Small bowel evaluation is a challenging task and has been revolutionized by high-quality contrasted sectional imaging (CT enterography - CTE) and magnetic resonance enterography (MRE) as well as by small bowel capsule endoscopy (SBCE).
The decision of which technique to employ during the investigation of small bowel diseases is not always simple or straightforward. Moreover, contraindications may preclude the use of these techniques in some patients, and although they are noninvasive procedures, may present with various complications.
SBCE plays a crucial role in the investigation of both obscure gastrointestinal bleeding and Crohn's disease, but it is also useful for surveillance of patients with Peutz-Jeghers syndrome, while CTE is very accurate in small bowel tumours and in established Crohn's Disease, and its use in patients presenting with gastrointestinal bleeding is increasing. MRE, an expensive and not widely available technique, is essential for the study of patients with Crohn's Disease, and presents an attractive alternative to SBCE in Peutz-Jeghers syndrome surveillance.
These diagnostic modalities are often not competitive but synergistic techniques. Knowing their characteristics, strengths and limitations, indications, contraindications and potential complications, as well as the adaptation to local availability and expertise, is essential to better select which procedures to perform in each patient, both safely and effectively, in order to optimize management and improve patient outcomes.
A investigação do intestino delgado, previamente difícil e limitada, sofreu uma revolução com o aparecimento de técnicas imagiológicas contrastadas de elevada qualidade, como a enterografia por tomografia axial computadorizada (enteroTC) e a enterografia por ressonância magnética (enteroRM), assim como pela enteroscopia por cápsula (EC).
A decisão na escolha da técnica a utilizar nas diferentes patologias do intestino delgado não é na maioria das vezes simples ou óbvia. Adicionalmente, a presença de contraindicações pode restringir o uso destas técnicas em alguns doentes, e apesar de não serem consideradas técnicas invasivas, não são isentas de riscos e complicações.
A EC tem um papel crucial na investigação da hemorragia digestiva de causa obscura e da doença de Crohn, mas tem-se revestido também de utilidade na vigilância de doentes com síndrome de Peutz-Jeghers; a enteroTC revelou uma elevada capacidade diagnóstica para neoplasias do intestino delgado e na doença de Crohn estabelecida, e a sua utilização na hemorragia digestiva de causa obscura tem vindo a expandir. A enteroRM, apesar de dispendiosa e de disponibilidade limitada, tem uma elevada eficácia no estudo da doença de Crohn, e é uma alternativa válida à EC no síndrome de Peutz-Jeghers.
Estas técnicas diagnósticas são frequentemente singergísticas e complementares, ao invés de competitivas. O reconhecimento das suas características, das suas capacidades e limitações, assim como das indicações, contraindicações e potenciais complicações, e aliado à adaptação à disponibilidade e competências locais, é essencial na correcta escolha de procedimentos seguros e eficazes para cada doente, de forma a optimizar a abordagem e o prognóstico.
The study of the small bowel has been traditionally limited to low yield techniques, such as push enteroscopy and small bowel follow-through, or invasive techniques such as intraoperative enteroscopy.
By the end of the past century, cross-sectional imaging techniques with excellent resolution, namely CT enterography (CTE) and magnetic resonance enterography (MRE), were developed to better observe and characterize small bowel pathology, while a new contender, small bowel capsule endoscopy (SBCE) has been shown to provide excellent diagnostic yield in a myriad of small bowel diseases.
The choice of what technique or even which combination to use for small bowel study in the different clinical settings encountered daily is often challenging, and depends on technical characteristics but also on local expertise and availability.
