were excluded, resulting in the final data sample came from a total of 50 participants (HTR: N = 28, LTR: N = 22), aged between 19 and 25 years (M = 21.07, SD = 1.41). Considering that all the subjects included in this study were college students, we did not measure the income, education and socioeconomic status levels of the subjects. The HTR showed higher RRS, PHQ-9 and SAS scores compared with the LTR (Table S2 of Supplementary materials).
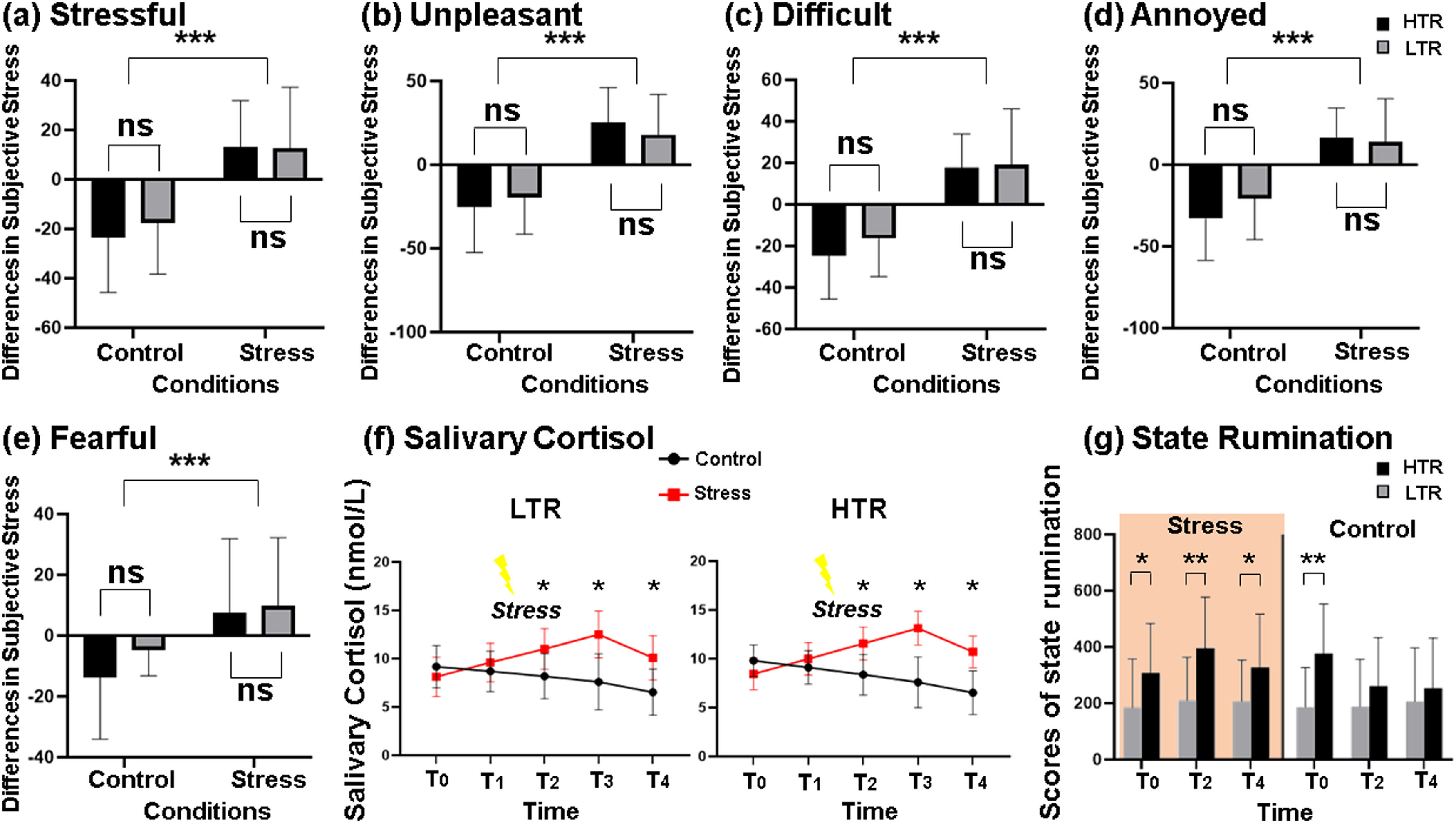
Successful stress and state rumination inductionThe changes in subjective stress levels showed a significant main effect of conditions. Participants under the stress condition reported more stressful (F = 64.057, p < 0.001, η2=0.572, Fig. 2a), unpleasant (F = 99.310, p < 0.001, η2=0.674, Fig. 2b), difficult (F = 69.248, p < 0.001, η2=0.591, Fig. 2c), annoyed (F = 63.341, p < 0.001, η2=0.569, Fig. 2d) and fearful (F = 24.404, p < 0.001, η2=0.337, Fig. 2e) perception. The main effect of the groups and the interaction effect between the groups and the conditions were not significant (Table S3 of Supplementary materials). Furthermore, significant main effects were found for both conditions (F = 420.05, p < 0.001, η2=0.897) and time points (F = 71.232, p < 0.001, η2=0.597) on the change in salivary cortisol levels, while neither the main effect of groups nor the interaction effect were significant. Results from paired t-tests indicated that the levels of salivary cortisol collected after the TSST were significantly higher than those obtained under the control condition (Fig. 2f). The significant increases in subjective stress level and salivary cortisol after completing the TSST task confirmed the successful stress induction procedure in the current study.
