Patients with end stage liver disease (ESLD) referred for liver transplantation (LT) are forwarded to pulmonary evaluation before being operated. ESLD is associated with muscle wasting, reduced exercise tolerance and aerobic capacity.
ObjectivesWe assessed the association between aerobic capacity (AC), liver disease severity and postoperative LT outcomes in a series of LT candidates in a university affiliated hospital in Brazil.
MethodsPre-LT oxygen uptake at peak (pre-VO2peak), liver disease severity, and early pos-LT outcomes such as length of intensive care unit (ICU) stay, <5 and ≥5 days and hospitalization, <20 and ≥20 days and postoperative mortality were compared. Pre-VO2peak was measured through the cardiopulmonary exercise testing (CPET). Severity of liver disease was estimated by the Model for End-Stage Liver Disease (MELD) categorization into MELD<18 and MELD≥18 groups. Student's t-test was used to compare these groups. A logistic regression model was built to verify the effect of those variables on the length of ICU stay, length of hospitalization and postoperative mortality.
ResultsA total of 47 patients were include in analysis. Pre-VO2peak was similar to that of healthy sedentary individuals (75±18%) and worse in the MELD≥18 group as compared to the MELD<18 group (19.51±7.87 vs 25.21±8.76mL/kg/min, respectively; p=0.048). According to the multivariate analysis, only a lower pre-VO2peak (<20.09±4.83mL/kg/min) was associated to a greater length of hospitalization (p=0.01).
ConclusionsIn LT candidates, a reduced pre-VO2peak may predict a higher risk of greater pos-LT length of hospitalization. The length of ICU stay and postoperative mortality were not associated with variables studied. This finding should be evaluated in other studies before making specific recommendations about a routine use of CPET in LT candidates.
Pacientes com doença hepática em estágio final (DHEF), referenciados para transplante hepático (TH), são encaminhados para avaliação da função pulmonar antes de serem operados. DHEF está associada à fraqueza muscular, redução da tolerância ao exercício e capacidade aeróbica (CA).
ObjetivosFoi avaliada a associação entre capacidade aeróbica, a gravidade da doença hepática e os resultados pós-operatórios do TH em uma serie de candidatos a TH em um hospital universitário no Brasil.
MétodosO consumo de oxigênio num teste limitado por sintomas (VO2pico) pré-TH, a gravidade da doeça hepática, e os resultados precoces pós-TH como tempo de permanênica na unidade de cuidados intensivos (UCI), <5 e ≥5 dias, tempo de permanência hospitalar, <20 e ≥20 dias e mortalidade foram comparados. O VO2pico pré-TH foi medido através do teste de exercício cardiopulmonar (TECP). A gravidade da doença hepática foi estimada pelo Modelo para doença hepática em estágio final [Modelo de End-Stage Liver Disease (MELD)] e categorizada nos grupos MELD<18 e MELD≥18. O teste t de Student foi utilizado para comparar esses grupos. Um modelo de regressão logística foi construido para verificar o efeito dessas variáveis sobre o tempo de permanência na UTI, tempo de internação e mortalidade pós-operatória. Resultados: um total de 47 pacientes foram incluídos nas análises. O VO2pico pré-TH foi semelhante ao de indivíduos saudáveis sedentários (75±18%) e pior no grupo MELD≥18 em comparação com o MELD<18 (19.51±7.87 vs 25.21±8.76mL/kg/min, respectivamente; p=0.048). De acordo com a análise multivariada, somente um menor VO2pico (<20.09±4.83mL/kg/min) pré-TH foi associado a um maior tempo de internação (p=0,01).
ConclusãoEm candidatos a TH, uma redução do VO2pico pré pode predizer um maior risco de maior permanência hospitalar pós-TH. Esta constatação deve ser avaliada em outros estudos antes de fazer recomendações específicas sobre o uso rotineiro do TECP para candidatos TH. O tempo de permanência na UTI e mortalidade pós-operatória não foram associados com as variáveis estudadas.
