The pandemic caused by the SARS-CoV-2 virus has had a serious impact on the functioning of gastrointestinal endoscopy Units. The Asociación Española de Gastroenterología [Spanish Association of Gastroenterology] (AEG) and the Sociedad Española de Endoscopia Digestiva [Spanish Association of Gastrointestinal Endoscopy] (SEED) have proposed the EPAGE guidelines for managing postponed colonoscopies.
ObjectiveTo evaluate the EPAGE guidelines as a management tool compared to the immunologic faecal occult blood test (iFOBT) and compared to risk score (RS) that combines age, sex and the iFOBT for the detection of colorectal cancer (CRC) and significant bowel disease (SBD).
MethodsA prospective, single-centre study enrolling 743 symptomatic patients referred for a diagnostic colonoscopy. Each order was classified according to the EPAGE guidelines as appropriate, indeterminate or inappropriate. Patients underwent an iFOBT and had their RS calculated.
ResultsThe iFOBT (P < .001), but not the EPAGE guidelines (P = .742), was an independent predictive factor of risk of CRC. The ROC AUCs for the EPAGE guidelines, the iFOBT and the RS were 0.61 (95%CI 0.49−0.75), 0.95 (0.93−0.97) and 0.90 (0.87−0.93) for CRC, and 0.55 (0.49−0.61), 0.75 (0.69−0.813) and 0.78 (0.73−0.83) for SBD, respectively. The numbers of colonoscopies needed to detect a case of CRC and a case of SBD were 38 and 7 for the EPAGE guidelines, 7 and 2 for the iFOBT, and 19 and 4 for a RS ≥ 5 points, respectively.
ConclusionThe EPAGE guidelines, unlike the iFOBT, is not suitable for screening candidate patients for a diagnostic colonoscopy to detect CRC. The iFOBT, in combination with age and sex, is the most suitable strategy for managing demand for endoscopy in a restricted-access situation.
La pandemia producida por el virus SARS-CoV-2 ha generado un grave impacto en el funcionamiento de las unidades de endoscopia digestiva. La AEG-SEED han propuesto la utilización de la guía EPAGE para la gestión de las colonoscopias pospuestas.
ObjetivoEvaluar la guía EPAGE como herramienta de gestión en comparación con el test de sangre oculta en heces inmunológico (TSOHi) y con una calculadora de riesgo (CR), que incluye la edad, el sexo y el TSOHi, para la detección de cáncer colorrectal (CCR) y lesión significativa colónica (LSC).
MétodosEstudio unicéntrico prospectivo. Se incluyeron 743 pacientes derivados para una colonoscopia diagnóstica. Se clasificó cada solicitud según EPAGE en apropiada, indeterminada e inapropiada. Se les entregó un TSOHi y se calculó el valor de la CR.
ResultadosEl TSOHi (P < ,001), pero no EPAGE (P = ,742), fue una variable independiente de riesgo de CCR. El AUC ROC de EPAGE, TSOHi y CR fue: 0,61(IC95% 0,49–0,75), 0,95(0,93–0,97) y 0,90(0,87–0,93) para CCR; y 0,55(0,49–0,61), 0,75(0,69–0,813) y 0,78(0,73–0,83) para LSC, respectivamente. El número necesario de colonoscopias para detectar un CCR y una LSC fue de 38 y 7 para EPAGE, de 7 y 2 para TSOHi, y de 19 y 4 para CR ≥ 5 puntos, respectivamente.
ConclusiónEPAGE, a diferencia del TSOHi, no es adecuado para seleccionar a los pacientes candidatos a colonoscopia diagnóstica para la detección de CCR. El TSOHi, en combinación con la edad y el sexo, es la estrategia más adecuada para gestionar la demanda de endoscopia en un escenario de acceso restrictivo.
The situation of health emergency due to the COVID-19 pandemic, caused by the SARS-CoV-2 virus, has had a serious impact on the activity of gastrointestinal endoscopy units worldwide. The need to limit access to the hospital and the identification of gastrointestinal endoscopies as high-risk procedures1 have led to the cancellation of practically all of these examinations, with only those considered emergencies procedures being carried out under strict protective measures.
