Gastrointestinal mixed adenoneuroendocrine carcinomas (MANECs) are rare tumors and, as the name implies, are characterized by the presence of both components, exocrine and neuroendocrine, each one representing at least 30% of the lesion.1 It is unclear if they originate from proliferation of different cell lineages or from stem cells capable of differentiating along multiple cell lineages. They may present as polypoid lesions, with variable size. Treatment and prognosis is determined by the more aggressive component.2
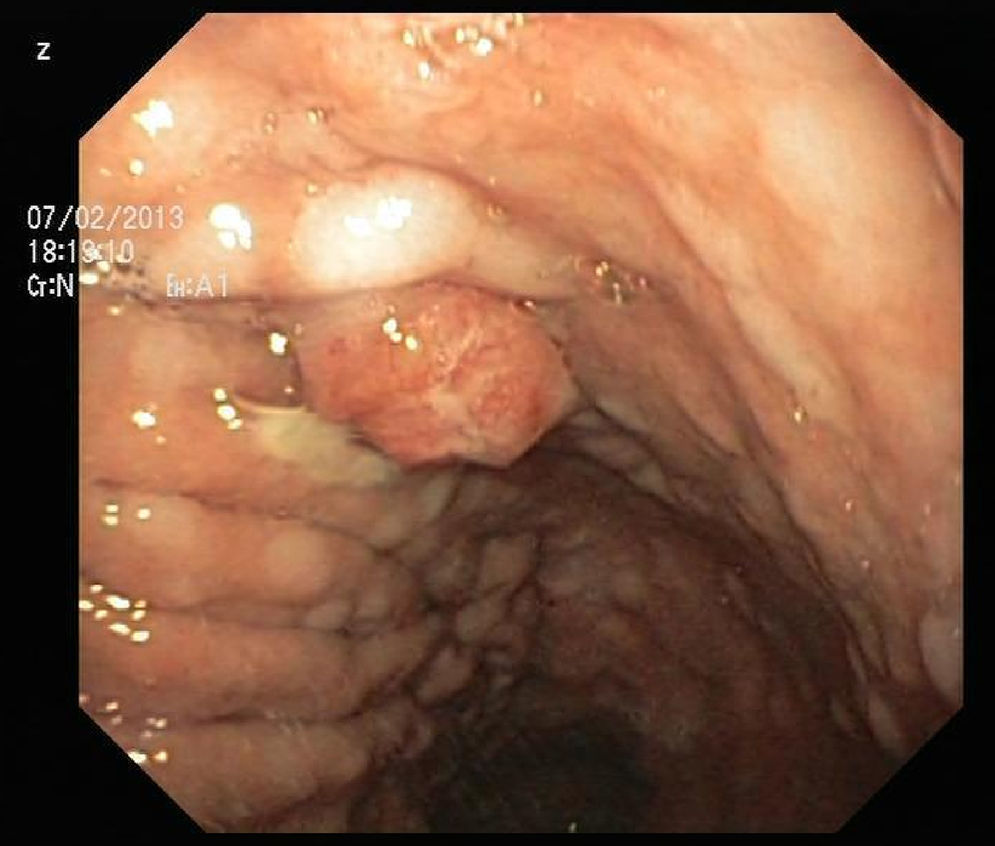
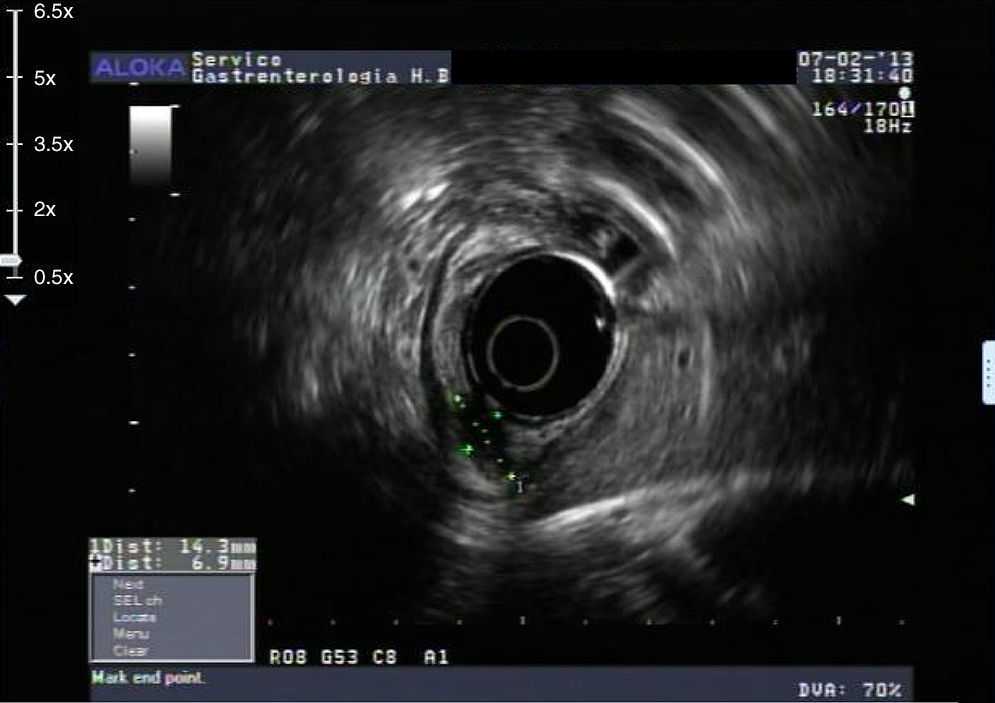
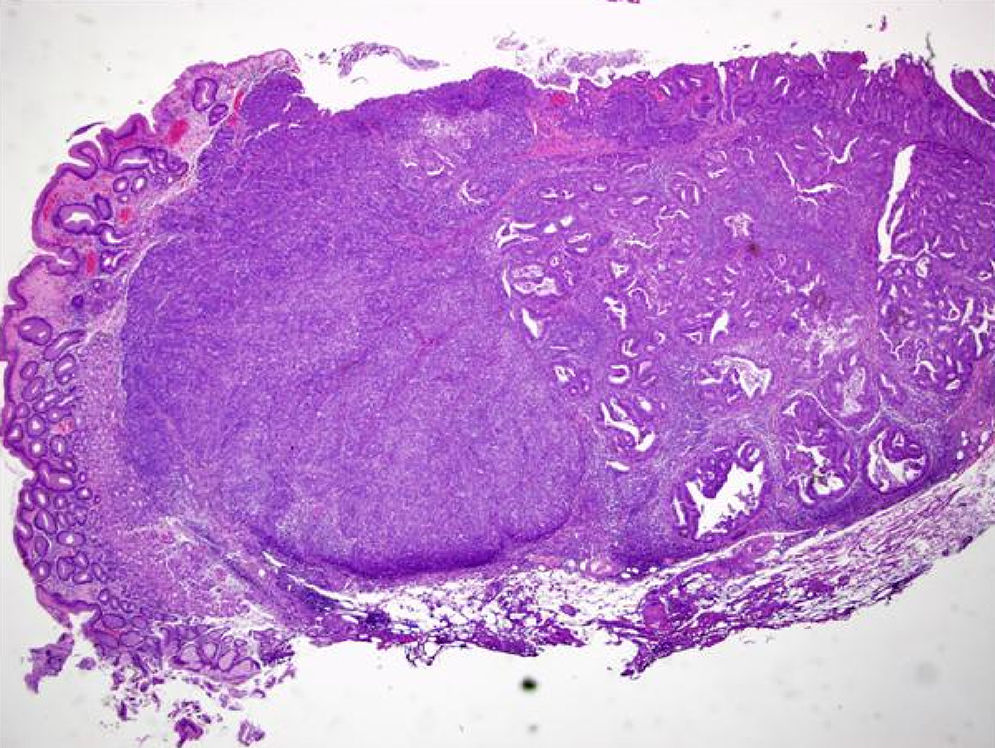
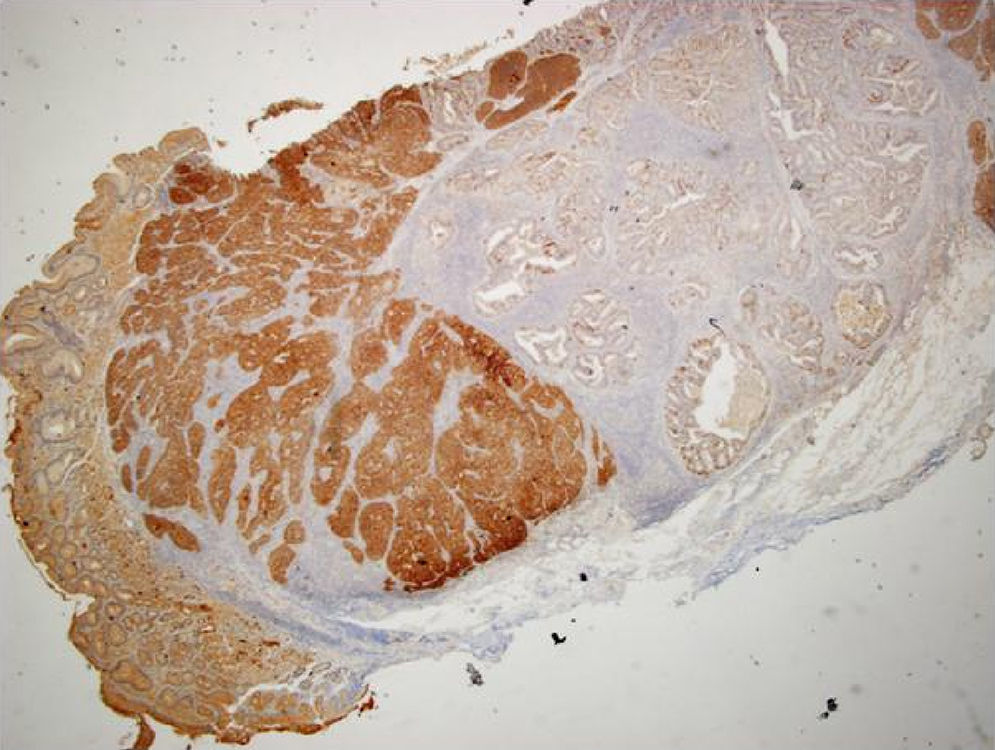
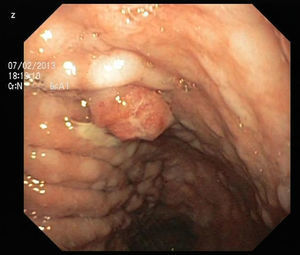
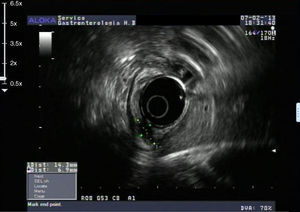
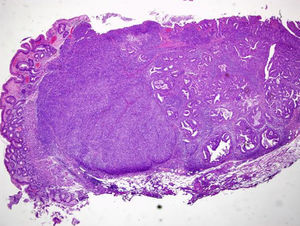
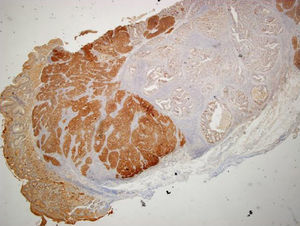
An 84-year-old woman was referred to upper gastrointestinal endoscopy for evaluation of dyspepsia. Endoscopic examination showed a 15mm polypoid lesion with central ulceration in the anterior wall of gastric body and papulous gastropathy (Fig. 1). Biopsies of the gastric lesion showed a neuroendocrine tumor and random biopsies of the gastric mucosa showed chronic atrophic gastritis and intestinal metaplasia. Serum antibodies to parietal cells and to intrinsic factor were negative. Serum level of