Successful stress induction. The main effect of the experimental conditions was significant on the five subjective stress indicators, including (a) stressful, (b) unpleasant, (c) difficult, (d) annoyed and (e) fearful. (f) The main effects of the experimental conditions were significant on the change of salivary cortisol levels in two groups. (g) The change of state rumination collected at T0, T2 and T4 in two groups. LTR, low trait ruminators; HTR, high trait ruminators; ns means no significant difference; * p < 0.05, ** p < 0.01, *** p < 0.001.
Regarding state rumination, the results indicated a significant main effect of groups (F = 9.609, p < 0.01, η2=0.167). The levels of state rumination in the HTR were generally higher than in the LTR. Furthermore, there were significant interaction effects of time points × groups (F = 11.976, p < 0.001, η2=0.200) and conditions × time points × groups (F = 7.980, p < 0.01, η2=0.143) (Table S4 of Supplementary materials). Specifically, the levels of state rumination collected at T2 and T4 in the HTR were significantly higher than those in the LTR under the stress condition, whereas no significant difference was observed under the control condition (Fig. 2g).
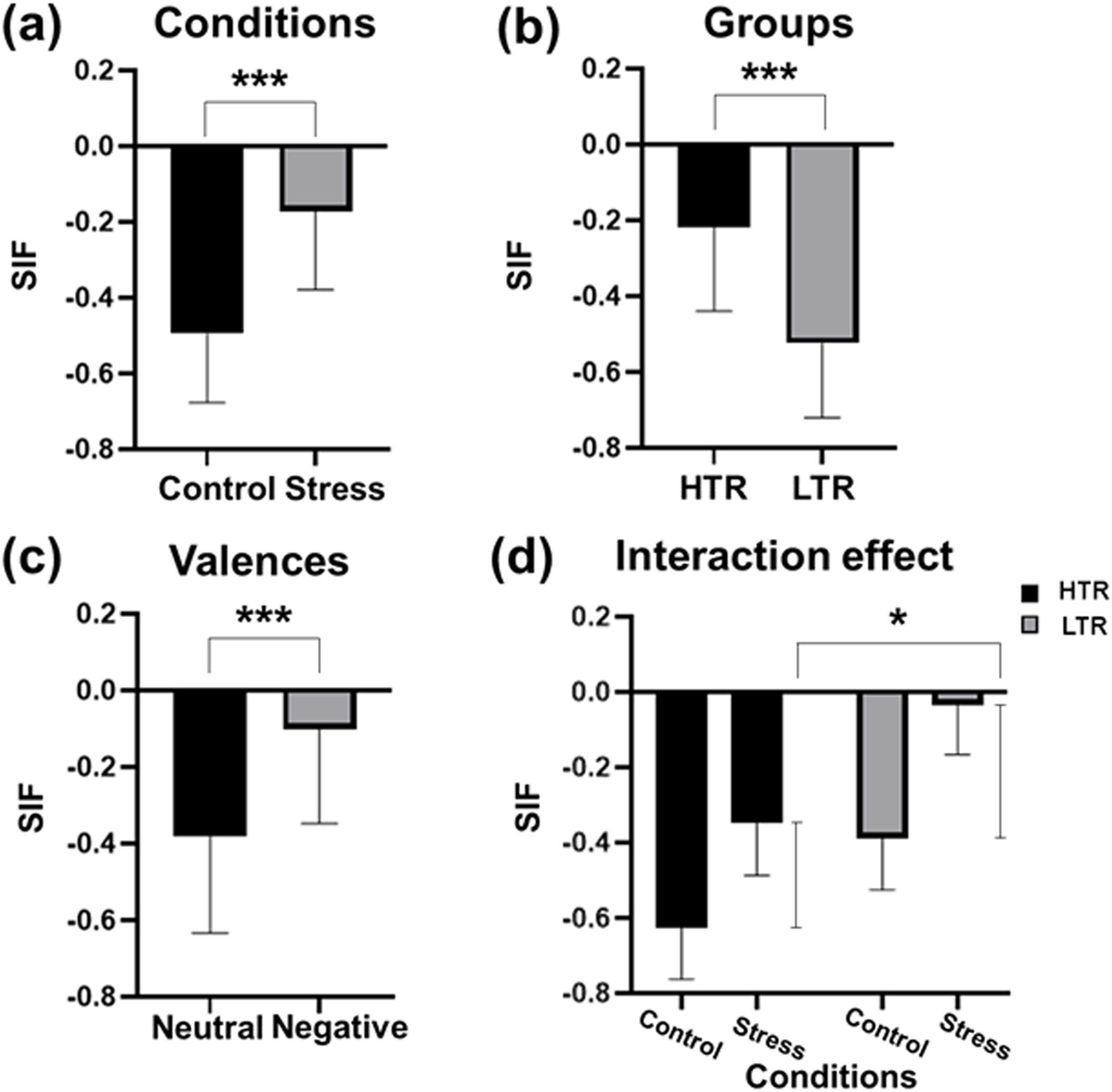
Behavioral performance of intentional memory suppressionThe behavioral results showed significant main effects of conditions (F = 290.387, p < 0.001, η2=0.838), valence of the stimulus materials (F = 40.545, p < 0.001, η2=0.396), and groups (F = 221.418, p < 0.001, η2=0.744) (Fig. 3). Specifically, for the conditions, the SIF indexes of subjects under the stress condition were significantly higher than those under the control condition, indicating a poorer performance in the TNT task. For the groups, the HTR exhibited a poorer performance in the TNT task compared to the LTR. In addition, behavioral performance in both the HTR and LTR was worse during the TNT trials applying negative stimuli. More importantly, the interaction effect between the conditions and the groups was significant (F = 4.263, p < 0.05, η2=0.086). Compared to the LTR, there was a more significant difference in the behavioral performance in the HTR between the stress and control condition, while the other interaction effects were all not significant (Table S5 of Supplementary materials).
Results of behavioral performance in TNT task. The main effects of (a) the conditions, (b) the groups and (c) the valence of stimulus materials on suppression-induced forgetting index were significant. (d) The interaction effect between the experimental conditions and the groups on suppression-induced forgetting index was significant. LTR, low trait ruminators; HTR, high trait ruminators; ***p < 0.001.
