The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was responsible for the outbreak of the 2019 coronavirus disease (COVID-19), which is now considered as a pandemic. The prevention strategies adopted have included social distancing measures and the modification, reduction or interruption of a large proportion of routine healthcare activity. This has had a significant impact on the care provided in Gastrointestinal Motility Units. Having passed the peak, in terms of mortality and infections, a gradual reduction in transmission figures has been observed in Spain and other European countries. The risk of reactivation, however, remains high, so it is necessary to have a plan in place that allows healthcare centres to safely resume, for their patients and professionals, instrumental examinations linked to the management of motor pathology.
Based on the available scientific evidence and the consensus of a panel of experts, the Spanish Association of Neurogastroenterology and Motility (ASENEM) has drawn up a series of practical recommendations, which have been adapted to the risks inherent in each activity. These include individual protection proposals, as well as organisational and structural measures, which are conceived to allow for the gradual resumption of examinations while minimising the possibility of contagion.
El coronavirus del síndrome respiratorio agudo severo tipo 2 (conocido por sus siglas en inglés, SARS-CoV-2) ha sido responsable del brote de la denominada enfermedad por coronavirus de 2019 (COVID-19), que ha llegado a tener la consideración de pandemia. Las estrategias adoptadas para su prevención han incluido medidas de distanciamiento social, así como la modificación, reducción o interrupción de gran parte de la actividad sanitaria habitual. Esto ha afectado de forma muy notable a la asistencia prestada en las Unidades de Motilidad Digestiva.
Superado el pico de mortalidad y contagios por la infección, se ha observado durante las últimas semanas en España y otros países europeos, una reducción paulatina en las cifras de transmisión. Sin embargo, el riesgo de reactivación sigue siendo alto, por lo que es necesario disponer de una planificación que permita a los centros sanitarios reiniciar con seguridad para pacientes y profesionales, las exploraciones instrumentales vinculadas al manejo de la patología motora.
La Asociación Española de Neurogastroenterología y Motilidad (ASENEM) ha elaborado una serie de recomendaciones prácticas basadas en la evidencia científica disponible y en el consenso de un panel de expertos, y adaptadas a los riesgos inherentes a cada actividad. Se incluyen propuestas de protección individual, pero también medidas organizativas y estructurales, cuyo objetivo es permitir reanudar progresivamente las exploraciones, minimizando la posibilidad de contagio.
The COVID-19 pandemic has required a redistribution of healthcare resources and implementation of severe social distancing measures. These demands have altered or even disrupted a great deal of usual healthcare activity. Although a sustained improvement in the propagation of the infection has been seen in recent days, there is not yet any fixed date for completely ending lockdown restrictions and returning to what was normal before the pandemic began. However, delaying procedures for diagnosing and treating other diseases, such as gastrointestinal disorders, until the risk of contagion disappears completely is not an option.
In this scenario, the Spanish Association of Neurogastroenterology and Motility (ASENEM), formerly the Spanish Gastrointestinal Motility Group (GEMD), feels it is appropriate to provide physicians with a number of recommendations based on experience and the available scientific literature, to ensure that activity in our specialisation resumes gradually and is as safe as can be for patients, healthcare professionals and the community at large.
These recommendations contain structural and organisational measures that must be subjected to regular evaluation, as they may be modified depending on the changing course of the pandemic and the epidemiological parameters available in each period and geographic location.
MethodologyA number of clinical questions were posed to a panel of expert physicians from the Spanish Association of Neurogastroenterology and Motility (ASENEM), who were asked to answer the questions through recommendations for management based on the best scientific evidence available. Where this evidence did not exist, the participating authors were asked to provide an answer consistent with their experience. Subsequently, a first draft was prepared with all the information collected. Matters with discrepancies or greater uncertainty were debated remotely until the panel established a set of consensus recommendations.
Need for restarting activityNeurogastrointestinal diseases and diseases of gastrointestinal motility encompass, among other things, serious gastrointestinal motility diseases, such as achalasia, gastroparesis, chronic bowel pseudo-obstruction, megacolon and serious congenital diseases such as Hirschsprung disease. These diseases may have serious consequences for the health of the individual, both directly and due to complications deriving from them. Although the study of these diseases, as well as the preoperative and postoperative evaluation of other conditions, need not be done on an emergency basis, except in rare cases, they also should not be delayed excessively, so as to prevent the development of serious complications and stop the deterioration of quality of life linked to these diseases.
