Electrosurgical units (ESUs) are indispensable devices in our endoscopy units. However, many endoscopists are not well-trained on their use and their physical bases are usually not properly studied or understood. In addition, comparative data concerning the settings that may be applied in different circumstances are scarce in the medical literature. Given that it is important to be aware of their strengths and risks, we conducted a review of the available information and research on this topic.
Las unidades electroquirúrgicas (UES) son dispositivos indispensables en nuestras unidades de endoscopia. Sin embargo, muchos endoscopistas no están bien entrenados en su uso y sus bases físicas generalmente no son estudiadas o comprendidas adecuadamente. Además, los datos comparativos sobre los ajustes que pueden aplicarse en diferentes circunstancias son escasos en la literatura médica. Dado que es importante conocer sus fortalezas y riesgos, realizamos una revisión de la información existente y la investigación sobre este tema.
When we were trainees, we observed that one of the main tasks undertaken by the senior endoscopists involved the usage of electrosurgical units (ESUs). In fact, a wide number of the procedures in our endoscopy units required diathermy. However, when we asked our mentors how the ESUs works or why to apply any given setting, the answer was usually quite unsatisfactory with a few honorable exceptions. This situation was really striking, particularly when you consider all the endoscopic resections, sphincterotomies and hemostatic procedures that we performed every day. As the years go by, more and more therapeutic interventions are scheduled in our endoscopy rooms and the common general knowledge on ESUs remains to be improved. Why on earth a racing driver would steer his car without fully understand the machine? A lack of specific training, confusing terminology and varied proprietary technologies may contribute to this picture.
While the final electrosurgical result in the tissue depends on numerous factors concerning the patient, the lesion, the user technique and the device, an adequate knowledge of our ESU is key. The lines below are an attempt to explain some physical bases and electrosurgical principles to improve our understanding and management of the ESUs and, therefore, our patients’ safety. Definitely, when we know better, we perform at our best.
Principles of electrosurgeryThe physics: basic principlesThe physical basis of electrosurgery is the use of high frequency alternating current that is delivered to the tissue and transformed into thermal energy. The heating that we produce in the cells is used to cut, coagulate or ablate by adjusting various parameters. Consequently, snares, endoknives and sphincterotomes are the active electrodes of this electrical circuit.
The governing concept that applies to electricity is Ohm's law and its three variables affect the temperature that the tissue reaches. Thus, voltage (V; measured in volts=joules/coulomb) is the force required to push the flow of electrons (I; current intensity measured in amperes=coulombs/second) through a resistance (R; measured in ohms) along the circuit. Briefly, V=I×R. Therefore, as long as resistance (impedance in alternating currents) increases, if voltage remains constant, the current intensity decreases. The latter equation explains one endoscopist's common observation: as tissue becomes more coagulated, it becomes more resistant to electrical flow decreasing the effectiveness during the polypectomy. Since voltage is the main force that pushes the electrons forward, it is commonly used to increase the thermal injury to achieve hemostasis. However, we should be aware that higher voltages also increase the risk of wall burns.1,2
Additionally, power (or work; W; measured in watts=joules/second) is the product of voltage (V) and current intensity (I): W=V×I. When we substitute the terms with the Ohm's law, then W=V×(V/R)=V2/R. Consequently, power is directly proportional to voltage and inversely proportional to tissue resistance (impedance). In other words, as resistance increases, power decreases, and as voltage increases, so does the power.3
Finally, the thermal energy (Q) that is released when the electrical current flows through a resistor is determined by Joule's law and is the product of power and time; Q=W×t. Again, when Ohm's law elements are used in this equation, then Q=(V×I)×t=(I×R)×I×t=I2×R×t. For this reason, the resistor (our endoscopic device) is heated and emits energy. This thermal energy is directly proportional to the resistance (impedance) and to the length of time during which such current intensity is flowing through. This effect is easily understood bearing in mind the function of light bulbs. In short, Joule's heat produced by the transformation of an electric current enables us to create the cut/coagulation effect to tissues with our ESUs. From another standpoint, tissue resistance transforms electrical current into calorific energy that increases the tissue temperature and allow us to achieve the desired effects.
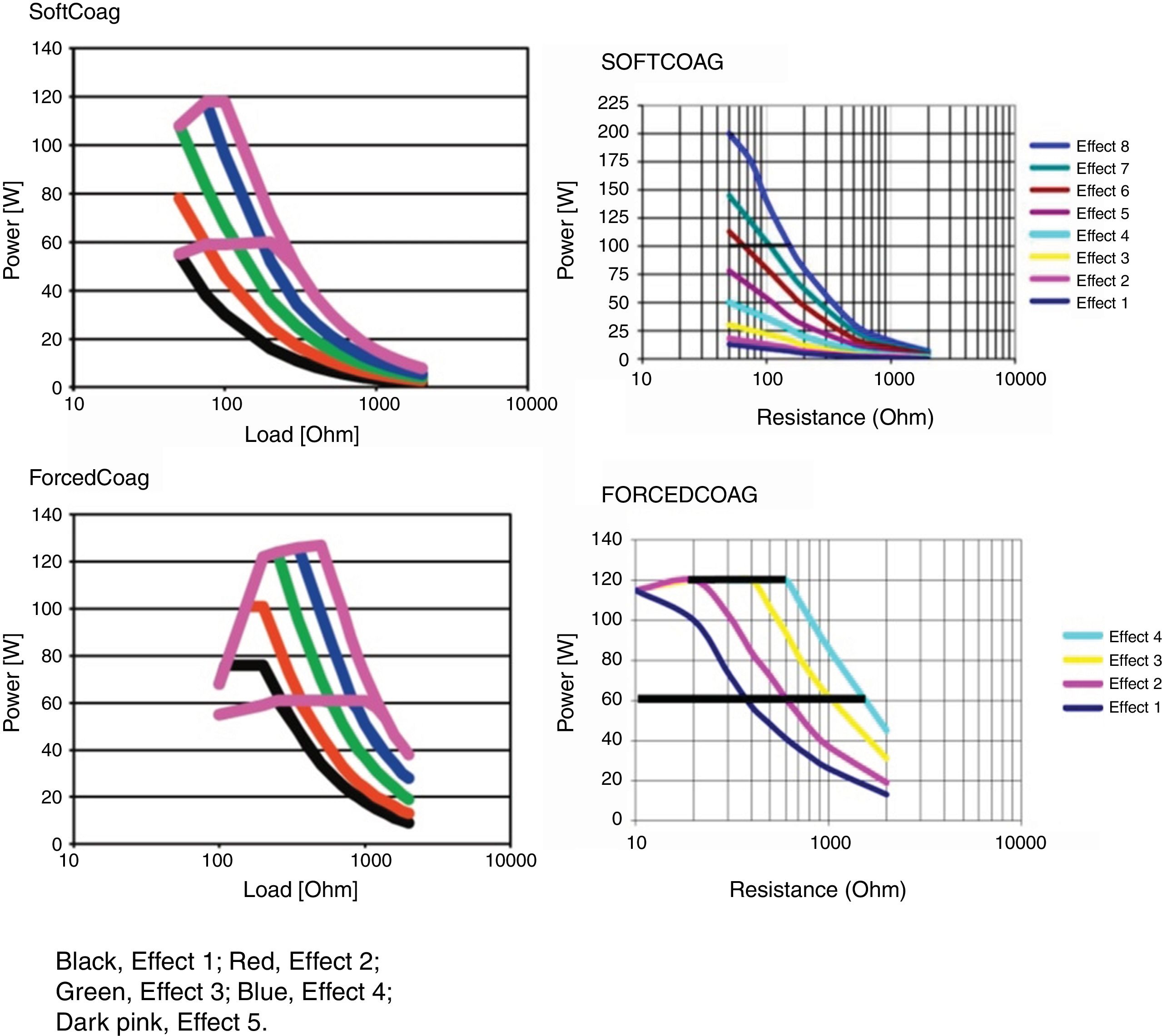
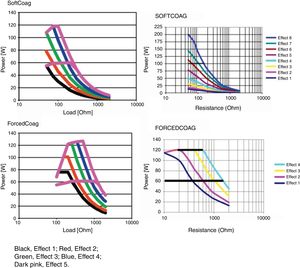