2Technical characteristicsCTE was developed in 1997 to better assess small bowel Crohn's Disease (CD).1 The preparation for CTE includes a clear liquid diet for the 4–6h previous to the examination, as well as the administration of 1000–2000cc of an oral contrasting agent, usually over 45–60min, followed by intravenous contrast during image acquisition.2 Oral contrast is used in order to accomplish bowel distension and maximize the contrast between the lumen and the bowel wall.2 CT enteroclysis, where oral contrast is administered through a nasojejunal tube, allows for superior jejunal distension,3 but poor patient tolerance and low efficiency limits its use.2 A number of oral neutral contrasting agents are available, including methylcellulose, polyethylene glycol, manitol, low-density barium solution and water alone – the latter is rapidly absorbed, resulting in poor distension, particularly in the distal ileum, and may contribute to fluid overload.2 For the intravenous contrast, 100–150cc of an iodine-based contrasting agent is used, and as in regular CT, caution should be employed in the prevention of iodine nephropathy, particularly in diabetic and elderly patients.2,4 A prokinetic, such as metoclopramide (10–20mgpo), is often administered at the start of the oral contrasting agent ingestion, and hyoscine butylbromide (20mgiv) at the start of the intravenous contrasting agent in order to reduce peristalsis imaging artefacts.2
MRE was more recently made available to clinicians, but the same core principles apply in regards to CTE. After a 4–6h fast, 1000–2000cc of a biphasic water-based oral contrast similar to the ones used during CTE is administered, often with metoclopramide (10–20mgpo), followed by a gadolinium-based intravenous contrast (0.65mg/kg) together with either hyoscine butylbromide (20mgiv) or glucagon (0.5mgiv).5,6
Small bowel capsule endoscopy (SBCE) was first unveiled in 2001.7 For the procedure, ESGE guidelines support the indication for a clear liquid diet on the day preceding the exam, as well as a 12h fast.7 A purgative bowel preparation (commonly using 1000–3000cc polyethylene glycol-based solutions) is often administered before capsule ingestion as it was shown to significantly improve the diagnostic yield (OR 1.68; 95% CI 1.16–2.42).8 Just as well, the antifoaming agent simethicone (300mgpo before capsule ingestion) results in a significantly better mucosal visualization by reducing the presence of air bubbles inside the intestinal lumen.8–10 To prevent incomplete SBCE, domperidone (10mgpo) has been advocated, for its use was significantly associated with a higher rate of procedure completion by reducing gastric transit time,11 despite the fact that newer capsules, with prolonged battery duration, may in the future obviate the need for prokinetics in SBCE.12
3Contraindications and complicationsImmediate complications related to CTE and MRE are rare, and most frequently associated with intravenous contrast administration. When transient physiologic responses to the contrast (e.g. localized warmth sensation) were excluded, immediate adverse reactions following intravenous contrast administration were recently reported to be as low as 0,6%13,14 in two large series of paediatric and adult populations, and resolved without complications after prompt treatment administration (corticosteroids or epinephrine) in the vast majority of the cases. Moreover, severe adverse effects are rare (<1 in 1000),13,14 and a large scale Japanese study showed no deaths attributed to adverse reactions to intravenous fluid injection in 170,000 patients.15 Nevertheless, history of asthma, allergy requiring medication or previous allergic reaction to the contrast should alert the clinician to either avoid administration of the contrast or consider the use of premedication with corticosteroids, as well as having the equipment for resuscitation made available.15 Contrast nephropathy occurs in 1.6–2.3% of the patients,16 but this risk is sharply increased in patients with impaired renal function, diabetes or elderly age.
Delayed complications may occur in up to 50% of the patients,2,17 including skin rash, fever, musculoskeletal pain, nausea, vomit, while very late adverse reactions are rare but significant, such as iodine-based thyrotoxicosis and gadolinium-associated nephrogenic systemic fibrosis. Cumulative ionizing radiation exposure constitutes another complication of CTE. In fact, up to 2% of the neoplasia incidence may be due to radiation, and the lifetime risk of neoplasia resulting of CT procedures is 2–4 in 10,000 patients.18 This risk is dose but also age-related, with the paediatric population representing the highest risk group, and patients aged over 40 having a very low risk of future CT radiation implications.19
With capsule endoscopy, the most frequently observed complication is capsule retention, defined as the presence of the capsule within the patient's bowel after 14 days of capsule ingestion, with an overall incidence of 1–2%.20 Such risk is very low among healthy volunteers, as well as in patients presenting with OGIB or suspected CD, but increased in patients with history of NSAID consumption, abdominal surgery or radiation, and particularly in patients with established CD or small bowel neoplasias, in which the incidence of retention may reach 13% and 25%, respectively.7,20,21 Other complications, such as bowel perforation or capsule aspiration, are exceedingly rare.22,23
Current capsule endoscopy guidelines consider the following as contraindications to capsule endoscopy: pregnancy, suspected bowel obstruction, swallowing disorders and imminent MRI procedure.7,21 Nevertheless, there is a possibility for endoscopic placement of the capsule in the duodenum for patients with swallowing disorders,21 which should also be considered in patients with previous gastric surgery (e.g. partial gastrectomy with Billroth II anastomosis)24 and patients with delayed gastric emptying.21 Additionally, no complications were reported in pregnant women submitted to SBCE during the first trimester,7 and in certain settings, SBCE was proven to be safe and feasible even in patients with known small bowel stenoses.25 Finally, paediatric age21 and pacemakers or implantable cardioverter defibrillators7,26 are no longer considered contraindications to SBCE.