Need for developing special recommendationsThe SARS-CoV-2 virus, responsible for COVID-19, has proven to be highly contagious, and to cause severe respiratory distress in up to 10%-15% of cases, with a mortality rate in Spain of around 10% of confirmed cases.1
Transmission of the virus essentially occurs via the respiratory tract, through expulsion of microdroplets when speaking (range of 1−2metres), coughing or sneezing (range of up to 8m), and is even more likely when under these or other circumstances (orotracheal intubation, bronchoscopy or use of nebulisers) aerosols are generated. Some published studies have found that the virus is stable at a pH of 3–10, and may remain in the environment in aerosol form for three hours. It has been suggested that transmission occurs not only through respiratory secretions and saliva, but also direct contact with contaminated surfaces (on stainless steel and plastic it is detected up to 5–7 days following experimental inoculation). Furthermore, the virus’s RNA has been detected in faeces, although to date faecal–oral transmission has not been demonstrated.2–5
The percentage of healthcare staff infected in Spain, relative to all confirmed cases, is around 22%.3 Whether the risk is in relation to direct patient contact and/or improper use of protective equipment is unknown.
Gastrointestinal motility, anorectal biofeedback and gastro-oesophageal reflux studies and breath tests are likely to cause contamination, either due to aerosols which may be generated in some examinations with oral or nasal intubation, or to direct contact with materials and tubes with patient secretions.
Assessing the patient’s condition with regard to SARS-CoV-2In order to assess the risk associated with a diagnostic test, it is important to be familiar with the patient’s situation with respect to COVID-19. In this regard, four identifiable types of patient may be encountered:
- •
Patients with a known current infection (a positive polymerase chain reaction [PCR] test result) or suspected current infection (fever, cough, difficulty breathing or recent fatigue, anosmia, or acute diarrhoea). These patients carry a very high risk of contagion.
- •
Patients who are asymptomatic but have recently been in close contact with an infected subject (living with an individual who had the infection in the past two weeks).
- •
Patients who are likely to have immunity, as they have recovered from the disease and/or have a test result showing that they have immunity against SARS-CoV-2 (specific IgG with negative IgM). In these cases, the risk of contagion would be virtually zero. However, to date, the exact extent and duration of immunity are unknown; hence, at the time of writing, it is recommended that precautions also be taken in these patients.
- •
Patients whose immunological status against the infection is unknown and who are not aware of having, or having had, COVID-19. As long as mass population testing is not done, this will be the most common type of patient, though this scenario will change as the situation unfolds.
Experts from the Johns Hopkins Center for Health Security1 have proposed that healthcare services as well as social and occupational services be reopened gradually, in four phases:
- 1
Phase 1: the mitigation phase. During this phase, maximum social distancing (lockdown and closure of public spaces) is needed in order to slow rates of infection transmission. This prevents the collapse of healthcare systems, helping them to adapt and improving their capacity for performing as many diagnostic tests as possible. The objectives are to diagnose and treat all cases and, secondarily, to actively search for infection carriers and those in close contact with them.
- 2
Phase 2: In this phase, reopening of services begins, social distancing is maintained (1−2m) and meetings are limited. Initiating this phase may be considered if the following criteria are met:
- a)
The number of new cases has been decreasing on a daily basis over the course of the past 14 days.
- b)
Rapid diagnostic tests are sufficient for investigating at least all symptomatic patients, individuals in close contact with them and essential service workers.
- c)
The healthcare system is capable of safely caring for patients; this includes having sufficient personal protective equipment (PPE) for workers.
- d)
The national health system is capable of contact tracing that identifies those in close contact with new cases.
- 3
Phase 3: This is started when an effective treatment or vaccine is available. The social distancing rules in the prior phase are relaxed.
- 4
Phase 4: In this phase, health policies for addressing future threats similar to the current one should be established.
The recommendations set out below are applicable as of Phase 2 of this classification.