Microprocessor controlled ESUs: constant power vs. constant voltageTo regulate all these physical parameters, contemporary ESUs use microprocessors. This technology controls output power, adjusting voltage and intensity to the predefined algorithm, by frequently sensing tissue impedance. As tissue impedance changes, the microprocessor's software controls the energy being delivered to the target. For this reason, when we adjust the power setting in our electrosurgical source, this value serves only as an upper limit. The power to impedance curve sets by the algorithm design does all the rest.4 Power curves for different settings are included in the user manuals of our ESUs. These figures show us how our unit behaves in response to increasing tissue resistance. Narrow type power curves are used for effective coagulation: as the impedance increases because of hemostasis, the power drops quickly limiting depth thermal injury. On the other hand, broad type power curves maintain an effective cutting power as resistance increases and are commonly used for polypectomy or sphincterotomy3 (Fig. 1).
There are two types of ESUs nowadays. Each one of them deliver the power to the tissue in different ways. The conventional generator delivers a pre-determined wattage setting dialed in on the unit. This type of unit delivers constant power and the voltage fluctuates to keep the power as selected. Thus, voltage varies to maintain constant wattage. These types of ESUs may cause more unpredictable effects on tissues during therapeutic endoscopy and, therefore, deeper thermal injuries are more likely.
The second type of generator (automatic or microprocessor controlled) keeps the voltage constant and allows the power to fluctuate, according to the needs of the tissue. The integrated microprocessor reads the tissue response in milliseconds at the active electrode contact point. The impedance in this area is detected by the microprocessor and it adjusts the power on demand. Although you set a pre-defined wattage in the ESU, this selection acts as the maximum power to be delivered. Since voltage remains constant, thermal effects are more predictable.
The circuit: monopolar and bipolar systemsESUs may operate in monopolar or bipolar modes. When using diathermy, the tissue and, of course, the patient forms an integral part of a current circuit. The components of this circuit are: the ESU itself, the active electrode, the patient and the dispersive electrode. The first generators were ground-referenced systems which involved a ground pad to close the circuit.5 These systems introduced the risk of alternative routes for electricity such as electrocardiographic electrodes or metal objects worn by the patient or in the operating bed. Since the advent of isolated generators, more than 40 years ago, the current returns via the dispersive electrode to an isolated transformer within the ESU. With these present-day units, when the flow of electrons does not find its way through the dispersive electrode the generator typically does not get activated.
In our endoscopy units, the most commonly used mode is monopolar. As stated before, the current pass through the active electrode (e.g.: snare, endoknife, etc.) to the target tissue and then, to the dispersive electrode to reach the generator at the end of the circuit. The entire patient is involved in this mode and a plate is required.6
Bipolar modes are mainly applied in endoscopy as contact coagulation. The bipolar devices bear the active and the return electrodes very close in their tips. Thus, the current pass through a small amount of tissue between them and the current flow returns to the ESU by the device itself. We use no plates with bipolar instruments and lower voltage settings are commonly delivered. The latter leads to a main drawback: we need more time to achieve an effective coagulation and the risk of tissue adherence to the device is higher. In addition, including cutting properties in a bipolar device may be challenging although it is possible to do. Theoretically, with endoscopic bipolar cutting instruments, the energy reaching deeper layers is reduced. At least, one device for endoscopic submucosal dissection has been developed for this purpose.7
Electric current: types and frequencySince current flow is the movement of electrons and/or ions we must create an environment which favors it. This condition is achieved by including a positive and a negative pole in the circuit. On one hand, when direct current (DC) is used, such as batteries, the polarity remains constant and the flow of electrons is unidirectional. On the other hand, alternating current (AC) is created when the power source generates energy by switching from positive to negative for each of the two poles. The latter is the case of wall outlet sources in our homes. Actually, there is no net flow of electrons in AC circuits but oscillations without directionality. This is the case in our electrosurgical generators.
Another important concept is that of current frequency which reflects the changes in output polarity. It is measured in hertz (Hz) equal to one cycle per second. In Spain, power outlet is 50Hz. This frequency allows the nerve and muscle cells to depolarize and thus, it has a stimulating effect. As you can guess, its impact on human myocardium does not allow us to use it for electrosurgical purposes. Thus, the frequency of AC must be high enough to ensure that no neuromuscular stimulation is induced. Therefore, high frequency currents in the range of 300–1000kHz are commonly applied in electrosurgery. Hence, the term radiofrequency electrosurgery comes from the frequencies used in AM radio broadcasting that are within that range.2
Tissue effectsThe different effect of electrical current depends largely on the temperature that the target tissue reaches. Thus, as voltage is increased in our ESU, so the tissue temperature does.