Pregnancy is also a contraindication to CTE, not only secondary to ionizing radiation,18 but because iodinated contrast may depress neonatal thyroid function,27 as well as to MRE, due to concerns regarding the teratogenic potential of gadolinium.27 Other contraindications to CTE include known allergy to iodine-based contrast14 and impaired renal function18; young age, particularly in the setting of inflammatory bowel diseases or Peutz-Jeghers syndrome, where multiple small bowel evaluations are warranted throughout a lifetime, may also constitute a relative contraindication to CTE.28,29 MRE is contraindicated in patients with metallic foreign objects and depressed renal function,30 but may be used in claustrophobic and younger patients if light sedation is available.30
4Inflammatory bowel diseaseBoth cross-sectional imaging and SBCE have uses in a multitude of diseases, but nowhere is the clinical decision of which technique to employ more frequent and influential than on patients with inflammatory bowel diseases.
CTE exhibits high spatial resolution, allowing for accurate imaging of mural and extra-luminal diseases, especially when a multidetector row is used.2,4 The sensitivity for CTE in the context of established CD is up to 90%,31,32 and typical radiological findings include mucosal hyperenhancement, mural thickening (>3–4mm is considered abnormal, and up to 2cm may be observed in CD), ulceration, mesenteric inflammation and engorgement of vasa recta (resulting in the classical “comb sign”)2,33; extra-luminal CD findings, such as abdominal abscesses and fistulae may also be observed and characterized.2 Likewise, MRE allows for the diagnosis of both intestinal and extra-intestinal abnormalities in CD, and the radiological findings are similar between the two techniques. MRE, in particular, exhibits a very high sensitivity and specificity (up to 84% and 100%, respectively) in the evaluation of intra-abdominal fistulae.4 A common concern among CD patients submitted to CTE is the cumulative radiation exposure, particularly as these patients are often of younger age and present with multiple relapses of disease activity.4
CD is one of the main indications for SBCE, both for diagnosis as well as for known disease. It allows for mucosal evaluation of the entire small bowel, and for the detection of CD lesions such as villous oedema, erosions, ulcers and stenoses.7 SBCE has been shown to display a high sensitivity for CD, significantly superior to other diagnostic modalities, including both CTE34 and MRE,35 particularly for proximal and superficial lesions,35,36 resulting in a very high negative predictive value, ranging from 96 to 100%.37 In fact, SBCE has recently been shown to be equivalent to ileocolonoscopy in the diagnostic yield of small bowel CD.38 Furthermore, inflammatory activity on the proximal small bowel detected on SBCE has been shown to have a significant impact on disease course and was independently associated with a significant risk of relapse,39 and directly contributed to changes in the therapeutical approach in up to 30% of patients with known CD.40 Inflammatory activity scores such as the CECDAI (or Niv Score)41 and the Lewis Score42–44 have been developed and validated in order to quantify inflammation severity, extent and distribution.41,43
Nevertheless, when compared to both CTE and MRE, SBCE presents a lower specificity,33,45 in part due to the fact that up to 20% of healthy subjects may present small bowel erosions during SBCE,46 but also because small bowel lesions akin to CD may be encountered in other entities, such as during non-steroidal anti-inflammatory drug use.47 Additionally, SBCE in the setting of established CD is usually limited to patients with non-stricturing non-penetrating diseases, although recent evidence suggests that the capsule may be able to traverse CD small bowel stenoses in the majority of the patients.25 The use of the patency capsule, a device with dissolvable components precluding the occurence of obstruction, identifies patients with an increased risk of SBCE retention, and it is currently recommended by the ESGE guidelines before performing SBCE in patients with established CD.47
In patients with suspected CD with a negative ileocolonoscopy, current ECCO48 and ESGE47 guidelines consider SBCE to be a first-line examination in the absence of obstructive symptoms, whereas in such patients, cross-sectional imaging should be preferred. On the other hand, in patients with established CD, cross-sectional imaging, with the potential to assess both intestinal and extra-intestinal disease, should be the modality of choice to evaluate the small bowel, preferably MRE due to absence of ionizing radiation. SBCE should be reserved to patients with unexplained symptoms or OGIB, when MRE/CTE are inconclusive.