RecommendationsGeneralHow should healthcare activity be restarted?- •
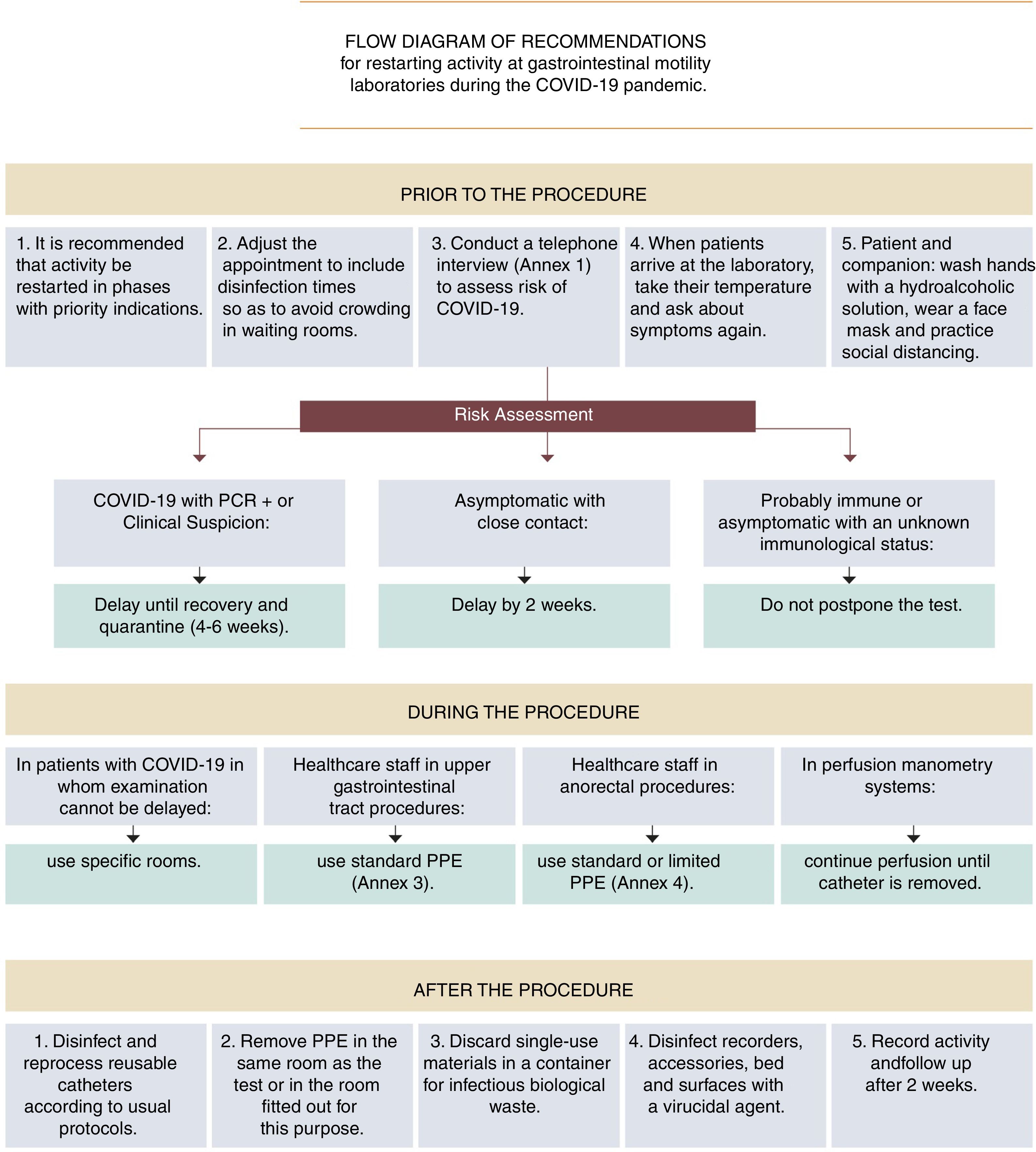
It is recommended that activity in gastrointestinal motility testing be restarted in a manner that is staggered and adapted to the SARS-CoV-2 infection epidemiological situation.
Due to the temporary suspension of examinations during Phase 1 of the pandemic, initial demand could exceed healthcare supply; hence, it may prove necessary to prioritise requests based on the estimated severity of signs and symptoms (Table 1 and Fig. 1).
ASENEM recommendations for resuming activity at gastrointestinal motility laboratories during Phase 2 of the COVID-19 pandemic.