Liver transplantation (LT) is largely indicated for patients with end-stage liver diseases (ESLD).1 The proportion of patients for whom this therapy has been indicated has increased worldwide, although the number of viable grafts has not in the same proportion.2 Due to this discrepancy, several scores has been developed in order to better assess the severity of ESLD. Previous studies had shown that the Model for End-Stage Liver Disease (MELD) – the most used one – has a strong association with pre-transplant mortality rate.3 In spite of that, it does not accurately predict complications after LT.4
The amelioration of operative techniques and the recent advances in the immunosuppressive therapy has consistently contributed to the better rates of survival and the prevention of graft dysfunction. Although LT success is traditionally measured using variables related to graft function along with patient survival, it may be accounted in terms of health-related quality of life scores or even through cardiopulmonary parameters such as aerobic capacity (AC), exercise capacity, skeletal muscle strength and body composition.5–7
An effective interaction of several physiologic mechanisms is necessary to sustain exercise and maintain gas exchange between the environment and the cells.8 The gold standard for AC analysis is the oxygen uptake at peak exercise (VO2peak) during symptom-limited cardiopulmonary exercise testing (CPET). CPET provides an integrated evaluation of muscle and cardiorespiratory responses to effort.9 AC has been used as a prognostic factor in LT and is associated with liver disease severity as measured by MELD.5,6 AC is reduced in patient with chronic liver disease by several mechanisms: metabolic disorders, malnutrition, decreased muscle strength, cirrhotic cardiomyopathy and anemia.10–13
Evaluation of VO2peak before LT may be useful in prognostic evaluation and for the prescription of supervised exercise programs in both pre- and postoperative periods, thus increasing the possibility of restoring the AC of these individuals.13–15
Therefore, the aim of this study is to verify the association between AC and the severity of liver disease (MELD) and its relation to the immediate clinical outcomes after LT: length of ICU stay and of hospitalization and postoperative mortality.
2Materials and methodsThis is a transversal study. Patients aged more than 18 years who were active on the LT list and were undergoing regular monitoring at the outpatient clinic of the Liver Transplantation Unit of Clinics Hospital of the Federal University of Minas Gerais were invited to participate in the study from May 2010 to October 2011.
Patients who were on the waiting list to undergo liver re-transplantation, those who were hospitalized at the time of enrollment, those who had moderate-to-severe hepatic encephalopathy or were unable to perform the CPET, and those with indication for combined transplant (liver plus another organ) were excluded from the study.
In the first visit, data from the physical examination, anthropometric data, comorbidities, medicines in use, and calculation of the Child-Turcotte-Pugh (CHILD) score and MELD classifications were performed.
Patients included in the study were classified into groups MELD <18 or ≥18. After transplantation length of ICU stay and length of hospitalization were assessed and grouped in <5 and ≥5 days and <20 or ≥20 days, respectively.
CPET tests were performed according to international guidelines9 using an integrated stationary metabolic ErgoPC Elite system for effort and metabolic gas analysis (Micromed, Brasilia, Brazil; expired gas analyzer: MetaLyzer® 3B Cortex, Cortex, Leipzig, Germany). All equipments were calibrated before all examinations. On a treadmil, individualized speed ramp protocol was used and the duration of the test was limited by maximal exertion or if safety criteria were fulfilled. Maximal exertion was defined as a failure to maintain the ergometer speed for more than 30seconds or the development of symptoms of fatigue, pain or lightheadedness.9 One single professional conducted the tests, but the records were analyzed independently by two professionals using the Metasoft® Cortex software (Cortex,Leipzig, Germany).
In the case of divergence between the reports, the records were re-analyzed together by both professionals in order to reach a consensus.
The minute ventilation, respiratory rate, tidal volume, oxygen uptake at peak exercise during symptom-limited CPET –VO2peak, carbon dioxide production (VCO2), ventilatory equivalents (VE/VO2 and VE/VCO2), and end tidal pressure (PETO2 and PETCO2) were measured using face masks that allowed the passage of exhaled air in breath-to-breath intervals with a cut-off point every 15seconds for analysis. The VO2peak was considered according to the average of the last 30seconds of exercise that was interrupted by signs of maximum effort tolerance: exhaustion, fatigue or lower limb pain that prevented continuation of the exercise. The anaerobic threshold (AT) was determined using the visual-graphic method (V-slope method) and the tabular method.9 In addition to the values provided by Metasoft, the VO2-predicted percentage of sedentary healthy individuals was calculated using the formula proposed by Bruce et al.16 The VO2 in the AT was presented as absolute values and as percentage of the predicted VO2.
