Immune checkpoint inhibitors (ICIs) are effective agents against several malignancies. However, they are associated with gastrointestinal and liver immune-related adverse events (GI-IrAEs and LI-IrAEs), which can lead to their temporary or permanent discontinuation.
AimThe aim of this study was to evaluate the efficacy and gastrointestinal and liver toxicity of ICIs in oncological treatments in actual clinical practice.
Material and methodsPatients with advanced cancer who received at least 1 ICI dose between May 2015 and September 2018 were retrospectively assessed.
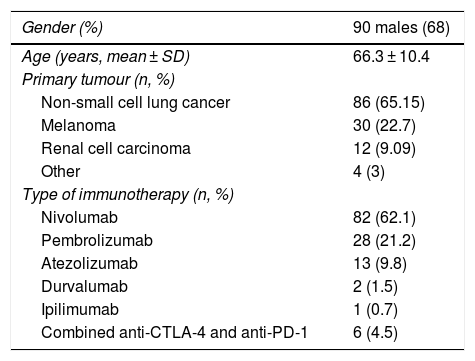
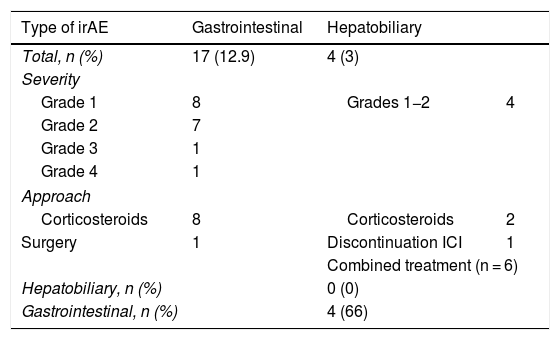
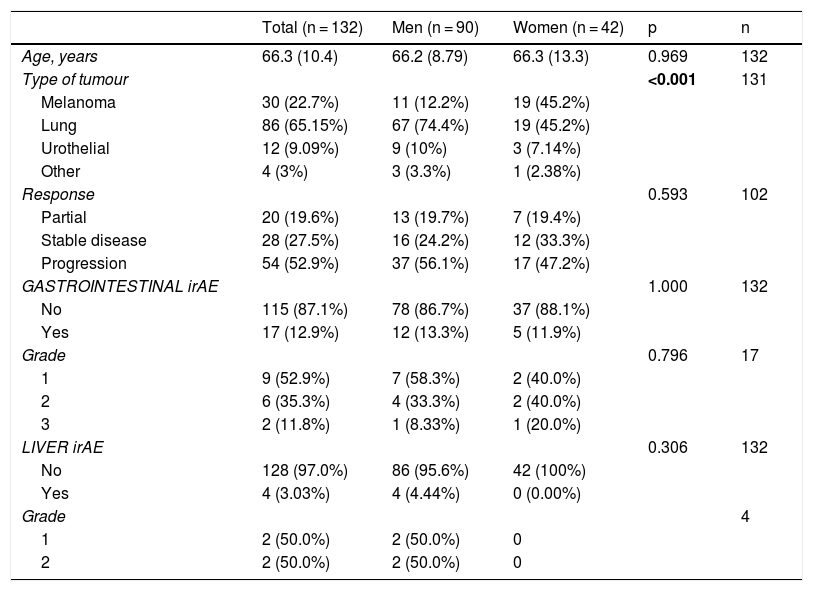
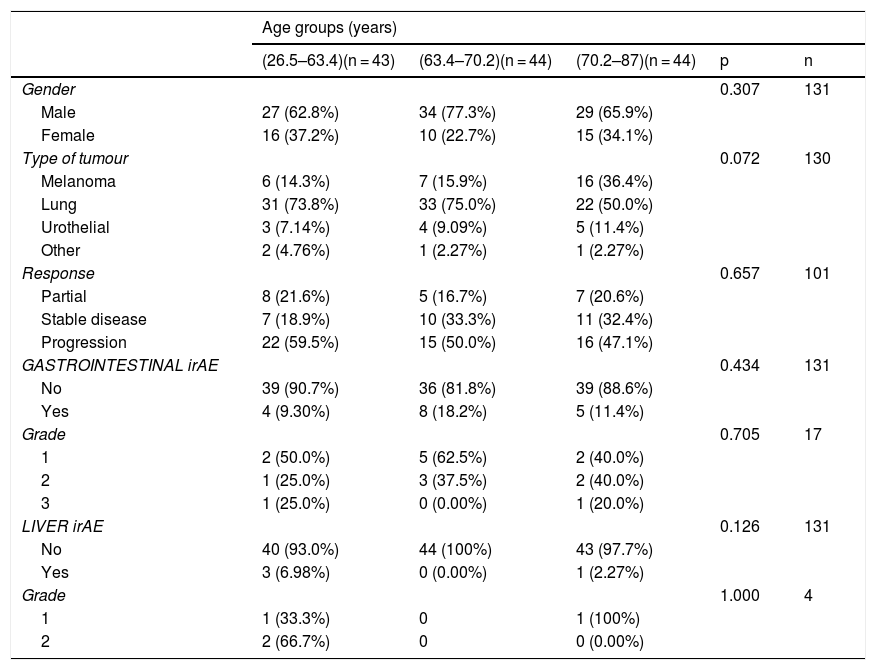
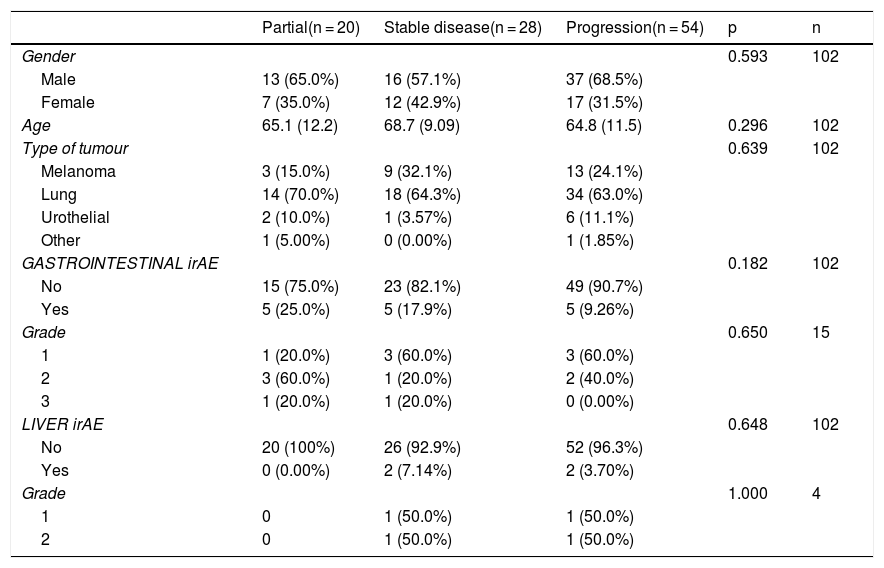
Results132 patients with non-small cell lung cancer (65.15%, n = 86); melanoma (22.7%, n = 30); renal carcinoma (9.09%, n = 12); and other tumours (3%, n = 4) were included. The treatments administered were nivolumab (n = 82), pembrolizumab (n = 28), atezolizumab (n = 13), durvalumab (n = 2), ipilimumab (n = 1) and the antiCTLA-4/PD-1 combination (n = 6). In total, 51 patients (38.6%) developed IrAEs, 17 (12.9%) of which experienced GI-IrAEs. Of these, 8 (47%) needed steroids and 1 patient required surgery due to intestinal perforation. Grade I Li-IrAEs were observed in 4 patients (3.03%): 2 (50%) required corticosteroids and 1 patient had to discontinue treatment. Four patients (66.6%) who received combination therapy experienced GI-IrAEs. IrAE incidence were not associated with age, gender or drug response.
ConclusionsGI-IrAEs are one of the most common adverse events in patients receiving ICIs. A multidisciplinary approach and a greater understanding of these events could help to reduce morbidity and therapy discontinuation.
