Colorectal cancer (CRC) is a global public health problem, given its high incidence and mortality rates. More than 1.2 million new cases were estimated in 2008, and 608700 deaths due to CRC were certified.1 Over 25000 new cases are diagnosed every year in Spain alone, which accounts for 15% of the annual total of all different types of tumours, and CRC is the second leading cause of cancer-related death— after lung cancer — with more than 13000 deaths annually.
The aetiology of this disease is complex and involves interaction of environmental and genetic factors. Most tumours (60%) have a sporadic origin with regard to environmental factors2; 27% of cases appear to be familial, caused by alterations in known susceptibility genes; and 10% of cases are hereditary, the main examples of which are familial adenomatous polyposis (FAP) and hereditary nonpolyposis colorectal cancer (Lynch syndrome).3,4
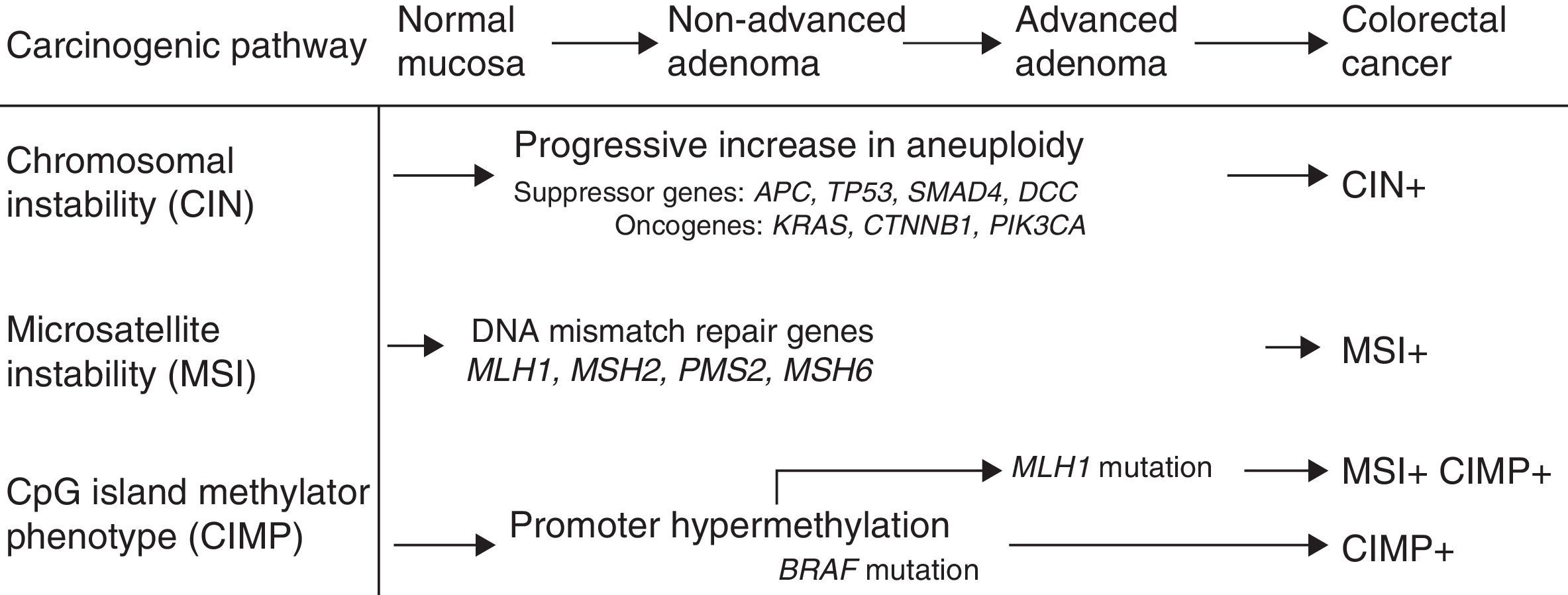
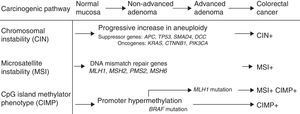
Mechanisms of carcinogenesis and genes implicatedFollowing the description of the suppressor pathway in 1990 by Fearon and Vogelstein, corresponding to the adenoma–carcinoma sequence,5 a total of 3 carcinogenesis pathways have been described (Fig. 1). In the suppressor or chromosomal instability pathway, the tumours often present alterations in karyotype, with chromosome gains and losses, as well as translocations. The factor presumed to initiate this pathway is the inactivation of a suppressor gene, such as the APC gene. Most cases of sporadic CRCs (close to 85%) fall within this group, as do cases of FAP caused by germline mutations of the APC gene.