During this time, several authors have reported a high risk of contagion and transmission of the SARS-CoV-2 virus on endoscopy units, due to the generation of microdroplets in the air.1–3 Infections due to the production of periendoscopic aerosols for up to three to six hours in closed rooms have been reported.1–3 There have also been findings of live viruses in the faeces of patients with COVID-19.1–4 Therefore, the risk of transmission during lower gastrointestinal endoscopy procedures cannot be underestimated.
In the context of the progressive decline in the number of cases of hospitalisation for COVID-19, it is now a challenge to resume activity in endoscopy rooms severely depleted by multiple limitations. First, there is the reduction in available personnel (nursing, endoscopy and anaesthesia), due to SARS-CoV-2 infection itself or assignment to care rooms for patients with COVID-19. Second, mechanical ventilation equipment has been temporarily allocated to makeshift intensive care areas. Third, the need to reduce patient exposure in waiting and recovery rooms and to ensure the cleanliness of endoscopy rooms, as well as the requirement to use protective equipment, is limiting the number of examinations per room. Finally, the risk of transmission of SARS-CoV-2 has caused a change in the risk–benefit ratio of endoscopic examinations. Added to these structural problems, which preclude resuming activity at maximum capacity, is the lengthening of waiting lists for examinations not performed in this period.
Faced with this worrying situation, different scientific societies such as the Societat Catalana de Digestologia [Catalan Society of Gastroenterology] (SCD),5 the Sociedad Española de Endoscopia Digestiva [Spanish Society of Gastrointestinal Endoscopy] (SEED)6 and the Asociación Española de Gastroenterología [Spanish Association of Gastroenterology] (AEG)6 have focused their efforts on developing prioritisation protocols for examinations with a higher diagnostic and therapeutic performance. Specifically, in the case of diagnostic colonoscopy, the AEG and the SEED recommend assessing the correct indication based on the results of the European Panel on the Appropriateness of Gastrointestinal Endoscopy II (EPAGE).7 These are guidelines updated in 2008 that determine the appropriateness of the indication for endoscopy based on the scientific evidence evaluated by an international expert committee.8 In the case of diagnostic colonoscopy, they focus on the value of abdominal symptoms with greater precision in the identification of colorectal cancer (CRC).9–12
In recent years, scientific evidence has proliferated on the usefulness of the immunological faecal occult blood test (iFOBT) in detecting CRC or significant colonic lesion (SCL), finding a better discriminatory capacity than abdominal symptoms.13–16 In addition, a risk calculation (RC) based on the iFOBT, age and sex has been described that offers a simple, precise way of estimating the risk of advanced neoplasia (AN), that is, advanced adenoma or cancer.16
Due to the exceptional situation in which we find ourselves, efficient waiting list management is critical to prevent diagnostic delay in patients with CRC or other significant lesions and to avoid performing unnecessary tests that compete with those indicated as preferential or priority. The objective of our study, therefore, is to evaluate whether the iFOBT or the above-mentioned RC is a better tool for prioritising and determining the appropriateness of diagnostic colonoscopy for the detection of CRC and SCL compared to the EPAGE guidelines.
Material and methodsStudy designThis is a post hoc analysis of a previous study that described the value of the iFOBT as a strategy for prioritising diagnostic colonoscopies for the detection of AN in symptomatic patients.16 In short, it is a prospective single-centre study in which all patients over 18 years of age for whom a diagnostic colonoscopy had been ordered at Hospital Universitari de Bellvitge [Bellvitge University Hospital] between September 2011 and October 2012 were invited to participate. Those who had required colonoscopy for the follow-up of polyposis syndromes, post-endoscopic polypectomy surveillance or as surveillance after CRC resection were excluded. Hospitalised patients who had undergone colectomy or with a personal history of inflammatory bowel disease (IBD) were excluded as well. Individuals who, due to their symptoms or reason for requiring a colonoscopy, could not be classified in any EPAGE category were also disqualified from inclusion. The patient selection flow chart is shown in Appendix B, Supplementary data.