Descriptive results were presented as absolute values and relative frequency for the characteristics of various qualitative variables (i.e., death, gender, and etiology of cirrhosis) and of the computation of arithmetic mean, median, standard deviation, and interquartile interval for the following quantitative traits: age, MELD and CHILD scores, length of ICU stay, length of hospitalization, VO2peak, %predicted VO2peak, VO2 in AT, and VO2 in %predicted AT.
All analyzed data were assessed using the Shapiro–Wilk test in order to evaluate the normality of data distribution. For comparisons between MELD <18 and MELD ≥18 groups, the Mann–Whitney test or Student's t-test for independent samples was used. A statistical significance level of 0.05 was adopted for those analyses. A logistic regression model was used to verify the effect or influence of each variable on the length of ICU stay (<5 or ≥5 days) and length of hospitalization (<20 or ≥20 days) and postoperative mortality. Only patients who had performed the CPET less than 90 days before LT and had not died in the operative room were considered for analyses. All variables that were significant at the level of 0.25 were regarded as candidate variables for the multivariate model, except for those that were a linear combination of other variables, i.e., age, sex, and VO2 in the % predicted AT. The covariables that independently had a p-value <0.05 were maintained for the next stage. For statistical analyses, the following programs were used: Statistical Package for the Social Sciences, version 17.0 (SPSS Inc., Chicago, IL, USA) and R Development Core Team 2010 (R Foundation for Statistical Computing, Vienna, Austria).
All the participants agreed to participate and signed the informed consent form. The study protocol and the informed consent form were approved by the Research Ethics Committee of Federal University of Minas Gerais (document: ETIC no. 134/09, dated May 29, 2009).
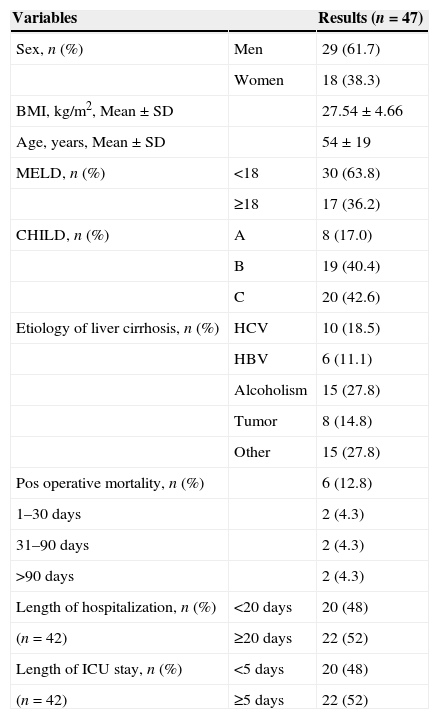
3ResultsBetween May 2010 and October 2011, 111 patients underwent LT in our hospital; of these, 47 patients participated in this study and performed CPET before LT. Sixteen patients don’t meet the inclusion criteria (4 due to combined transplant (liver-kidney), 5 were hospitalized, 4 with moderate-to-severe hepatic encephalopathy and 3 were not able to perform the CPET). In the remaining 48, the CPET was temporarily unavailable. As two patients did not reach the AT during CPET, two died during the perioperative period, and one had performed CPET more than 90 days before LT, the length of ICU stay in the ICU of hospitalization could be assessed in only 42 patients. Patients characteristics and outcomes are shown in Table 1.