Microsatellite instability (MSI) occurs due to hereditary or acquired impairment of the DNA mismatch repair (MMR) system, which is made up of various genes including MLH1, MSH2, MSH6, and PMS2. Defects in this system lead to the accumulation of alterations in the microsatellites, short repetitive nucleotide sequences that are distributed along the entire genome.6 Tumours with MSI are currently characterised by high instability or loss of expression of one or more MMR proteins in the immunohistochemical (IHC) study carried out on the tumour specimen.7 MSI is present in 15% of CRCs.8 It can be caused by germline mutations in the repair genes (Lynch syndrome) or by somatic inactivation of the repair genes in relation to epigenetic mechanisms such as promoter hypermethylation of repair genes (most often the MLH1 gene). This mechanism explains sporadic tumours with MSI, which present a clinical phenotype characterised by localisation in the right colon, high histological grade and abundant lymphocyte infiltration in the tumour specimen.
In recent years, the carcinogenesis pathway associated with methylation at CpG islands — the CpG Island Methylator Phenotype [CIMP] pathway — has been described.9 This is characterised by hypermethylation of the promoter region and the initial exons of a large number of genes that contain a high concentration of cytosine and guanine dinucleotide islands linked by phosphates, resulting in their inactivation. This pathway has been detected in 35% of patients with CRC,10 and is also called the serrated pathway, since it could explain the carcinogenesis of serrated lesions in the colon.11
The activation or inactivation of oncogenes or suppressor genes plays an essential role in the development of CRC. Thus, the Ras oncogenes (HRAS, NRAS, KRAS) encode membrane proteins with guanosine triphosphatase activity involved in the intracellular processing of extracellular signals, which include various growth factors. Mutations in codons 12 and 13 of exon 2 of the KRAS gene have been detected in approximately 40% of CRCs. More recently, mutations in the KRAS gene have been described outside exon 2, and also in the NRAS gene (codon 61), although these are less common. The BRAF gene is a member of the Raf kinase family that is responsible for regulating the MAP kinase/ERK signalling pathway. The most common mutation is detected in codon V600E (exon 15) of the BRAF oncogene, and is found in up to 30%–40% of CRCs.12 These mutations are often associated with MSI in the absence of a family history of CRC.13 Mutations in KRAS and BRAF do not coexist in most tumours, so they are considered mutually exclusive.14 The function of the TP53 suppressor gene is to block cell proliferation in the presence of damaged DNA and promote DNA repair. It can also cause cellular apoptosis if this repair is insufficient. Mutations in TP53 have been detected in up to 70% of cases of CRC.15
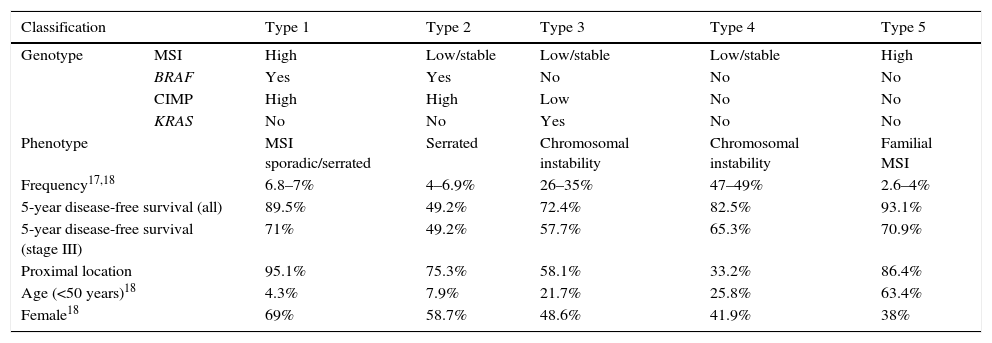
Molecular classification and prognosisIn 2007, Jass16 formulated a classification of CRC based on MSI status, CIMP profile, APC and TP53 suppressor genes and the KRAS and BRAF oncogenes. Based on these molecular alterations, he sub-classified CRC into 5 types (Table 1), and found a correlation with various clinical and pathological characteristics: precursor lesion, histology, age at diagnosis, sex and location. Two articles have recently examined this topic in greater depth, identifying an association between this classification and patient prognosis, both overall and in stage III.17,18
Molecular classification of colorectal cancer and prognostic implications.