chromogranin A level was 129.6ng/mL (normal level<80ng/mL). Thoracic and abdominal CT revealed neither locoregional adenopathies nor distant metastasis. Single-photon emission computed tomography showed no lesion with uptake of 99mTc-tektrotyd. Endoscopic ultrasound (EUS) revealed that the gastric lesion was an uT1N0 tumor with 14×7mm (Fig. 2). The gastric lesion was resected endoscopically in one fragment using the “lift and cut” technique. Histological examination of the resected specimen showed a mixed adenoneuroendocrine carcinoma, comprising two closely juxtaposed elements each representing almost 50% of the tumor: (i) tubular well-differentiated adenocarcinoma; (ii) neuroendocrine carcinoma staining positively for chromogranin A and synaptophysin and with a mitotic index higher than 20 mitoses per 10 high-power fields (Figs. 3 and 4). The tumor invaded the submucosa and was intercepted by the deep and lateral margins of resection (Fig. 3) and so the patient was proposed for surgery. We decided for laparoscopic atypical gastrectomy which has significant lower rate of complications than the recommended complete or partial gastrectomy (en bloc resection) with local lymph node resection due to patient's age and poor performance status, the low risk (11%) of lymph node metastasis in cases of tumors with <2cm and no extension into muscle on EUS,3,4 and the absence of adenopathies in EUS and abdominal CT. The surgical specimen showed no residual lesion.
Histological examination (hematoxylin–eosin staining; magnification 20×) of the resected specimen showing a mixed carcinoma comprising a tubular well differentiated adenocarcinoma (on the right) and a neuroendocrine carcinoma (on the left), invading the submucosa and intercepted by the deep and lateral margins of resection.
The natural history of the gastric MANECs is unclear, but is mainly determined by the more aggressive component (the neuroendocrine component in our case). Like the pure gastric neuroendocrine tumor, the prognosis of MANEC depends primarily of stage (TNM) and also of histologic grading and mitotic count/Ki67 index. Despite the scarce information about this type of cancer, the survival rate at five years was slightly better than pure gastric adenocarcinoma, 33.3% vs 24% in one study.5
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Financial disclosuresThis work had no grant support or other assistance.
Author contributionsAll the authors (Dália Fernandes, João-Bruno Soares and Carla Rolanda) contributed for conception and design, analysis and interpretation of the data, drafting and critical revision of the manuscript for important intellectual content and final approval of this article.