Clinical and demographic characteristics of patients undergoing liver transplantation in the study.
| Variables | Results (n=47) | |
|---|---|---|
| Sex, n (%) | Men | 29 (61.7) |
| Women | 18 (38.3) | |
| BMI, kg/m2, Mean±SD | 27.54±4.66 | |
| Age, years, Mean±SD | 54±19 | |
| MELD, n (%) | <18 | 30 (63.8) |
| ≥18 | 17 (36.2) | |
| CHILD, n (%) | A | 8 (17.0) |
| B | 19 (40.4) | |
| C | 20 (42.6) | |
| Etiology of liver cirrhosis, n (%) | HCV | 10 (18.5) |
| HBV | 6 (11.1) | |
| Alcoholism | 15 (27.8) | |
| Tumor | 8 (14.8) | |
| Other | 15 (27.8) | |
| Pos operative mortality, n (%) | 6 (12.8) | |
| 1–30 days | 2 (4.3) | |
| 31–90 days | 2 (4.3) | |
| >90 days | 2 (4.3) | |
| Length of hospitalization, n (%) | <20 days | 20 (48) |
| (n=42) | ≥20 days | 22 (52) |
| Length of ICU stay, n (%) | <5 days | 20 (48) |
| (n=42) | ≥5 days | 22 (52) |
BMI: body mass index; SD: standard deviation; MELD: Model for End-Stage Liver Disease; Child: Child-Turcotte-Pugh; HCV: hepatitis C virus; HBV: hepatitis B virus; Other: primary biliary cirrhosis, hemochromatosis, Caroli disease, cryptogenic cirrhosis; Tumor: hepatocellular carcinoma or hemangioendothelioma; ICU: Intensive Care Unit.
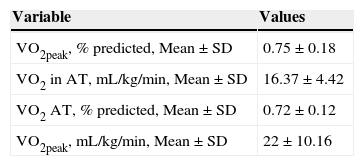
There were no adverse events during CPET tests. We analyzed 45 patients because two patients did not reach the AT. In this sample, VO2peak values were similar to those predicted for healthy sedentary individuals, although they presented high percentage of VO2peak values even during submaximal effort (Table 2).
Aerobic capacity of 45 patients who have performed CPET.
| Variable | Values |
|---|---|
| VO2peak, % predicted, Mean±SD | 0.75±0.18 |
| VO2 in AT, mL/kg/min, Mean±SD | 16.37±4.42 |
| VO2 AT, % predicted, Mean±SD | 0.72±0.12 |
| VO2peak, mL/kg/min, Mean±SD | 22±10.16 |
CPET: cardiopulmonary exercise testing; SD: standard deviation; VO2peak: oxygen uptake at peak exercise; AT: anaerobic threshold.
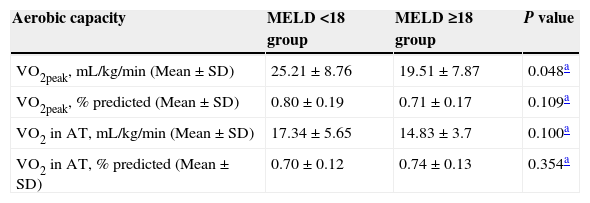
Patients who underwent both LT and CPET were analyzed according to the cirrhosis severity: MELD<18 group (22 patients) and MELD≥18 group (23 patients; Table 3). VO2peak was significantly higher in MELD<18 group. In the evaluation of AC, regardless of MELD, patients reached, on average, over 70% of the VO2peak value (Table 3).
Aerobic capacity of patients in groups categorized by MELD (n=45).
| Aerobic capacity | MELD <18 group | MELD ≥18 group | P value |
|---|---|---|---|
| VO2peak, mL/kg/min (Mean±SD) | 25.21±8.76 | 19.51±7.87 | 0.048a |
| VO2peak, % predicted (Mean±SD) | 0.80±0.19 | 0.71±0.17 | 0.109a |
| VO2 in AT, mL/kg/min (Mean±SD) | 17.34±5.65 | 14.83±3.7 | 0.100a |
| VO2 in AT, % predicted (Mean±SD) | 0.70±0.12 | 0.74±0.13 | 0.354a |
MELD: Model for End-Stage Liver Disease; SD: standard deviation; VO2peak: oxygen uptake at peak exercise; AT: anaerobic threshold.