As we all know, normal body temperature is around 36.5°C. When we have a fever, it rises and can reach 40°C. However, we do not experience any cell injury in these circumstances. If temperature rises above 50°C, cell death will happen in several minutes because of molecular changes within the cells and in the membrane proteins. This effect will be almost instantaneous around 60°C. Below 100°C, two different processes take place in live tissues. On one hand, cellular water loss through the thermal-induced damaged cell membrane lead to drying up, desiccation or dehydration, so the tissue collapses. On the other hand, the bonds between protein molecules are broken leading to denaturation and the extracellular collagen becomes coagulated. As this structure cools, a homogeneous gelatinous matter is built up. Such a process is remarkably useful to achieve occlusion within the blood vessels and allows the hemostatic effect. If tissue temperature continues to rise over 100°C, intracellular water starts boiling and vaporization of the cell occurs in a cloud of steam, organic matter and ions released in the blast. Finally, at about 150–200°C, live tissues are broken down in its essential carbon compounds and black carbonization appears. This latter effect is often useless for therapeutic purposes since it increases impedance and hinders the current to pass through the tissue.2,8
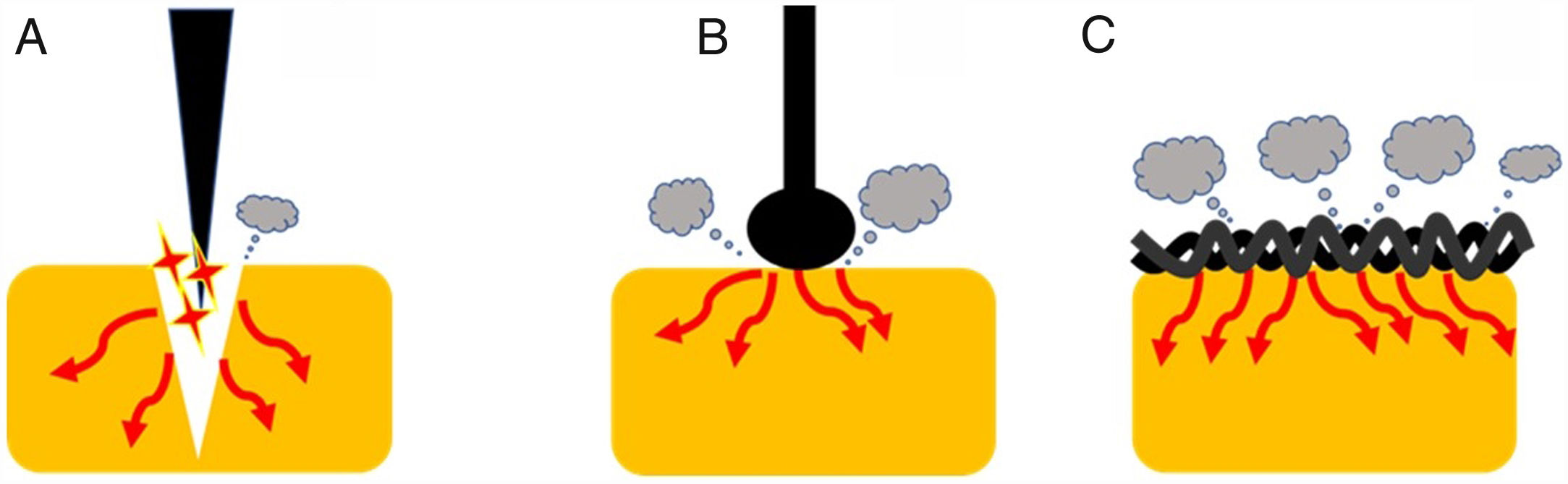
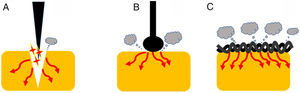
Current densityOne of the most important variables that can affect the tissue effect is current density. This term is defined, in electrical circuits, as current amount per unit area of cross section [D=Intensity (amperes)/area (cm2)]. Therefore, as current intensity increases, so current density does. Additionally, as the area of application increases, current density decreases.1 This concept has important practical implications (Fig. 2). Thus, when we use a device with a larger contact area with the target tissue, the current density becomes reduced increasing its coagulation ability. Therefore, different designs lead to different thermal effects. Generally speaking, a braided snare has a higher coagulation effect than the monofilament alternative. Similarly, a ball-tipped knife for endoscopic submucosal dissection increases its hemostatic capability when compared with needle-tipped versions.9 In addition, the amount of tissue captured is also important. For this reason, when we ensnare a thick polyp stalk and a large amount of tissue is in contact with a braided snare or it is compressed too tightly, current density decreases, and tissue coagulation is favored. This situation decreases cutting efficiency and may lead to entrapment of the device into the tissue.3,6,10 This concept is also applied when snare tip soft coagulation is used to achieve effective hemostasis11 or generate tissue ablation to reduce adenoma recurrence.12,13 Finally, at the place where the dispersive electrode is positioned, current density is lower because its surface area is larger. So, when properly placed, skin burns are exceptional.
Endoscopic devices with different designs. Needle-tipped knives (A) keep electrical current strongly focused in a targeted tissue increasing current density and promoting the cutting effect. Ball-tipped knifes and braided snares (B and C, respectively) increase its contact with the surface area that lead to lower current density and induces higher coagulation effect.