| RECOMMENDATIONS |
|---|
| It is recommended that activity in gastrointestinal motility testing be restarted in a staggered manner adapted to the SARS-CoV-2 infection epidemiological situation. |
| It is recommended that motility examinations with an appropriate indication NOT be postponed indefinitely. |
| For the moment there is NO conclusive scientific evidence that supports pre-procedure microbiological testing for the diagnosis of SARS-CoV-2 with a view to modifying personal protective measures against the infection. |
| PRIOR TO THE PROCEDURE |
|---|
| It is recommended that clinical risk of COVID-19 infection be systematically assessed before any motility study is performed. |
| In patients with current COVID-19 infection, whether confirmed by microbiological testing or simply suspected (consistent signs and symptoms without microbiological confirmation), it is recommended that the test be delayed until the lapse of at least four weeks from the onset of symptoms, regardless of any negative PCR test results before the full four weeks have elapsed. |
| In asymptomatic patients who have had close, prolonged contact with an infected individual without the proper protection in the past two weeks, it is recommended that the test be delayed until at least two weeks have elapsed since the date of the risk contact. If during this period the patient shows symptoms, the postponement should be lengthened to four weeks from the onset of symptoms. |
| In cases of patients who probably have immunity (patients who have recovered from the disease and have passed the infection risk period, and asymptomatic patients in whom testing reveals IgG immunity against SARS-CoV-2 and IgM negativity), postponing the appointment for the test is NOT indicated. |
| In cases of asymptomatic patients whose immunity is unknown, postponing the appointment for the test is NOT indicated. |
| It is recommended that the time allocated for the procedure be adjusted, taking into account the measures needed to prevent COVID-19 infection. This will probably entail increasing the time allocated to each procedure and reducing the total number of procedures. |
| Tell patients exactly what time they must arrive at the motility unit, to avoid crowds in the waiting room. |
| Inform patients that they are to come alone. Should patients need to be accompanied, each patient should have only one companion, preferably under the age of 60, with no high-risk comorbidities and no signs suggestive of COVID-19. |
| Provide patients and their companions with means to wash their hands with a hydroalcoholic solution when they enter the waiting room and when they leave the motility unit. |
| Both patient and companion should wear a face mask as long as they are at the hospital facilities. |
| To the extent possible (for example, during anorectal examinations), it is advised that patients also keep their face mask on throughout the entire performance of the test. |
| It is recommended that all healthcare and administrative staff on the unit not directly involved in the procedure wear a face mask and wash their hands regularly. |
| No healthcare professional with symptoms consistent with COVID-19 should perform motility examinations. |
| No healthcare working having been in close, prolonged contact with a COVID-19 patient without suitable protection (e.g. in a family environment) should perform motility examinations until after the quarantine period has elapsed, or until their capacity to transmit the virus has been ruled out by means of microbiological testing. |
| In the waiting room, spaces and seats should be spread out so as to maintain a safe distance between individuals of at least 1.5m (ideally two metres). |
| In the waiting room there should be no magazines or other objects that might act as fomites. |
| Take all patients’ temperature before entering the motility laboratory. For patients with a temperature in excess of 37.2€°C, except in cases of special emergencies, the examination should be suspended and the appointment should be rescheduled for a time after the appropriate periods have elapsed and/or the diagnostic tests indicated have been performed to ensure that the infectious period has passed. |
| It is recommended that patients be asked about any onset in the past few hours (since the telephone call for screening) of symptoms suggestive of COVID-19 before they access the motility laboratory, and that similar action to that specified in the previous section be taken should patients reply affirmatively. |
| DURING THE PROCEDURE |
|---|
| Motility tests are done on an emergency basis only in rare cases; hence, it is recommended that in cases of SARS-CoV-2 infection they be delayed until the infection has been cured and the recommended quarantine period has elapsed. |
| In the rare event that a motility procedure cannot be delayed in a patient with suspected or confirmed COVID-19 infection, it is recommended that the procedure be performed in an endoscopy room or operating theatre fitted out for this purpose (ideally, a negative-pressure room), in accordance with the same recommendations as in patients with COVID-19 infection who undergo emergency endoscopy. |
| Encourage implementation of basic hygiene measures in order to prevent healthcare staff from becoming infected. |
| Use of personal protective equipment (PPE) by all healthcare staff involved in an endoscopic procedure |
| Minimise the risk of the patient accidentally coughing on the examiner or other staff member by adjusting the bed such that the top of the patient’s head is below the examiner’s chin, and by avoiding standing in front of the patient when placing the catheter. |
| In all upper gastrointestinal motility procedures standard PPE is advisable. |
| In manometry and anorectal biofeedback, wearing a surgical face mask, two pairs of gloves and a non-waterproof coat may be sufficient. If defecatory manoeuvres are performed, it is advisable to add eye protection with goggles or a face shield and to opt for a FFP2−3 or N95 face mask. |