| Classification | Type 1 | Type 2 | Type 3 | Type 4 | Type 5 | |
|---|---|---|---|---|---|---|
| Genotype | MSI | High | Low/stable | Low/stable | Low/stable | High |
| BRAF | Yes | Yes | No | No | No | |
| CIMP | High | High | Low | No | No | |
| KRAS | No | No | Yes | No | No | |
| Phenotype | MSI sporadic/serrated | Serrated | Chromosomal instability | Chromosomal instability | Familial MSI | |
| Frequency17,18 | 6.8–7% | 4–6.9% | 26–35% | 47–49% | 2.6–4% | |
| 5-year disease-free survival (all) | 89.5% | 49.2% | 72.4% | 82.5% | 93.1% | |
| 5-year disease-free survival (stage III) | 71% | 49.2% | 57.7% | 65.3% | 70.9% | |
| Proximal location | 95.1% | 75.3% | 58.1% | 33.2% | 86.4% | |
| Age (<50 years)18 | 4.3% | 7.9% | 21.7% | 25.8% | 63.4% | |
| Female18 | 69% | 58.7% | 48.6% | 41.9% | 38% | |
CIMP: CpG island methylator phenotype; MSI: microsatellite instability.
The study by Phipps et al.17 included 2706 patients with CRC diagnosed between 1998 and 2007, who were classified into the 5 subtypes previously described by Jass.16 Thus, patients with CIMP-positive tumours (types 1 and 2 CRC) were older at diagnosis and were usually women. Tumour lesions in MSI-high or CIMP-positive patients (types 1, 2 and 5) were located predominantly proximal to the splenic flexure. Moreover, in type 2 (MSI-low, CIMP-high, BRAF mutation), patients were diagnosed at a more advanced stage, and had the lowest 5-year survival rate. In contrast, survival was longer in MSI-high CRCs (subtypes 1 and 5). Finally, mortality was significantly lower in subtype 3 (MSI-low, CIMP-negative, KRAS mutation) when compared with subtype 4 (MSI-low, CIMP-negative, no mutations), with a proportional hazard ratio of 1.32.
These results were corroborated by the Sinicrope group18 in a similar study that analysed the molecular characteristics (BRAF and KRAS mutations, MSI and MLH1 promoter methylation) in 2720 stage III CRCs treated with adjuvant chemotherapy (Table 1). In this study, the clinical characteristics of each of the different subtypes were consistent with the results described in the study by Phipps et al.,17 as shown in Table 1. MSI-low patients with mutations in BRAF (Hazard Ratio [HR] 1.43; 95% confidence interval [CI] 1.11–1.85) or KRAS (HR 1.48; 95% CI 1.27–1.47) had poorer 5-year survival than those with no mutations in these genes. However, disease-free survival in MSI-low patients with no mutations in BRAF or KRAS was similar to that of MSI-high patients.