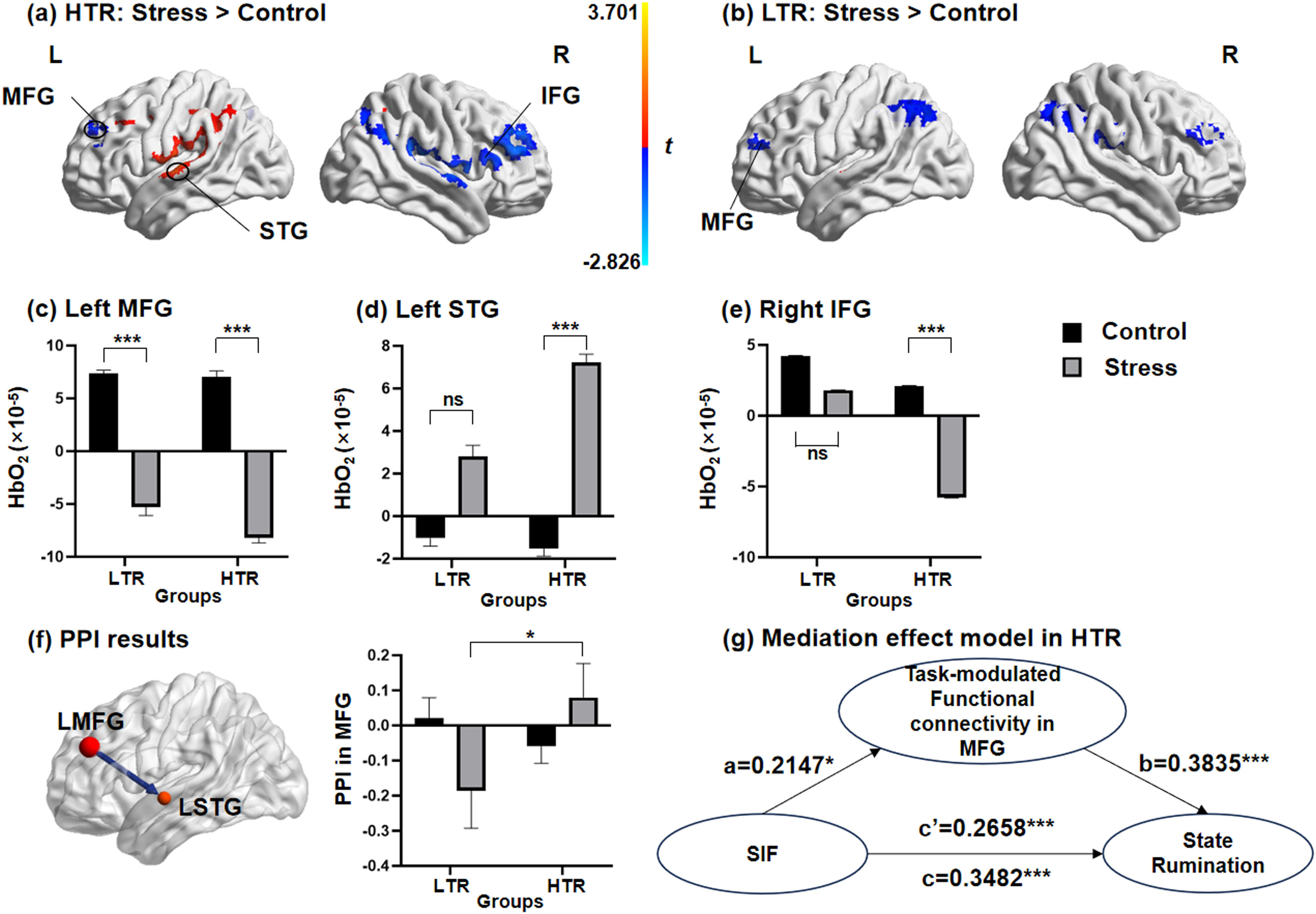
Significant main effects of the conditions in the left SPL (F = 4.934, p < 0.05), the right MFG (F = 3.792, p < 0.05) and the right SPL (F = 4.25, p < 0.05, Table S6 of Supplementary materials), and significant main effects of the groups in the left MFG (F = 10.905, p < 0.01) and the left STG (F = 7.089, p < 0.05, Table S7 of Supplementary materials) could be observed. More importantly, the results indicated a significant interaction effect of conditions × groups in the left MFG (F = 4.086, p < 0.05), the left STG (F = 4.724, p < 0.05) and the right IFG (F = 4.179, p < 0.05, Fig. 4a-b, Table S8 of Supplementary materials). Considering that the main effect of valence of stimuli and the interaction effects between the valence of stimuli and the other two factors were all not significant, the data of neutral and negative stimulus materials were combined in the subsequent analyses. We further performed simple effects analyses of the activation in the left MFG, the left STG and the right IFG (Table S9 of Supplementary materials). Results from the simple effect analyses found that in the HTR, the simple effect of the conditions was significant in the left MFG, the left STG and the right IFG. In the HTR, activation in the MFG and IFG during the TNT task under the stress condition was significantly lower than those under control condition, with the activation in the STG significantly increasing. In contrast, in the LTR, the simple effect of the conditions could only be found in the left MFG. The brain activation in the left MFG of the LTR under the stress condition was significantly lower than that under the control condition (Fig. 4c-e).