| It is recommended that training periods for medical residents be maintained, with application of the recommended personal protective equipment (PPE) and safety distance measures. |
| AFTER THE PROCEDURE |
|---|
| Disinfection and reprocessing of reusable catheters in motility examinations is to be carried out according to the usual protocols. |
| When perfusion catheters are used for manometry, it is advisable to maintain perfusion once the catheter has been removed from the patient, and to avoid, as far as possible, opening the water pump during the procedure. |
| Disconnect the pH meter or impedance pH meter probe from the recorder before removing it, to make it easier to quickly discard it in a plastic bag. |
| The pH meter or impedance pH meter, as well as the cover and the strap, must be left in a container to be disinfected using a 70% alcohol solution or another virucidal agent before the data dump is performed. |
| Disinfect the bed, railings, and other elements that have come into contact with the patient or the examiner with the virucidal agents recommended by the Spanish Ministry of Health. |
| Avoid sharing tools (telephones, computers, writing materials, etc.), or disinfect them regularly before and after using them. |
| Healthcare staff should take off their PPE in the same room in which the procedure was performed, or in a room specifically fitted out for this purpose, depending on availability. |
| Single-use materials should be discarded in a container for biological waste considered to be infectious (Category B, UN 3291). |
| Bed linen should be treated as high-risk textiles and stored in a sealed plastic bag, if the patient has confirmed or strongly suspected COVID-19. |
| It is advisable to contact the patient 15 days after the procedure and assess their COVID-19 infection status, in order to monitor any possible nosocomial transmission (contact tracing). |
| It is advisable to keep a record of activity and ensure that this is being done safely. |
| SPECIAL CONSIDERATIONS IN PARTICULAR PROCEDURES |
|---|
| Exhaled hydrogen and breath testing for Helicobacter pylori |
| It is recommended that protocols for acquisition, management and analysis of samples be reviewed and, where applicable, modified to reduce the risk of direct transmission by aerosols and to prevent contamination of equipment and adjacent areas. |
| During sample collection, extreme measures, depending on local methodology and characteristics, must be taken to prevent aerosol propagation. |
| Once samples are received, it should be ensured that they are stored in airtight containers and transported safely to the laboratory for testing. |
| Exhaled breath measurement is to be done according to the usual protocol. |
| Discarded materials must be deposited in a specific container for processing of potentially contaminated biological products. |
| It is recommended that any staff who instruct patients in taking samples wear an FFP2 (N95) or FFP3 face mask and gloves. |
| The staff who process the sample and the staff who remain with patients during the exhalation manoeuvres should wear standard PPE. |
| Once the analysis has been completed, the same disinfection measures recommended for motility tests must be taken. |
| Instrumental studies in oropharyngeal dysphagia |
| These have a high potential for generating aerosols; hence, it is recommended that the same protective measures as for upper gastrointestinal tract motility procedures be taken. |
- •
It is recommended that motility examinations with an appropriate indication NOT be postponed indefinitely.
In Phase 1 of the pandemic, motility laboratories were closed. For the reasons set out in point 2, they should gradually reopen in Phase 2, with application of appropriate safety measures, which are described below.
Prior to the procedureShould all patients be screened for SARS-CoV-2 infection prior to undergoing a motility procedure?- •
It is recommended that clinical risk of COVID-19 infection be systematically assessed before any motility study is performed.
It is proposed that a telephone call be made prior to the appointment (and as close as possible to the appointment itself) to determine the patient’s COVID-19 status, asking about symptoms commonly associated with COVID (recent fever, cough, dyspnoea or fatigue and anosmia), enquiring as to potential recent instances of close constant (the past two weeks) and finding out whether the patient has had PCR or immunological tests against SARS-CoV-2 (Annex 1).
- •
For the moment, there is NO conclusive scientific evidence that supports pre-procedure microbiological testing for diagnosis of SARS-CoV-2 infection with a view to modifying personal protective measures against the infection.
A recent study, based on a mathematical model, concluded that performing PCR on all candidate patients for an endoscopy (risk of virus transmission similar to motility tests) could be a useful strategy for more efficient use of PPE when the prevalence of COVID-19 is reduced. However, it was admitted that the risk of contagion among healthcare workers increases when prophylactic measures are not taken, and that this may be efficient in a private healthcare system, but is socially unacceptable.4
PCR’s sensitivity for the diagnosis of COVID-19 has been estimated at 66.7% in symptomatic patients in the first week and 54% in the second week. Other more recent, not-yet-published studies could raise this figure to 78%, whereas specificity in symptomatic individuals could be close to 99%, and negative predictive value could be 96%. Nonetheless, the percentage in asymptomatic carriers remains uncertain, as the actual number of infected individuals in the population is not known, and all percentages would be dependent on variations in prevalence.5–13 Considering these data, it is not possible to ascertain that PCR is a sufficiently precise tool for determining those situations in which PPE should be used. Moreover, there is a risk of identifying asymptomatic patients who do not really have an infection as positive, which would result in a need for isolation of the patient and those close to the patient.