5Obscure gastrointestinal bleedingOGIB has been defined as bleeding of unknown origin that persists or recurs after a negative initial endoscopy study (esophagogastroduodenoscopy and colonoscopy).49 OGIB may be responsible for up to 5% of all gastrointestinal haemorrhage,50 and in the vast majority of the cases, originates within the small bowel.7 Currently, SBCE is considered to be the first-line investigation in patients presenting with OGIB,47 demonstrating very high sensitivity and specificity (up to 95% and 75%, respectively) when compared to the gold standard of intraoperative enteroscopy,51 and resulting in clinical management changes in two thirds of the patients.52
The diagnostic yield of SBCE in OGIB has been reported to be up to 60% in recent meta-analysis and systematic reviews,20,53 significantly superior to other diagnostic modalities, such as push-enteroscopy (56% vs. 26%)54 and angiography (53% vs. 20%)55 and non-inferior to double balloon enteroscopy (62% vs. 56%).53 Independent risk factors associated with an increased diagnostic yield of SBCE in OGIB include the presence of overt OGIB,56 shorter interval between presentation and the procedure,57 recurrent OGIB with >6 months of duration or more than one bleeding episode,21 advanced age50 and antithrombotic use.58 The diagnostic yield may be further improved by the use of chromoendoscopy techniques, such as the Flexible Spectral Imaging Colour Enhancement (FICE, Fujinon Corporation®, Saitama, Japan), incorporated in Given Imaging® (Yoqneam, Israel) software, that enhance surface patterns to better observe mucosal lesions,59–61 and the use of such techniques should be considered in patients where a strong suspicion for small bowel abnormalities remain despite a negative SBCE.
Nevertheless, a negative SBCE does not always preclude important small bowel lesions, and while OGIB recurrence has been shown to be less likely in this setting,62 a recent study reported rebleed rates of up to 25% during long-term follow-up.63 In these patients, further investigation may be warranted, and CTE presents as a valid alternative. Studies have shown that SBCE is associated with an increase in diagnostic yield of 20–40%64,65 when compared to CTE, and this advantage was even more pronounced in superficial lesions with no luminal repercussion, such as vascular malformations,65 the most frequently observed origin of OGIB (20–55%).66 However, CTE may be the superior diagnostic modality in some cases, particularly during massive overt OGIB, where SBCE may be unable to locate or define the origin of the bleeding,67 and in patients with small bowel tumours.68
Few studies have reported on the usefulness of MRE in the context of OGIB, but its use it limited by a lower spatial resolution than CTE,5 reduced availability in the urgent setting,6 and cost.69 When compared to SBCE, MRE demonstrated a significantly inferior diagnostic yield (53% vs. 21%).70
6Small bowel tumours and Peutz-Jeghers syndromeSmall bowel tumours are rare, accounting for less than 5% of gastrointestinal neoplasias and less than 0.5% of all tumours.5,7,71 The most common indication for investigation in patients presenting with small bowel tumours is OGIB, in 70 to 90% of the cases.7 In fact, small bowel tumours are responsible for OGIB in 10–20% of the patients, second in frequency to vascular malformations, and ahead of CD (2–10%) and NSAID enteropathy (5%).66 Moreover, in patients under 40 years, small bowel tumours overcome vascular malformations as the leading cause of OGIB originating in the small bowel.66
Nevertheless, a low prevalence of small bowel tumours, coupled with their predominant location in the jejunum, nonspecific presentation of OGIB, abdominal pain and weight loss, frequently leads to delayed investigation and advanced neoplasia at diagnosis.71
The advent of SBCE allowed a paradigm shift, and recent studies have shown SBCE to detect small bowel neoplasia in patients with a previous work-up of 2–4 procedures,71 and impact management decision in 55–80% of the cases.71,72 There are, however, limitations to SBCE in the diagnosis of small bowel tumours: SBCE exhibits a false negative rate of up to 66% in the proximal small bowel or in submucosal lesions, even in large and protruding lesions,73 because of limited field of vision, folds and loop angulations, poor bowel preparation, rapid transit time, non-continuous image capture and incomplete examination; additionally, SBCE is unable to adequately characterize both the size and the location of the tumour,21,73 and presents a retention rate of up to 25% in such patients.7