Brain activation patterns and task-modulated functional connectivity during memory suppression. (a) In HTR, activations in the MFG and IFG significantly decreased under stress condition, while activation in the STG significantly increased. (b) In LTR, only the activation in the MFG can be found significantly higher under the stress condition than control condition. The simple effect of the conditions was found differentially significant in (c) the left MFG, (d) the left STG and (e) the right IFG in HTR and LTR. (f) The psychophysiological interaction between the seed of the left MFG with the left STG was negatively correlated in the LTR, but positively correlated in the HTR. (g) The effect of memory suppression performance on state rumination of individuals was significantly mediated by the task-modulated functional connectivity between the MFG and STG in HTR. MFG, middle frontal gyrus; STG, superior temporal gyrus; IFG, inferior frontal gyrus; LTR, low trait ruminators; HTR, high trait ruminators; SIF, suppression-induced forgetting; PPI, psychophysiological interaction; *** p < 0.001.
The interaction effect between conditions and groups was only significant in the task-modulated functional connectivity between the left MFG and left STG (F = 4.908, p < 0.05; Fig. 4f). Specifically, for the LTR, the task-modulated functional connectivity between the left MFG and left STG was negatively correlated and increased significantly under the stress condition compared to those under the control condition. For the HTR, the functional connectivity was positively correlated under the stress condition. Significant interaction effects between other seeds and any other ROIs were not observed (Table S10 of Supplementary materials).
Correlation analyses and mediation effect model resultsThe SIF indexes of subjects were negatively correlated with the brain activations in the left MFG (r=−0.218, p = 0.027) and the right IFG (r=−0.219, p = 0.027), while they were positively correlated with activation in the left STG (r = 0.342, p = 0.001) (Figure S3, Table S11 of Supplementary materials). Furthermore, the level of state rumination was positively correlated with SIF (r = 0.348, p < 0.001) and task-modulated functional connectivity in the MFG (r = 0.441, p < 0.001). There was also a significant correlation between memory suppression performance and PPI in MFG (r = 0.215, p < 0.05). In the mediation effect model, SIF was set as the independent variable, with the task-modulated functional connectivity in the MFG included as the mediator. Examination of the total effect revealed that SIF significantly predicted state rumination in the HTR (β = 0.3482; 95 % CI [0.6457, 2.1600]). The indirect effect of SIF on state rumination through PPI in the MFG was significant (β = 0.0823; 95 % CI [0.0288, 0.1410]). The direct effect of SIF on state rumination after accounting for PPI in the MFG remained significant (β = 0.2658; 95 % CI [0.3567, 1.7856]). Task-modulated functional connectivity in the MFG as a mediator, accounted for 23.64 % (Effect ratio = 0.2364) of the variance in the relationship between memory suppression performance and state rumination in the HTR (Fig. 4g).
DiscussionThe current study applied fNIRS to explore the impact of acute stress on intentional memory suppression in HTR and its underlying neural correlates. We found that the HTR exhibited more severe impairment in memory suppression after exposure to acute stress compared to the LTR. Additionally, more aberrant activation patterns and task-modulated functional connectivity in the MFG and STG were observed in the HTR than the LTR during intentional memory suppression under the stress condition, further resulting in a poorer memory suppression performance. These findings may provide some insights into the potential brain mechanisms underlying the process of active suppression of unwanted memories in the HTR under acute stress conditions.
At the behavioral level, the current research showed impairment in suppressing unwanted memories and increased state rumination in individuals after exposure to acute stress. Compared to the LTR, the HTR were more sensitive to acute stress, exhibiting more severe impairment in memory suppression and reporting higher levels of state rumination under stress. These findings suggest that the detrimental effects of acute stress on intentional memory suppression are somewhat regulated by the trait rumination of individuals, which may explain the phenomena of memory flashbacks and rumination on negative memories in HTR, particularly when such individuals have recently experienced stressful events (Rosenbaum et al., 2020).
At the neural level, activation in the left MFG and right IFG during intentional memory suppression in the HTR under the stress condition was significantly lower than those under the control condition, with a significant increase in the activation in the left STG. In the LTR, significant lower brain activation was only observed in the left MFG under the stress condition than those under the control condition. The MFG and the IFG have been shown to be important brain regions in the DLPFC, which is highly associated with cognitive control functions (McEwen & Morrison, 2013). The DLPFC region was significantly activated while individuals performed the TNT task, so as to recruit sufficient psychological resources to intentionally suppress unwanted memories (Webler et al., 2022; Weidler et al., 2022). However, the activation in DLPFC is downregulated under the influence of acute stress, which further impairs memory suppression performance (McEwen & Morrison, 2013). Compared to the LTR, the HTR were more sensitive to acute stress, resulting in a lower activation in the DLPFC and a poorer performance in the TNT task.