Serology and antigen-detection tests have low sensitivity in the initial stages of the disease, even in symptomatic patients, compared to PCR. Furthermore, antigen detection becomes negative before PCR does.14,15
How should appointments be scheduled according to the information gathered in screening for the infection?- •
In patients with current COVID-19 infection, whether confirmed by microbiological testing or simply suspected (consistent signs and symptoms without microbiological confirmation), it is recommended that the test be delayed until the lapse of at least four weeks from the onset of symptoms, regardless of any negative PCR test results before the full four weeks have elapsed.
COVID-19’s symptomatic period usually lasts around 10 days. Once the symptomatic period has passed, shedding of viral RNA is detected for a further 10 days in 90% of patients, but is no longer detected after 15 days in nearly 100% of patients.6,7
In general, it is recommended that the appointment for the motility procedure be delayed four weeks from the onset of symptoms, or from a positive result for any microbiological testing indicating a current infection, if the test was performed in an asymptomatic individual and the disease did not subsequently develop. In patients who have required hospitalisation, it is probably prudent to postpone the appointment for the procedure until six weeks have elapsed since the onset of symptoms (virus excretion has been reported in such cases up to 37 days later).6,9
Some patients have shown persistent positive PCR results in faecal samples following negative results for samples taken from the respiratory tract.10,11 Moreover, a negative result on PCR diagnostic tests lacks sufficient sensitivity to rule out the disease in all cases.
- •
In asymptomatic patients who have had close, prolonged contact with an infected individual without the proper protection in the past two weeks, it is recommended that the test be delayed until at least two weeks have elapsed since the date of the risk contact. If during this period the patient shows symptoms, the postponement should be lengthened to four weeks from the onset of symptoms.
Although the possibility of more prolonged incubation periods (up to 24 days) has been reported, the WHO has determined the mean SARS-CoV-2 infection incubation period to be 5.2 days, with a range of 1–14 days.5,6
- •
In cases of patients who probably have immunity (patients who have recovered from the disease and have passed the infection risk period, and asymptomatic patients in whom testing reveals IgG immunity against SARS-CoV-2 and IgM negativity), postponing the appointment for the test is NOT indicated.
- •
In cases of asymptomatic patients whose immunity is unknown, postponing the appointment for the test is NOT indicated.
A study conducted in northern Italy during the time of the pandemic’s exponential spread to patients of unknown immunological status found that performing endoscopic procedures with PPE (face masks of different types, gloves, coat and goggles) was associated with a risk of potential contagion of 1% among patients, and 4.3% among healthcare workers. This was lower than the risk of 10% stated for all other healthcare workers in the same geographic location.16
Is it necessary to modify the usual time assigned to each motility procedure?- •
It is recommended that the time allocated for the procedure be adjusted, taking into account the measures needed to prevent COVID-19 infection. This will probably entail increasing the time allocated to each procedure and reducing the total number of procedures.
The time required for disinfection will depend on the type of patient examined, the conditions for ventilating the working room, and each centre’s capacity for expeditious changes of PPE and disinfection of potential fomites. As a guideline, these actions may require 15−30min per procedure.
What other general rules should be taken into account during the appointment?- •
Tell patients exactly what time they must arrive at the motility unit, to avoid crowds in the waiting room.
- •
Inform patients that it is advisable for them to come alone. Should patients need to be accompanied, each patient should have only one companion, preferably under the age of 60, with no high-risk comorbidities and no signs suggestive of COVID.
Age over 60 years and diseases such as diabetes, obesity, hypertension, chronic bronchopathy, heart disease and diseases compromising immunity have been associated with a worse course of the disease. Therefore, affected individuals should avoid situations that may foster contagion, such as visits to hospital centres, especially if they are to spend time in crowded waiting rooms.2,6
Should any additional protective measures be recommended for patients entering the motility unit or its waiting room (Annex 2)?- •
Provide patients and their companions with means to wash their hands with a hydroalcoholic solution when they enter the waiting room and when they leave the motility unit.
- •
Both patients and companions should wear a face mask as long as they are at the hospital facilities.
- •
To the extent possible (for example, during anorectal examinations), it is advised that patients also keep their face mask on while the test is being performed.
- •
It is recommended that all healthcare and administrative staff on the unit not directly involved in the procedure wear a face mask and wash their hands regularly.