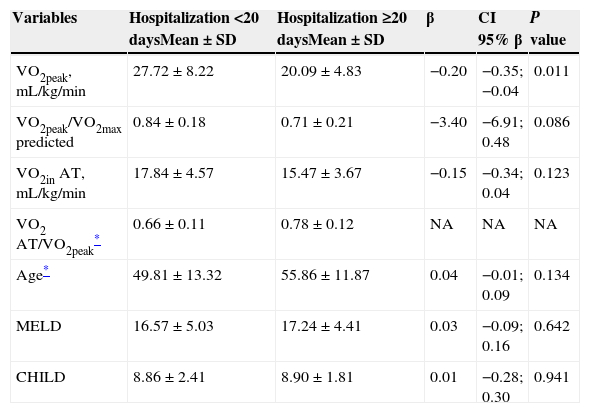
The univariate analysis is presented in Table 4. One patient who underwent CPET more than 90 days before the LT and two patients who died during the perioperative phase were excluded from this evaluation. The sample consisted of 22 patients in the ≥20-day hospitalization group and 20 patients in the <20-day hospitalization group. The variables VO2peak, mL/kg/min, VO2peak/VO2peak predicted and VO2in AT, mL/kg/min were significant in the univariate analysis and were selected for multivariate analysis.
Univariate analysis of the quantitative variables and length of hospitalization (n=42).
| Variables | Hospitalization <20 daysMean±SD | Hospitalization ≥20 daysMean±SD | β | CI 95% β | P value |
|---|---|---|---|---|---|
| VO2peak, mL/kg/min | 27.72±8.22 | 20.09±4.83 | −0.20 | −0.35; −0.04 | 0.011 |
| VO2peak/VO2max predicted | 0.84±0.18 | 0.71±0.21 | −3.40 | −6.91; 0.48 | 0.086 |
| VO2in AT, mL/kg/min | 17.84±4.57 | 15.47±3.67 | −0.15 | −0.34; 0.04 | 0.123 |
| VO2 AT/VO2peak* | 0.66±0.11 | 0.78±0.12 | NA | NA | NA |
| Age* | 49.81±13.32 | 55.86±11.87 | 0.04 | −0.01; 0.09 | 0.134 |
| MELD | 16.57±5.03 | 17.24±4.41 | 0.03 | −0.09; 0.16 | 0.642 |
| CHILD | 8.86±2.41 | 8.90±1.81 | 0.01 | −0.28; 0.30 | 0.941 |
NA: was not able to adjust in the univariate analysis; SD: standard deviation; VO2peak/VO2max predicted: relationship between O2 consumption at peak exercise and the consumption predicted for healthy sedentary individuals; VO2 AT: O2 uptake in anaerobic threshold; VO2 AT/VO2peak: O2 uptake anaerobic threshold regarding the peak consumption; OR: odds ratio; β: beta coefficient; CI 95% β: confidence interval of beta coefficient; MELD: Model for End-Stage Liver Disease; Child: Child-Turcotte-Pugh.
The multivariate regression model is presented in Table 5. The only variable that remained significant for the length of hospitalization was the VO2peak (mL/kg/min). The risk of being hospitalized for more than 20 days decreases by 0.20 as the VO2peak increases by 1mL/kg/min. There were not found any variables independently associated to length of ICU stay and postoperative mortality in the final model.
Final model of logistic regression to explain the length of hospitalization (n=42).
| Characteristics | Hospitalization<20 daysMean±SD | Hospitalization≥20 daysMean±SD | β | CI 95% β | P value |
|---|---|---|---|---|---|
| VO2peak, mL/kg/min | 27.72±8.22 | 20.09±4.83 | −0.20 | −0.35; −0.04 | 0.011 |
P value=0.941 of the Hosmer–Lemeshow Test of Goodness of Fit; SD: standard deviation; β: beta coefficient; CI 95% β: confidence interval of beta coefficient at 95%; VO2peak: oxygen uptake at peak exercise.
The main results of this study were that patients in the waiting list of LT, the AC as measured by the VO2peak was comparable to that of healthy sedentary individuals, AC was worse in the MELD ≥18 group, and an inverse relationship between length of hospitalization and VO2peak was observed.