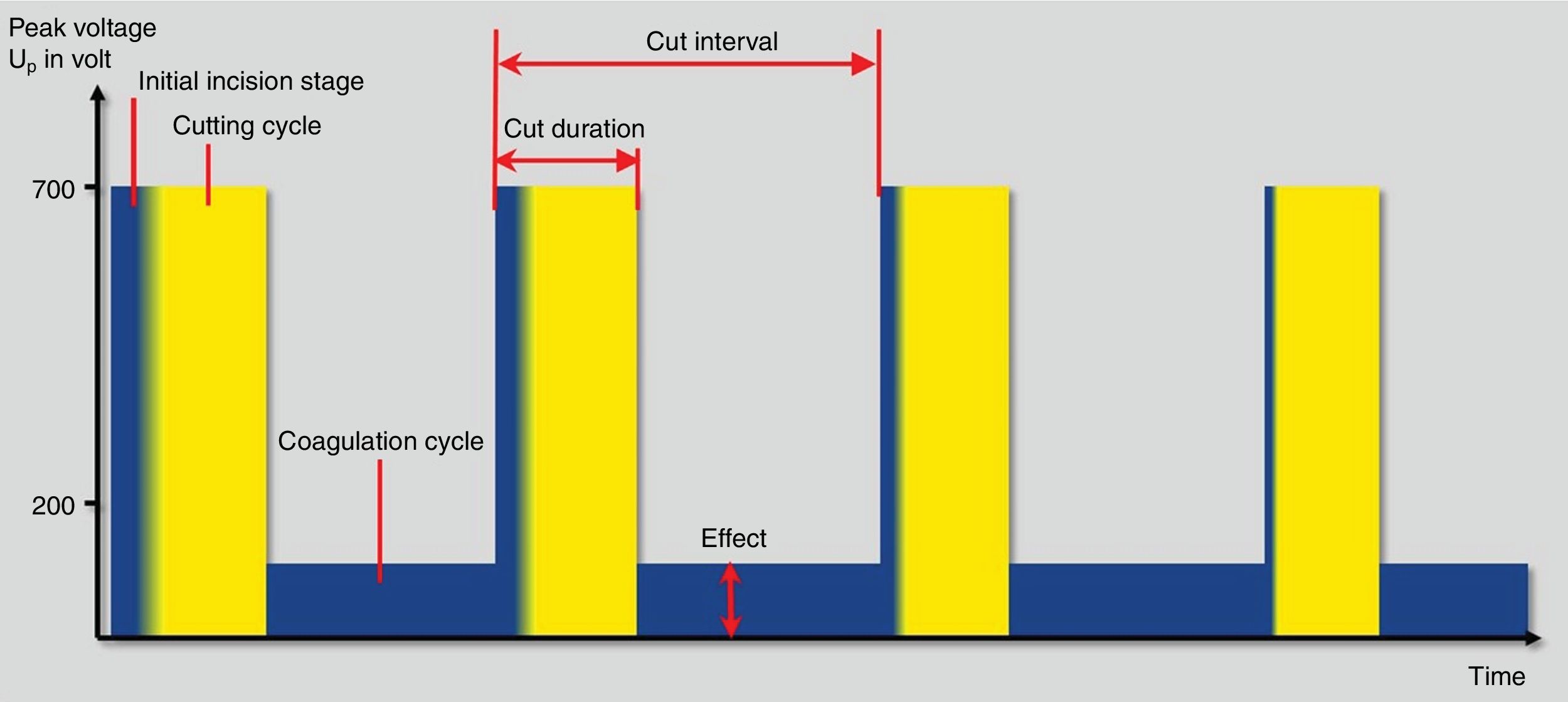
As mentioned above, the thermal effects of the cutting and coagulation currents are defined by the percentage of time in which they are released into the tissue as well as their voltage. This concept is defined by the duty cycle. Therefore, the currents that are released during the entire activation period (duty cycle 100%) are the so-called pure cutting currents, because they do not incorporate pause times (no current released) where the target tissue has chances to cool down. So, the longer pause times between current release pulses (lower duty cycle) the more coagulation power is produced. Thus, coagulation currents have a lower duty cycle (between 6% and 20%, generally). On the other hand, the so-called blend current currents are those whose duty cycle is between 12 and 80%.1,14
The crest factor is another concept that defines the behavior of currents waves, which refers to the ratio of the maximum peak amplitude between the average amplitude of the current wave. So, the cutting currents are those defined by a low crest factor (less than 2) unlike those with a high crest factor, which has greater coagulation power (from 7 to 8). For mixed currents the crest factor is defined between 2 and 5 (Table 1).
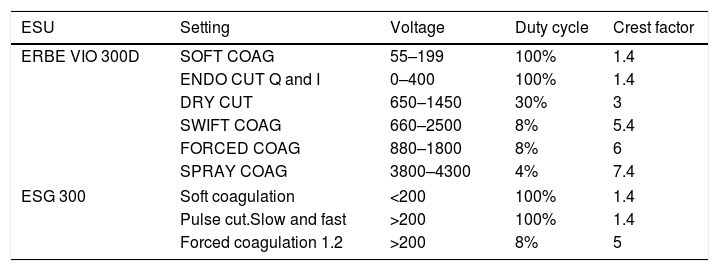
Outputs for commonly used generator models ERBE VIO 300D/ESG-300.
| ESU | Setting | Voltage | Duty cycle | Crest factor |
|---|---|---|---|---|
| ERBE VIO 300D | SOFT COAG | 55–199 | 100% | 1.4 |
| ENDO CUT Q and I | 0–400 | 100% | 1.4 | |
| DRY CUT | 650–1450 | 30% | 3 | |
| SWIFT COAG | 660–2500 | 8% | 5.4 | |
| FORCED COAG | 880–1800 | 8% | 6 | |
| SPRAY COAG | 3800–4300 | 4% | 7.4 | |
| ESG 300 | Soft coagulation | <200 | 100% | 1.4 |
| Pulse cut.Slow and fast | >200 | 100% | 1.4 | |
| Forced coagulation 1.2 | >200 | 8% | 5 | |
ESU: electrosurgical unit.
One of the key parameters to consider when assessing the effects of different waveforms is voltage. A high frequency current over 200V will cause a spark and, consequently, a fast increase in temperature into the tissue with an immediate bursting effect into the cells and, therefore, cutting. However, in the peripheral areas of the target tissue where the current is applied, a smaller and more progressive temperature increase is generated which causes a coagulation effect at this level. For this reason, the concept of pure cut is never given unless a cold cut, such as cold snare polypectomy would take place. Additionally, current waveforms under 200 Vp will not generate a spark to induce a cutting effect. These latter low voltages are used in the soft coagulation setting.