- •
No healthcare professional with symptoms consistent with COVID-19 should perform motility examinations.
- •
No healthcare working having been in close, prolonged contact with a COVID-19 patient without suitable protection (e.g. in a family environment) should perform motility examinations until after the quarantine period has elapsed, or until their capacity to transmit the virus has been ruled out by means of microbiological testing.
- •
In the waiting room, spaces and seats should be spread out so as to maintain a safe distance between individuals of at least 1.5m (ideally 2m).
- •
In the waiting room there should be no magazines or other objects that might act as fomites.
- •
Take all patients’ temperature before entering the motility laboratory. For patients with a temperature in excess of 37.2€°C, except in cases of special emergencies, the examination should be suspended and the appointment should be rescheduled for a time after the appropriate periods have elapsed and/or the diagnostic tests indicated have been performed to ensure that the infectious period has passed.
- •
It is recommended that patients be asked about any onset in the past few hours (since the telephone call for screening) of symptoms suggestive of COVID-19 before they access the motility laboratory, and that similar action to that specified in the previous section be taken should patients reply affirmatively.
- •
Motility tests are done on an emergency basis only in rare cases; hence, it is recommended that in cases of SARS-CoV-2 infection they be delayed until the infection has been cured and the recommended quarantine period has elapsed.
- •
In the rare event that a motility procedure cannot be delayed in a patient with suspected or confirmed COVID-19 infection, it is recommended that the procedure be performed in an endoscopy room or operating theatre fitted out for this purpose (ideally, a negative-pressure room), in accordance with the same recommendations as in patients with COVID-19 infection who undergo emergency endoscopy.
- •
Encourage implementation of basic hygiene measures in order to prevent healthcare staff from becoming infected.
Insist on basic measures, such as hand-washing with soap, frequent use of disinfectant solutions and uniform changes at the end of each working day.5,6,17,18
- •
Ensure that all healthcare staff involved in an endoscopic procedure use personal protective equipment.
As a general rule, it is advisable to use a coverall and shoe covers, an FFP2-3 or N95 face mask (preferably FFP3 in cases of confirmed infection), goggles and/or a face shield, two pairs of gloves, leg coverings, a cap (optional), and a waterproof coat and/or a plastic apron5,6,19–26 (Annex 3).
- •
Minimise the risk of the patient accidentally coughing on the examiner or other staff member by adjusting the bed such that the top of the patient’s head is below the examiner’s chin, and by avoiding standing in front of the patient when placing the catheter.
- •
In all upper gastrointestinal motility procedures standard PPE is advisable (Annex 4).
- •
In manometry and anorectal biofeedback, wearing a surgical face mask, two pairs of gloves and a non-waterproof coat may be sufficient. If defecatory manoeuvres are performed, it is advisable to add eye protection with goggles or a face shield and to opt for a FFP2-3 or N95 face mask (Annex 4).
The risk of generating aerosols in these examinations is minimal. Under these conditions, surgical face masks and FFP2 (N95) masks have been shown to offer similar protection against infections caused by respiratory viruses.27–31 Thus, the greatest risk in this examination would be that deriving from contact with faecal matter. Although the virus’s RNA has been detected in faeces, no cases of disease transmission by this route have been reported. Some researchers have questioned the virus’s viability in faeces and its infectious capacity in a hospital environment, suggesting only a potential role in contagion in crowded situations.11
Can medical residents continue to pursue their rotations on motility units?- •
It is recommended that training periods for medical residents be maintained, with application of the recommended personal protective equipment (PPE) and safety distance measures.
Before patients leave the room, they will be advised to wash their hands, or gloves if any, with a hydroalcoholic solution.
How should the disinfection of devices, materials and surfaces be carried out (Annex 5)?- •
Disinfection and reprocessing of reusable catheters in motility examinations is to be carried out according to the usual protocols.
There is agreement on maintaining the usual disinfection protocols for endoscopies. Manometry probes can be subjected to the same process, or to high-level disinfection with a substance in which the active components are among those with declared efficacy against the virus.32
- •
When perfusion catheters are used for manometry, it is advisable to maintain perfusion once the catheter has been removed from the patient, and to avoid, as far as possible, opening the water pump during the procedure.
When catheters are washed by hand, it is advisable to maintain perfusion during the stages of cleaning with soap and disinfection. If catheters are processed using endoscope washers, with inner disinfection of the channels, perfusion may be stopped immediately following removal.
It is advisable to fill the manometer’s water pump before starting examinations for the day, in order to prevent it from opening during a procedure, insofar as this can be prevented.