Patients with ESLD have several factors that may lead to exercise limitation. The liver is the main organ involved in carbohydrate homeostasis.17–20 Moreover, these patients may have a resting hyperdynamic cardiovascular state, characterized by a decrease in pulmonary vascular resistance associated with increased cardiac frequency and cardiac index.11,21,22 All of these factors may contribute to disorganization of the muscular, pulmonary and cardiovascular components of exercise capacity and may lead to a reduced VO2peak.13 These factors may explain the lower VO2peak found in our study, in which patients had severe disease (MELD ≥18). This result is concordant with that of Dharancy et al. that reported a negative correlation between VO2peak and MELD.12 Lemyze et al. also found that the severity of the disease, as determined by MELD score, was an independent variable more closely associated with the changes in VO2peak23. Two other studies also found a significant negative correlation between severity of ESLD (CHILD classification) and the AC.24,25
During submaximal effort, the VO2peak of the sample was 16.37±4.4mL/kg/min. However, the MELD ≥18 group had lower values (median±II: 14.83±3.7mL/kg/min), although we did not find a significant difference between the groups. In a study carried out by Pieber et al., the average value of VO2peak found in the patients before LT was 10.3±3mL/kg/min. Nevertheless, they did not refer to any score of severity of the disease.10
CPET represents the gold standard for the determination of exercise capacity and cardiorespiratory reserve, which reflects the ability of the body to consume and use oxygen during exercise.9 In disagreement with all the current evidence, in our study, the patients showed a VO2peak similar to that of sedentary healthy individuals. The reasons that could explain this finding are not known and should be addressed in other studies.
In this study, multivariate analysis showed an independent association between AC and the length of hospitalization. A similar result was observed by Bernal et al. in the patients with impaired AC who had prolonged hospitalization after LT. The results of the present study suggest that the patient's evaluation by means of CPET before LT may contribute to the prediction of some outcomes after LT. Moreover, CPET may reflect the stress caused by LT and may successfully reveal the conditions that could compromise the final result of an LT, such as coronary heart disease and pulmonary hypertension.14,26
We did not find any relevant associated variables with length of ICU stay. It is well known that patients with reduced cardiorespiratory reserve have an ICU stay with a mean of 5.3 days longer as compared to patients with normal cardiorespiratory reserve, a finding that is important in terms of critical care management and costs of medical assistance.26 Otherwise, the prediction of early postoperative mortality is particularly important in the context of LT as the appropriate allocation of limited donor organs and (more recently) marginal grafts is critical. Prentis et al. have demonstrated that a cardiopulmonary reserve of more than 9.0mL/min/kg predicted reliably a reduced postoperative mortality risk.26 Regarding this topic, our multivariate analysis of postoperative mortality didn’t show any significant associated variables. It should be emphasized that the observed postoperative mortality in our sample was too small (only two deaths).
This research was innovative in that it revealed the real physical condition of the patients who were candidates for LT in our hospital. No adverse events were observed during the examinations; this had not been expected because our patients were at high risk of having complications in treadmill tests.
Among our limitations, the low sample size is partly explained by the reduced number of patients on the waiting list for LT in the State of Minas Gerais, Brazil, at the time of this study. Moreover, since the test was temporarily unavailable, we fail to perform 48 tests of patients who were able to transplant. This is because TCP is not routinely performed by these patients and is not a recommendation of the local protocol LT in our hospital. The number of prospective studies comparing LT outcomes with AC is low. Therefore, it is difficult to compare our results with the available reports in the medical literature.
In conclusion, in LT candidates, a reduced pre-VO2peak may predict a higher risk of greater pos-LT length of hospitalization. The length of ICU stay and postoperative mortality were not associated with variables studied. This finding should be evaluated in other studies before making specific recommendations about a routine use of CPET in LT candidates.
5Summary boxCPET represents the gold standard for the determination of exercise capacity and cardiorespiratory reserve, and it generates measures of a number of aspects of AC.
Chronic liver disease is associated with muscle wasting, reduced exercise tolerance and AC.
The VO2peak was significantly higher in the MELD <18 group.
The patients showed an AC closer to that of healthy individuals; however, those with poorer performance in CPET had a higher risk of greater length of hospitalization.
Conflicts of interestThe authors have no conflicts of interest to declare.