In order to increase the coagulation component and, consequently, the depth of the thermal damage, two factors can be modified: firstly, the waveform should change from continuous to discontinuous and secondly, the voltage intensity should increase progressively to enlarge the depth of the effect. This is because along the margins of the target tissue, there is an increase in impedance due to the coagulated tissue which must be defeated. The forced coagulation mode is a clear example of this type of setting and its main function is hemostasis.10 Nowadays, other coagulation modes with enhanced cutting properties are available such as Blend or SWIFT COAG modes.15
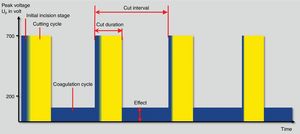
Finally, fractionated cutting coagulation modes (ENDO CUT® or Pulsecut®) are commonly used settings for polypectomy these days (Fig. 3). They are current modes that mixes cutting current pulses with coagulation phases. Three parameters may be set by users with these modes: (1) effect, it is the voltage of the coagulation current. Therefore, the higher the effect, the more coagulation power is delivered; (2) duration. It defines the length of cutting phase. The higher the duration, the longer the cutting width and (3) interval, which is defined by the time frame between two cutting phases. Thus, the longer interval, the lower the cutting speed. In the same way, when the value of this interval decreases, the cutting speed increases. Interestingly, the maximum peak voltage is different in I and Q modes. Therefore, for ENDO CUT I mode, the maximum Vp is 550V, comparing with Q mode in which the maximum Vp is 770V. These differences are kept regarding the setup: ENDO CUT I effect 1: none; effect 2: Soft 1; effect 3: Soft 2 and effect 4: soft 3. For ENDO CUT Q: Effect 1: none; Effect 2: soft; Effect 3: Forced 1; Effect 4: Forced 2.
Unfortunately, this wide range of possibilities lead to many different setting options amenable to use for the endoscopists, increasing the variability among them, hindering clinical trials and their comparability and, ultimately, preventing clear recommendations in clinical guidelines.
Safety recommendationsRisk of skin burnsThe fact that the monopolar current is the most frequently circuit used in endoscopic procedures helps to prevent possible damages caused by current dispersion. However, caution must be exercised in case that the active electrode (polypectomy snare) is connected to the electrosurgical generator and out of its point of action (e.g. on the stretcher in contact with the patient's skin). In this case, if the circuit were activated, a burn would occur in the contact area with the active electrode. Nowadays, dispersive electrodes have a visual and auditory alarm system, that detects impedance variations that could lead to heat dispersion in the contact area, to prevent the risk of burning.16
Electrosurgical generators and its interaction with implanted devicesBoth pacemakers and implantable defibrillators (AIDs) are devices that are sensitive to electrical currents generated in the heart. However, this also makes them sensitive to any type of electrical circuit. However, the new generation of pacemakers have been designed to be resistant to this type of electrical impulses. Despite this advance, the risk of possible interference exists and therefore requires a series of precautions that should be taken. In the case of pacemakers, the detection of an impulse from an electrosurgical source can be interpreted as an electrical noise causing an asynchronous rhythm. In case of an AID, this impulse can cause an inappropriate discharge. For this reason, following the scientific societies recommendations, any endoscopy unit in cooperation with hemodynamic and arrhythmia units should promote the creation of protocols to act properly in such cases.17
Positioning of the dispersive electrodeA key point for the proper functioning of electrosurgical sources is the positioning of the dispersive electrode, commonly called “plate”. Thus, we must ensure a uniform flow of current over its entire surface in order to avoid excessive temperature increases at specific points depending on its orientation and positioning. However, there is a subtype of neutral electrode that has an equipotential ring that makes the current distribution homogeneous, regardless of its orientation.
In the case of a pacemaker or patient with AID, the circuit between the active and dispersive electrodes should be positioned far away from the implantable device, and it should not include the device inside it. Therefore, it is advisable to place the dispersive electrode correctly positioned and as close as possible to the active electrode avoiding heat dispersion.6
Recommended settings for polypectomy and EMRPolypectomy is the most common therapeutic technique in gastrointestinal endoscopy. According to the European guidelines, for polyps larger than 10mm it is recommended to perform hot snare polypectomy. This technique is also indicated for polyps between 6 and 9mm in size, when cold snare polypectomy is not technically feasible.18 However, polypectomy adverse events are not negligible. In a review of retrospective studies evaluating major adverse events, Ko et al. reported bleeding (0.1–0.6%), perforation (<0.1%) and post-polypectomy syndrome (<0.2%) as the most serious gastrointestinal complications of colonoscopy, and they were mainly related to the use of electrosurgery.19 Similarly, in a review of the FDA Manufacturer and User Facility Device Experience database, complications related to the use of an electrosurgical unit (ESU) were mainly related to the operator or to the sub-optimal use of the devices.20 Thus, the use of diathermy is not currently recommended for removal of small and diminutive polyps. Additionally, tissue ablation of those small polyps is not acceptable because complete retrieval of specimens for histological assessment is necessary.
Type of currentMonopolar current is mainly universally used for polipectomy, but there is no homogeneity regarding the proper type of current or specific guidelines on this topic. Only few and low-quality data, as retrospective or small sample size studies, can be found in the literature. In fact, the only clear recommendation from ESGE and ASGE is to avoid pure cut current due to the higher risk of bleeding. Despite this, no other settings are specifically recommended.1,18 In consequence, the use of current type is variable. A survey conducted in 2014 among 189 American gastroenterologists referred 46% used pure coagulation current, 46% blended current, 3% pure cut current, and other different forms of current in 4% of the cases.15
Regarding the higher risk of bleeding with pure cut current, it was highlighted as a main risk factor in a multi-center Korean study including more than 9000 polypectomies.21 In contrast, Parra-Blanco et al., in a retrospective study including 4735 polypectomies performed in a single center from 1995 to 1998, suggested that pure cut current was safe. They described a bleeding rate of 1.1% when using a correct protocol. However, they advised against its use in case of low expertise in the application of clips.22
Other types of current have been compared without relevant differences. Van Gossum et al. compared the blended and coagulation current, in a retrospective study with 1485 procedures. The complication rate was similar, but the timing for bleeding differed: 2–8 days for pure coagulation current and within 12h in case of blended current.23
Polypectomy and microprocessor-controlled ESUsImportantly, the concern about the type of current can be partially overcome using microprocessor-controlled ESUs. Because polypectomy is a dynamic process, a proper current supply should also be active. In fact, at the beginning of the polypectomy, a higher cutting current is necessary to perform the incision as a large amount of tissue is in contact with the snare, what reduces the current density. Once the incision has started the intensity required to maintain the cut is at first reduced with respect to the first phase, but as polypectomy progresses, tissue impedance tends to rise as tissue dehydrates and consequently current intensity needs to increase.6
The microprocessor can monitor current changes, voltage, power and resistance of the tissue, and to automatically modify the required settings. When using fractionated cutting modes, the ESU initiates an incision phase and continues with short cutting cycles alternating with prolonged coagulation periods with limited voltage peaks. Theoretically, this reduces the risks of bleeding due to an extremely rapid cutting and the risk of perforation due to an intense coagulation. On one hand, a longer coagulation period prepares the tissue for the next cutting phase and decreases the risk of bleeding but, on the other hand, it increases the amount of current administered to the intestine wall increasing the risk of perforation or post-polypectomy syndrome.