- •
Disconnect thepHmeter or impedancepHmeter probe from the recorder before removing it, to make it easier to quickly discard it in a nearby plastic bag.
- •
The pH meter or impedance pH meter, as well as the cover and the strap, must be left in a container to be disinfected using a 70% alcohol solution or another virucidal agent before the data dump is performed.
- •
Disinfect the bed, railings and other elements that have come into contact with the patient or the examiner with the virucidal agents recommended by the Spanish Ministry of Health.
The list of recommended virucidal agents is available at: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Listado_virucidas.pdf.
The most commonly used virucidal agent is a dilution of 20mL of bleach per litre of water (0.2%).17,18,26,33
- •
Avoid sharing tools (telephones, computers, writing materials, etc.), or disinfect them regularly before and after using them.
- •
Healthcare staff should take off their PPE in the same room in which the procedure was performed, or in a room specifically fitted out for this purpose, depending on availability.
- •
Single-use materials should be discarded in a container for biological waste considered to be infectious (Category B, UN 3291).34
- •
Bed linen should be treated as high-risk textiles and stored in a sealed plastic bag, if the patient has confirmed or strongly suspected COVID-19.
- •
It is advisable to contact the patient 15 days after the procedure and assess their COVID-19 infection status, in order to monitor any possible nosocomial transmission (contact tracing).
- •
It is advisable to keep a record of activity and ensure that this is being done safely.
- •
It is recommended that protocols for acquisition, management and analysis of samples be reviewed and, where applicable, modified to reduce the risk of direct transmission by aerosols and to prevent contamination of equipment and adjacent areas.
There are different methods on the market for analysing breath tests, as well as different methods for collecting samples, including glass tubes into which patients blow directly and which are stoppered for subsequent analysis, air-tight bags for storing gases, and portable analysers into which patients blow directly through specific filters.
As the breath test involves analysis of air exhaled by patients, and as there are no comparative studies at present showing the possible advantages and disadvantages of each of these methods, the recommendation is to review the protocol for performing these tests at each laboratory in order to adapt the method used to the current circumstances, taking into account two basic parameters: a) the safety of the staff collecting and analysing the sample and b) the proper disinfection of the materials and sites where the tests are performed.
What procedure should be followed with regard to taking samples?- •
During sample collection, extreme measures, depending on local methodology and characteristics, must be taken to prevent aerosol propagation.
It is advisable to have a spacious, well-ventilated room that accommodates compliance with safe distancing. Methodology permitting, patients may perform the test at home, after visiting the motility laboratory to pick up the carbohydrate (in hydrogen tests) and receive instructions on sampling. Afterwards they are to return the samples to the department for subsequent analysis.
- •
Once samples are received, it should be ensured that they are stored in airtight containers and transported safely to the laboratory for testing.
- •
Exhaled breath measurement is to be done according to the usual protocol.
- •
Discarded materials must be deposited in a specific container for processing of potentially contaminated biological products.
- •
Once the analysis has been completed, the same disinfection measures recommended for motility tests must be taken.
In disinfecting portable monitors and their intermediate tubes, the use of alcohol should be avoided as it damages the sensor. In this case, it is advisable to use alcohol-free virucidal solutions or disinfectant wipes according to the manufacturer’s instructions.
Instrumental studies in oropharyngeal dysphagia- •
These have a high potential for generating aerosols; hence, it is recommended that the same protective measures as for upper gastrointestinal tract motility procedures be taken.
No external funding was received for the preparation of this manuscript.
Declaration of conflicts of interestThe authors declare that they have no conflicts of interest with regard to the drafting of this manuscript.
We would like to thank Drs Antonio Ruiz de León, Constanza Ciriza, Concepción Sevilla (Gastrointestinal Department at Hospital Clínico San Carlos [San Carlos Clinical Hospital] in Madrid), Miguel Mínguez (Hospital Clínic Universitari de València [University Clinical Hospital of Valencia]) and Marta Gracia (Hospital Universitario Miguel Servet [Miguel Servet University Hospital] in Zaragoza) for reviewing and contributing to this document.
Please cite this article as: Alcedo J, Serra J, Pérez de la Serna J, Mas P, Barba E, Suárez JF, et al. Recomendaciones de la Asociación Española de Neurogastroenterología y Motilidad (ASENEM) para el reinicio de la actividad de los laboratorios de motilidad digestiva, tras el confinamiento por el estado de alarma decretado a raíz de la pandemia por COVID-19. Gastroenterol Hepatol. 2020;43:485–496.