Los inhibidores del punto de control inmunitario son fármacos eficaces en el tratamiento de diversas neoplasias. Sin embargo, se han relacionado con eventos adversos inmunomediados (EAI) gastrointestinales y hepáticos que pueden desencadenar su interrupción temporal o definitiva.
ObjetivoEvaluar, en condiciones de práctica real, la eficacia y toxicidad gastrointestinal y hepática de los ICIs en tratamientos oncológicos.
Material y métodosEstudio retrospectivo con inclusión de pacientes con diagnóstico de neoplasia avanzada que habían recibido al menos una dosis de ICIs entre mayo de 2015 y septiembre de 2018.
ResultadosSe incluyeron 132 pacientes con neoplasia de pulmón no microcítico (65.15%, n = 86); melanoma (22.7%, n = 30); carcinoma renal (9.09%, n = 12); y otros tumores (3%, n = 4). Los fármacos empleados fueron nivolumab (n = 82), pembrolizumab (n = 28), atezolizumab (n = 13), durvalumab (n = 2), ipilimumab (n = 1) y la combinación antiCTLA-4/PD-1 (n = 6). El 38.6% (n = 51) desarrolló EAI, de tipo gastrointestinal en el 12.9% (n = 17). De ellos, un 47% (n = 8) requirieron esteroides, y en un paciente precisó cirugía por perforación intestinal. En el 3.03% (n = 4) se objetivaron EAI hepáticos grado I: el 50% (n = 2) requirió corticoterapia y en un paciente fue preciso interrumpir el tratamiento. Entre los pacientes con tratamiento combinado, el 66.6% (n = 4) presentó EAI gastrointestinales. La incidencia de EAI no se relacionó con la edad, sexo, ni con la respuesta al fármaco empleado.
ConclusionesLos EAI gastrointestinales son uno de los más frecuentemente observados en pacientes en tratamiento con ICIs. El manejo multidisplicinar y un mayor conocimiento de dichos eventos podría ayudarnos a reducir su morbilidad, así como las interrupciones del tratamiento.