The importance of the microprocessor was clearly demonstrated in a retrospective study conducted by Burgess et al. that included 1172 patients who underwent endoscopic mucosal resection for lesions over 20mm. In the multivariate analysis, blended (8.5%) and pure coagulation current (15.2%) were significantly associated with delayed bleeding when compared with a fractionated cutting current mode (5.8%) (OR 2.03; p=0.038).24
Additionally, fractionated cutting current modes might entail other benefits by obtaining high-quality pathological specimens. In a retrospective study by Fry et al. in which the pathological analysis was blinded to the polypectomy technique, specimens obtained with the ENDO CUT mode® showed higher quality of the sample, especially in terms of margins of resection, compared to those obtained with a conventional ESU.25 For all these reasons, the use of non-microprocessor-assisted ESUs should now be avoided for endoscopic procedure whenever possible.
Other factors affecting the electrosurgical outcomesThe electrosurgical outcome is not only determined by the setting of the chosen ESU (type of current, intensity and interval duration), but also by the characteristics of the snare used, the area of contact with the tissue and the duration of current transfer time.
Regarding the snare characteristics, a single or thin filament snare enhances the cutting properties. In contrast, a thick filament snare often presents a greater coagulation effect (see above). Given these premises, the intensity of the current set on the ESU should be related to the type of snare used.6
Once you have chosen the snare and the ESU settings, the snare should be correctly positioned, elevating the polyp above the muscularis propria to avoid wall injuries. After correct positioning, especially in case of large or pedunculated polyps, pressure around the polyp needs an initial tension and an appropriate speed that allows a correct transmission of current into the tissue. When the stalk is captured in the initial phase, a large area of tissue is in contact with the snare and therefore current density is reduced, enhancing its coagulation properties. For this reason, intensity needs to be higher during the initial phase. Then, as a result of tissue dehydration, impedance increases, leading to higher intensity requirements to maintain the cutting effect. It is therefore crucial, particularly for big pedunculated polyps, to avoid excessively rapid closure during cutting to avoid bleeding. At the other end of the spectrum, if snare closing is too slow, the incarceration of the snare in the stalk or its incomplete removal may occur.10
In the presence of sessile or flat lesions larger than 10mm and therefore suitable to EMR, the same general rules mentioned above regarding to the correct use of ESU should be applied, but the lesions are elevated with a submucosal injection. This allows to separate the mucosa from the underlying muscularis propria minimizing the chance of thermal or mechanical damage of the muscularis propria.18 In these cases, due to the absence of large amount of tissue and big feeding vessels and acknowledging that the current is deployed directly to the colonic wall, a faster closure might be appropriate. Examples of some recommended settings for polypectomy and EMR are shown in Table 2.
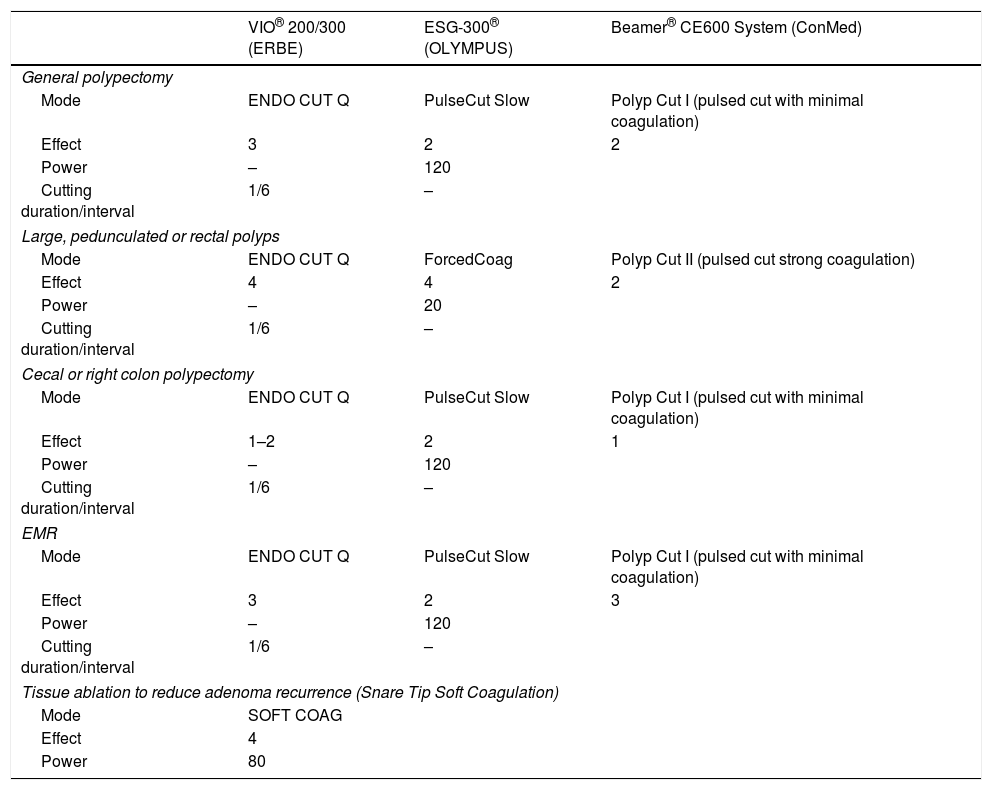
Examples of possible recommended settings for colonic polypectomy and EMR according to the most used ESUs.