CTE sensitivity and specificity for small bowel tumours have been reported to be up to 93% and 99%, respectively.2,74 Furthermore, CTE is able to adequately locate and characterize both location, size, extra-intestinal invasion and metastatic disease (lymphatic and disseminated).74 In a recent prospective study, CTE with a 64-section multidetector row demonstrated a superior diagnostic yield when compared to SBCE (88 vs. 38%), and this difference largely resulted from the detection of 100% of small bowel tumours compared to only 33% observed with SBCE,68 and these results were replicated in other published reports.2,5,74 As a result, CTE should be considered as a valid alternative to SBCE as a first line diagnostic procedure in younger patients presenting with OGIB, a population where small bowel tumours are the most prevalent finding.66
The use of MRE for small bowel tumour diagnosis is limited due to its lower spatial resolution and susceptibility for movement artefacts, but, similarly to CTE, it allows for the tumour characterization, as well as the assessment of metastatic disease.5,6
Peutz-Jeghers syndrome (PJS) is an autosomal dominant condition presenting with mucocutaneous pigmentation and gastrointestinal hamartomatous polyps.29 Almost 90% of PJS patients present with small bowel polyps, and the chief indication for small bowel surveillance is the significantly increased risk of intussusception,75 particularly in larger polyps76 – by age 20, intussusception has occurred in half the patients with PJS, and, in the majority of them, presented with acute abdomen requiring surgical approach.77In the European Mallorca consensus of 2007, the decision was made to use SBCE in the screening and surveillance of PJS every 2–3 years after 8 years of age and in symptomatic patients,76 and these recommendations were adapted and incorporated in the recently published ACG guidelines on the management of hereditary gastrointestinal cancer syndromes, allowing for the second small bowel SBCE to be delayed until the age of 18, if the first SBCE detected no polyps, and no symptoms developed.77
Nevertheless, SBCE has been shown to miss the detection of up to 20–40%78,79 of PJS small bowel polyps, including large polyps78 as well as those in the proximal small bowel.79,80 Recently, a number of authors have reported superior capabilities of SBCE versus MRE in polyps <10mm,81 equivalence of both techniques in polyps 10–15mm,81,82 but crucially, MRE superiority in diagnostic yield, as well as better size and location estimation, in larger small bowel polyps.29,47,81,82 This advantage of MRE was not observed in another prospective study, where a polyp of 30mm was missed by MRE, and identified during SBCE.83 Thus, current ESGE guidelines consider small bowel surveillance to be adequate with both SBCE and MRE, depending on availability and local expertise.47
No role exists for CTE in PJS, due to the repeated need for small bowel assessment in young patients, resulting in an unacceptable ionizing radiation exposure.29
7ConclusionIn conclusion, SBCE remains the first-line examination for OGIB, and plays a key role in the diagnosis of CD, as well as the surveillance of PJS. Its use on small bowel tumours and established CD, however, should be reserved to selected cases due to the risk of retention and inability to precisely define location and extra-intestinal involvement of either pathology.
CTE has proven to be the most effective modality in the study of small bowel tumours, and may be used in the diagnostic work-up of OGIB in patients where this diagnosis is more likely to occur. CTE should also be considered both in suspected and established CD, but ionizing radiation exposure should pose concerns, particularly in younger patients.
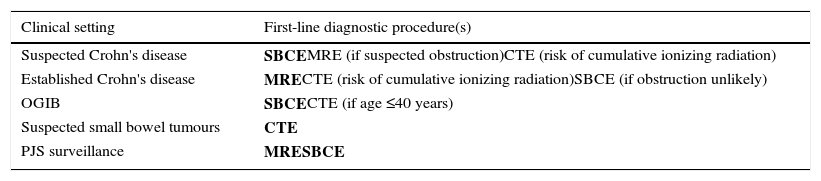
Finally, the importance of MRE in the study of CD is growing as a non-invasive non-ionizing technique with the possibility to characterize both mucosal injury and penetrating disease, and provides an alternative to SBCE in PJS patients. Nevertheless, local expertise and availability is asymmetric, and a limitation to the broad use of this technique. Table 1 summarizes the main indications for SBCE, CTE and MRE, as well as the first-line modalities for each clinical setting.
First-line diagnostic procedure(s) for the investigation of the small bowel.
| Clinical setting | First-line diagnostic procedure(s) |
|---|---|
| Suspected Crohn's disease | SBCEMRE (if suspected obstruction)CTE (risk of cumulative ionizing radiation) |
| Established Crohn's disease | MRECTE (risk of cumulative ionizing radiation)SBCE (if obstruction unlikely) |
| OGIB | SBCECTE (if age ≤40 years) |
| Suspected small bowel tumours | CTE |
| PJS surveillance | MRESBCE |
In clinical practice, SBCE, CTE and MRE are often not competitive but synergistic techniques; the knowledge of their characteristics, strengths and limitations, indications and contraindications, as well as the adaptation to local availability and expertise, is crucial to select the best sequence of examinations for each specific situation, in order to optimize diagnostic algorithms and ultimately clinical management and outcomes.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors declare no conflict of interests.