Moreover, task-modulated functional connectivity between the left MFG and left STG was negative in the LTR and positive in HTR. The activation of the STG, an important neural structure participating in memory-related processes (Taing et al., 2021; Wu & Buckley, 2022), has been found to be downregulated by the DLPFC during memory suppression (Benoit et al., 2016; Hermans et al., 2014). However, acute stress reduces activation in the DLPFC, further increasing activation in the STG and impairing memory suppression performance (Quaedflieg et al., 2015). The current research indicated that the negative functional connectivity between the DLPFC and the STG in the LTR increased under stress to facilitate memory suppression. In contrast, the positive functional connectivity between the DLPFC and the STG in the HTR under stress resulted in impaired downregulation of the DLPFC, leading the increased activation in the STG and poorer memory suppression performance.
Significantly, a negative correlation was found between activation in the left MFG and right IFG and the behavioral performance in memory suppression, while a positive correlation between activation in the left STG and the SIF index could be observed, indicating that the activation patterns in the DLPFC and the temporal gyrus may predict the performance of memory suppression. Considering the mediation effect model, the direct effect of memory suppression performance on state rumination was found to be significant in the HTR. The SIF index of the HTR positively predicted the levels of state rumination, meaning that the HTR tend to report higher state rumination with the higher SIF index and poorer memory suppression performance. Meanwhile, the significant indirect effect indicated that task-modulated functional connectivity between the MFG and STG mediated the influence of memory suppression performance on state rumination. The aberrant functional connectivity in the DLPFC impaired the ability of intentional memory suppression during the TNT task in the HTR, thus inducing higher levels of state rumination, which makes such individuals prone to exhibit more stress-related symptoms (Quaedflieg et al., 2020).
This study has certain limitations. First, considering that procedures of the TSST, which has been considered as the most effective paradigm for experimental stress induction, are not applicable to fMRI scenario, the current research applied fNIRS, which offers a more ecologically valid but relatively lower spatial resolution to collect signal changes in brain regions. Future research should consider the trade-off between temporal and spatial resolution. Second, as the HTR may be a potential at-risk group for developing stress-related disorders, it would be valuable to include a population of clinically diagnosed patients in future research. Comparing HTR and clinical patients may help to deepen our understanding of the process of HTR development in the population of clinically diagnosed patients, which may provide certain clinical insights for early prevention and intervention in this process.
ConclusionsIntentional suppression of unwanted memories is an important cognitive function which helps individuals to eliminate the negative impact of irrelevant information and maintain their mental health. Exposure to acute stress is believed to impair individuals’ memory suppression ability. The current research found that the HTR exhibited a greater degree of impairment of intentional memory suppression after exposure to stress compared to the LTR. Furthermore, an aberrant activation pattern and functional connectivity in the DLPFC were found in the HTR during the process of memory suppression under the stress condition, supporting the hypothesis that the impaired effect of acute stress on intentional memory suppression is modulated by the trait rumination of individuals. These findings could provide insights for the prevention and early intervention in the development of several stress-related disorders in individuals with high trait rumination.
CRediT authorship contribution statementJixin Long: Conceptualization, Investigation, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Lanxin Peng: Investigation, Writing – review & editing. Qian Li: Writing – review & editing. Lijing Niu: Writing – review & editing. Haowei Dai: Writing – review & editing. Jiayuan Zhang: Writing – review & editing. Keyin Chen: Writing – review & editing. Tatia MC Lee: Writing – review & editing. Meiyan Huang: Writing – review & editing, Resources, Supervision. Ruibin Zhang: Conceptualization, Resources, Supervision, Project administration, Funding acquisition.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
This study was supported by National Key R & D Program of China (STI2030-Major Projects 2022ZD0214300), Nature Science Foundation of China (refs: 32271139, 31900806), Guangdong Basic and Applied Basic Research Foundation (ref: 2023A1515011331), Science and Technology Program of Guangzhou, China (ref: 2023A04J1964), Guangzhou Philosophy and Social Science Project for 2022 Yangcheng Young Scholar during the fourteenth Five-year Plan Period (ref: 2022GZQN30). The funding organization played no further role in study design, data collection, analysis and interpretation, and paper writing.