| VIO® 200/300 (ERBE) | ESG-300® (OLYMPUS) | Beamer® CE600 System (ConMed) | |
|---|---|---|---|
| General polypectomy | |||
| Mode | ENDO CUT Q | PulseCut Slow | Polyp Cut I (pulsed cut with minimal coagulation) |
| Effect | 3 | 2 | 2 |
| Power | – | 120 | |
| Cutting duration/interval | 1/6 | – | |
| Large, pedunculated or rectal polyps | |||
| Mode | ENDO CUT Q | ForcedCoag | Polyp Cut II (pulsed cut strong coagulation) |
| Effect | 4 | 4 | 2 |
| Power | – | 20 | |
| Cutting duration/interval | 1/6 | – | |
| Cecal or right colon polypectomy | |||
| Mode | ENDO CUT Q | PulseCut Slow | Polyp Cut I (pulsed cut with minimal coagulation) |
| Effect | 1–2 | 2 | 1 |
| Power | – | 120 | |
| Cutting duration/interval | 1/6 | – | |
| EMR | |||
| Mode | ENDO CUT Q | PulseCut Slow | Polyp Cut I (pulsed cut with minimal coagulation) |
| Effect | 3 | 2 | 3 |
| Power | – | 120 | |
| Cutting duration/interval | 1/6 | – | |
| Tissue ablation to reduce adenoma recurrence (Snare Tip Soft Coagulation) | |||
| Mode | SOFT COAG | ||
| Effect | 4 | ||
| Power | 80 | ||
ESD technique consists of several well-established steps. The first stage, generally dispensable for colon, lies in marking the lesion margins typically by using the dissection knife. Once this step is accomplished, several agents or solutions must be injected directly into the submucosa. The third step consists of performing a mucosal incision around the lesion. This mucosal cut, along with the presence of the submucosal cushion, will allow us to get access to the proper submucosal plane and thus, perform the submucosal dissection. During this phase, we will operate beneath the lesion by coagulating and cutting the submucosal fibers until the complete resection of the lesion is achieved. Pre-coagulating the submucosal vessels lain within the dissection plane is an extremely important point, since it enables us to perform the procedure cleanly and safely. The dissection knife, along with hemostatic forceps whenever large vessels are involved, will be the tools commonly used for the pre-coagulation or treatment of any bleeding event.
The use of high-performance ESUs will make a remarkable impact on the success of the procedure due to their ability to adapt their performance to every condition and situation. Among the most widely used units we can find the ERBE VIO 200/300 and more recently the VIO 3 electrosurgical generators (ERBE, Tübingen, Germany). Alternatively, there are several studies carried out with Olympus ESG-100 units.26,27 Besides, a new model ESG-300 has been recently developed by this latter manufacturer (Olympus, Tokyo, Japan).
Additionally, the range of knives available for the ESD technique is extraordinarily broad. Most of them are typically monopolar devices with cutting and coagulating functions. No clear superiority of any of them over the others has been clearly proved. However, their technical features make some of them more suitable for certain steps or circumstances of the ESD procedure. Three different types of knives can be generally distinguished: needle-type (e.g. Flush knife BT, Dual knife J, Hook knife); insulated-type (e.g. IT2 knife) with a protective ceramic or plastic insulator around the electrode's tip; and finally scissor-type (e.g. SB knife) which allows us to grasp submucosal fibers before cutting with electric current. The ESU settings will vary depending on the knife chosen and its technical features.
As described above, the shape of the contact area between the knife and the tissue is one of the main characteristics that we must take into consideration when setting the cutting power. The narrower the contact area is, the higher the current density delivered and, thus, the higher the cutting ability.26 This is important to know because all the devices with higher cutting ability (e.g. needle-type knives) will demand a very accurate handling in order to minimize the risk of perforation.
Another important factor that will determine tissue response to current is the contact time between the knife and the tissue. This time will be determined by the knife's speed and by the current-application time (the time interval during which the foot pedal is pressed). Long application times may lead to the carbonization of the tissues or to long uncontrolled cuts. On the other hand, fast knife movements, especially for needle-type knives which present a narrower contact area and therefore a higher cutting ability, may lead to a shallower coagulation effect and thus, higher rates of intraoperative bleeding. As a rule, and especially for needle-type knives, performing slow knife movements with short current-application times (foot pedal pressed down) will be mandatory in order to complete the procedure cleanly and safely.28
Typically, the mucosa and submucosa of the gastrointestinal tract present a relatively low electrical resistance, giving rise to an adequate electric conductivity. Nevertheless, under certain conditions such as the presence of severe fibrosis or an excess of adipose tissue within submucosa, the electrical resistance gets higher. This same effect can also be demonstrated after performing prolonged hemostasis onto large vessels as a result of the coagulation and the severe dehydration induced in the tissues. All these conditions will have a significant impact on the knife's performance. An increase in the electric current voltage or the choice of current modes with predominant cutting effect will be needed in order to reach a better performance level.
Therefore, the ESU settings for the ESD technique, instead of remaining unchanged, will vary according to several factors: (1) the location within the gastrointestinal tract: since the walls of the esophagus and colon are thinner than those of the stomach, it will be necessary to adjust parameters at these locations in order to control the depth of cut and coagulation and thus to minimize the risk of perforation; (2) the type of knife: the technical differences between each knife will also determine the optimal settings to be chosen, differing from one device to another. Besides, for each knife there may even be a certain degree of variability according to the individual preferences of each endoscopist; (3) the phase of ESD involved: during the circumferential cutting we generally use settings where the cutting function prevails; during the dissection phases we prefer coagulation modes with cutting ability; finally, during the hemostasis phase we tend to opt for pure coagulation modes, and finally, (4) the tissue properties: conditions that increase impedance, such as severe fibrosis or adipose tissue in the submucosal layer will require enhanced cutting abilities.
Electrosurgical modes for ESDCutting modesTheir effect is usually restricted to a narrow area of tissue around the knife, thereby providing a clean incision and inducing minimal thermal damage to the adjacent tissues. Since the coagulation effect is minimal, this mode will not be suitable for performing hemostasis and could induce higher bleeding rates.
- 1.
ENDO CUT I. This mode alternates cutting current pulses with a low voltage coagulation current, which translates into a cutting mode with minimal coagulation effect causing little tissue damage when compared to the coagulation modes. This mode has been advocated for esophageal lesions spreading over 75% of the circumference where ESD has a considerable risk of developing strictures.29 The minimal coagulation effect of this mode would theoretically decrease the inflammatory response during the dissection phase and, thus, might reduce the appearance of fibrosis. This hypothesis has been confirmed by several studies in animal models, although its clinical implementation is not well determined yet.30–32 Thus, we can use this mode for circumferential cutting, submucosal dissection phase under conditions of increased electrical resistance (fibrosis) and, probably for the submucosal dissection phase in order to minimize the peripheral tissue damage.
- 2.
DRY CUT: cutting mode presenting a higher coagulation effect than ENDO CUT I. This guarantees a more efficient vessel-sealing during the cutting phase, which may result into a lower bleeding rate. It is commonly used for circumferential cutting and the submucosal dissection phase under conditions of increased electrical resistance.