The difference in survival detected between the 2 CIMP-positive subtypes (types 1 and 2) is significant. Although these 2 subtypes are similar with respect to clinical characteristics (advanced age, predominantly females and proximal location), the presence of high MSI increases the likelihood of survival. Most previous studies that had evaluated the prognostic importance of MSI, CIMP and mutations in BRAF and KRAS had evaluated these markers individually. A recent meta-analysis associated high MSI with a 40% increase in overall survival (95% CI 31%–47%),19 and the BRAF V600E mutation with poorer survival. In contrast, data from studies on survival in relation to positive CIMP and mutations in KRAS and NRAS have been inconsistent, probably because the other molecular characteristics were not taken into consideration.
The biological basis of the differences observed in the specific CRC subtype survival continue to be an important topic for future research. In fact, although subtypes 2 and 3 were diagnosed in an advanced stage in the study by Phipps et al., the authors suggest that this poorer prognosis is related with a more aggressive natural history, a hypothesis that was confirmed in the study by Sinicrope et al.17,18
Therapeutic implications of molecular classificationThe findings of the 2 articles provide a basis for establishing a prognostic value for molecular classification in CRC. However, these studies were not designed to analyse differences in response to treatment according to subtype. In this respect, MSI-high or CIMP-positive patients are known to show no response to 5-fluorouracil as monotherapy.20,21 For this reason, in intermediate stages (TNM II) with better prognosis, the MSI status should be evaluated before considering the need for and type of adjuvant chemotherapy.22,23 Similarly, the mutational status of the KRAS/NRAS gene is a predictor of response to specific therapies targeting the epidermal growth factor receptor, e.g. cetuximab and panitumumab. It is important to note that not only patients with the mutated KRAS/NRAS gene fail to respond to these treatments, but also patients with native KRAS/NRAS but a mutated BRAF gene have lower disease-free interval and overall survival.24 For this reason, mutations should be determined not only in KRAS/NRAS but also in the BRAF gene to guide therapy in all patients with metastatic CRC.
Another significant breakthrough in the treatment of CRC has been published recently. The programmed death-1 (PD-1) pathway is a negative feedback system that controls the Th1 cytotoxic immune response. This pathway is active in many tumours, causing inactivation of T-suppressor lymphocytes and disabling the immune response. Blockade with PD-1-targeted antibodies produces significant clinical responses in various tumours. However, the study by Topalian et al. found that only 1 out of 33 patients with CRC responded to PD-1 blockade, and that this response was associated with the presence of high MSI.25 For this reason, Le et al. conducted a phase II study to evaluate the clinical activity of pembrolizumab in 41 patients with metastatic cancers refractory to treatment, according to MSI status. In this study, patients with MSI-high CRC treated with pembrolizumab had a higher rate of response (40% vs 0%) and progression-free survival (78% vs 11%), and a lower risk of disease progression (HR 0.04; 95% CI 0.01–0.21) or death (HR 0.18; 95% CI 0.03–1.01) compared to patients with MSI-low CRC. Moreover, patients with MSI-high non-CRCs had similar responses to those with MSI-high CRC.26
In conclusion, the latest findings obtained, together with existing information, show the importance of molecular classification of CRC in establishing and reporting the prognosis of this disease to patients. Similarly, the molecular defects implicated in tumour development must be understood in order to choose the best therapy (adjuvant treatment, chemotherapy in metastatic CRC as first-line and after progression). In the near future, decision-making in patients with CRC will therefore be determined not only by the tumour stage, but also by the different molecular subtypes described in the literature.
FundingJoaquín Cubiella has received an enrichment grant through the BIOCAPS programme funded by the European Commission (FP-7-REGPOT 2012-2013-1, Grant agreement no. FP7-316265).
Please cite this article as: Areses Manrique MC, Iglesias Rey L, Cubiella J. El largo camino de la biología molecular a la práctica clínica en el cáncer colorrectal. Gastroenterol Hepatol. 2016;39:429–432.