As stated earlier in this paper, these modes lie in generating high-voltage bursts with cutting ability followed by inactive intervals. When increasing the “effect” we can intensify the peak voltage and, thus, the coagulation ability. Since higher peak voltages are delivered for these modes, theoretically, the risk of deep thermal damage may be increased.
These modes are typically used during the dissection phase, since they present a good cutting ability, albeit lower than that of cutting modes, coupled with significant simultaneous hemostasis which efficiently seals small vessels and prevents bleeding.
- 1.
SWIFT COAG: its peak voltage and therefore its coagulation ability are the lowest among the rest of modes of this group. On the other hand, its cutting ability is higher than that of FORCED COAG or SPRAY COAG. It is used for the submucosal dissection phase and mainly under conditions of high electric resistance.
- 2.
FORCED COAG: it presents a higher peak voltage and, therefore, a higher coagulation effect than SWIFT COAG. It is typically used for dissecting the submucosal layer where capillary vessels are widely distributed. Its effect can be enough to coagulate small capillary vessels with a similar diameter of the tip of the knife but not for larger vessels, since the cutting effect occurs before an adequate dehydration of the vessel takes place.
- 3.
SPRAY COAG: it delivers higher peak voltages than the two previous modes. It produces a mechanical cut and generates concurrent high coagulation whenever the knife contacts the tissue. In case of non-contact, it only produces coagulation, which could be useful for stopping diffuse capillary bleeding.27 This is the most frequently used mode for the dissection phase in the POEM procedure.
- 1.
SOFT COAG: it is a pure coagulation mode without cutting ability due to its low voltage (<200V) that prevents spark ignition. By intensifying the “effect” parameter we increase the voltage of this mode. Nevertheless, the lowest effect levels of this current mode are those attaining the highest depth of the coagulation effect. On the contrary, higher effect levels give rise to a shallower thermal damage. The latter are commonly used for the hemostasis of larger vessels, such as penetrating vessels, where the aim is to reduce the risk of deep thermal damage onto the muscle layer. Isolating and then coagulating these vessels with SOFT COAG has proven itself very useful in order to significantly decrease the intraoperative bleeding.33,34
One of the main limitations of this mode is that its prolonged use leads to tissue dehydration and to a progressive increase of its electrical impedance. Consequently, large vessels requiring very long application times will attain a higher resistance to the flow of current. Thus, the efficacy of the coagulation generated by the SOFT COAG mode will diminish and may even become insufficient to achieve the complete coagulation of the vessel. One of the alternatives examined in order to overcome this limitation is using a FORCED COAG mode with a low power setting (Effect 1, 10W) as a hemostatic current mode. The higher voltage generated by this mode allows us to maintain the current flow in operation for longer, despite increasing the electrical resistance of the tissue. Some studies in animal models have shown a wider and deeper effect than that of the SOFT COAG mode when applied with a Flush-knife35 without increasing the perforation risk.
Nonetheless, the use of the SOFT COAG mode with hemostatic forceps has proven to be the most effective method for preventing large vessels from bleeding.36,37 These monopolar devices present a wider contact area and a lower current density, which results into a lower warming rate and therefore gives rise to a better coagulation effect.
The preferred electrosurgical settings according to ESD stages are shown in Table 3.
ESD stages and preferred electrosurgical settings (VIO 300D). Note that these settings may differ depending on the knife, the location and the individual preferences. For further details about these parameters, we refer to the manufacturer's technical specifications.
| STAGE | MODE | SETTINGS |
|---|---|---|
| Marking | SOFT COAG | Effect 5, 50–100W |
| FORCED COAG | Effect 2, 20W | |
| SWIFT COAG | Effect 2, 40W | |
| Circumferential cutting | ENDO CUT I (Flush knife; Fujifilm, Tokyo, Japan) | Effect 2–4, Dur 3, Int 2–3 |
| DRY CUT (Dual knife; Olympus, Tokyo, Japan) | Effect 2–3, 80W | |
| Submucosal dissection | FORCED COAG (Flush knife; Fujifilm, Tokyo, Japan) | Effect 2–3, 50W |
| SWIFT COAG (Dual knife; Olympus, Tokyo, Japan) | Effect 3–4, 50W | |
| SPRAY COAG (Hook knife; Olympus, Tokyo, Japan) | Effect 2, 60W | |
| Severe/adipose fibrosis | SWIFT COAG (Flush knife; Fujifilm, Tokyo, Japan) | Effect 3–4, 50–100W |
| DRY CUT (Flush knife; Fujifilm, Tokyo, Japan) | Effect 3–4, 55–100W | |
| Hemostasis with knife | SOFT COAG | Effect 5, 80W |
| FORCED COAG (Flush knife; Fujifilm, Tokyo, Japan) | Effect 1, 10W | |
| SWIFT COAG (Dual knife; Olympus, Tokyo, Japan) | Effect 1, 15W | |
| SPRAY COAG (diffuse capillary bleeding) | Effect 3, 60W | |
| Hemostasis with forceps | SOFT COAG (Coag-grasper; Olympus, Tokyo, Japan) | Effect 5–6, 100W |
Recently, new endoscopic approaches have been developed for the resection of gastrointestinal tumors. Underwater EMR (UEMR) and full-thickness resections with the FTRD kit (OVESCO Endoscopy AG; Tübingen; Germany) are some examples.
When performing UEMR, marking is usually done with the tip of the snare in the SOFT COAG (effect 4; 40W) or FORCED COAG (effect 1; 20W) modes. Then, the setting is typically replaced by ENDOCUT Q (effect 3; duration 1; interval 6) when the lesion is ensnared.
The recommendations for full-thickness resections with the FTRD include the same marking settings as in the case of UEMR but it differs in the cutting current that is applied: ENDOCUT Q; effect 1; duration 4; interval 1.
ConclusionsAlthough evidence regarding the management of ESUs for the most common endoscopic procedures should be expanded and studies comparing outcomes for different settings are not enough in the scientific literature, knowing the basic principles of diathermy is mandatory for endoscopists. Choosing one mode and setting or the another may have clinical implications concerning the efficacy and potential adverse events of our therapeutic procedures. Definitely, knowledge on management of electrosurgery may be able to improve procedural outcomes and safety for our patients.
Conflict of interestsDr. Toyonaga invented the FlushKnife BT and FlushKnife BTS in conjunction with Fujifilm and receives royalties from its sale.
The remaining authors disclosed no financial relationships relevant to this publication.
The study was not funded.