Eligible patients were invited to a consultation with the researcher, in which they were administered a thorough questionnaire on their medical history, toxic habits and drug use, as well as gastrointestinal symptoms. Finally, the presence of iron deficiency anaemia was confirmed or ruled out in laboratory tests performed as a pre-anaesthetic evaluation prior to the endoscopy. After signing the informed consent form, the patients who agreed to participate received an Eiken Chemical OC-Sensor® faecal occult blood test, as well as instructions on how to perform it. Samples that were not properly collected or stored were excluded from the study. The rest were analysed by an expert technician using the OC-Sensor MICRO desktop analyser (Eiken Chemical 1 Co., Ltd., Tokyo, Japan). Both the technician and the endoscopist were blinded to patient data and iFOBT results.
All the colonoscopies were performed by experienced endoscopists. The colonoscopy was considered complete if caecal intubation was achieved, demonstrated by visualisation of the ileocaecal valve or the appendiceal orifice. Bowel preparation was considered adequate according to the validated Boston Bowel Preparation Scale. The data recorded included the number, size and histology of polyps and the presence or absence of CRC. The study was approved by the scientific committee of the Institut d'Investigació Biomèdica de Bellvitge [Bellvitge Biomedical Research Institute] (IDIBELL) of Hospital Universitari de Bellvitge and its ethics committee (reference PR283/11).
Study variablesThe dependent variables analysed were the detection of CRC and SCL (defined as the presence of CRC, advanced adenoma [AA] or IBD). AA was defined as the presence of an adenoma ≥10 mm, with a villous component or high-grade dysplasia. The following variables were recorded due to their potential association with CRC or SCL: sex, age, body mass index (BMI), diabetes mellitus, dyslipidaemia, nonsteroidal anti-inflammatory drugs (NSAIDs), antiplatelet or anticoagulant therapy, alcohol and tobacco use, family history of risk of CRC, personal history of colorectal adenomas, iron deficiency anaemia (IDA), abdominal signs or symptoms, and faecal haemoglobin concentration (f-Hb) measured by the iFOBT. The presence of significant IDA was conceptualised according to the definition of the National Institute for Health and Care Excellence (NICE) as a haemoglobin concentration ≤10 g/dl in women and ≤11 g/dl in men. The origin of the colonoscopy order and the reason for referral, as well as the appropriateness thereof, based on the EPAGE guidelines, were recorded. The iFOBT result was considered a quantitative and qualitative variable, using, in the latter case, the following cut-off points for f-Hb: ≥10 μg Hb/g of faeces, ≥15 μg Hb/g and ≥20 μg Hb/g.
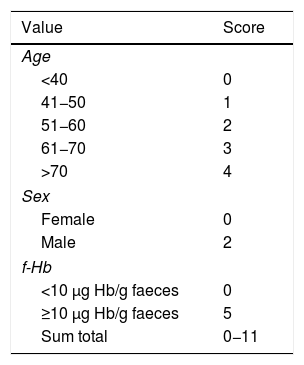
In addition, the value of a risk index for AN, that is, CRC or AA, was calculated based on age, sex and an f-Hb value ≥10 μg Hb/g of faeces, developed in a previous study.16 In summary, to perform this AN RC, each independent risk factor was assigned a weighted value according to the respective coefficients in the multiple logistic regression model.16 This risk scoring system has a range of 0–11 points depending on the presence or absence of a characteristic or risk factor in each subject. Table 1 shows the risk factors and the score assigned to each of them.
European panel on the appropriateness of gastrointestinal endoscopy IIThe EPAGE II guidelines are open-access online guidelines designed to help healthcare professionals evaluate the appropriateness of colonoscopy. They are based on a questionnaire that includes abdominal signs and symptoms, age, personal and family history of CRC, and previous examinations. A score of 1–9 is obtained; a score of 1–3 is considered inappropriate, a score of 4–6 uncertain and a score of 7–9 appropriate. To perform the statistical analysis, two EPAGE categories were used. The first, called EPAGE I-A, considered appropriate both colonoscopy orders classified as uncertain and those classified as appropriate by the questionnaire offered in the guidelines. The second, called EPAGE A, deemed appropriate only colonoscopy orders classified as appropriate.
Statistical analysisCategorical variables were presented in terms of number and proportion with respect to the study population (%). A univariate analysis of the categorical variables was performed using the X2 test and Fisher's F test, and of the quantitative variables using Student's t test. A multivariate analysis was performed using binary logistic regression to identify independent predictive factors of CRC and SCL, including the significant variables in the univariate analysis (P < .05). The variables that were not significant (but considered important in the previous scientific literature) were also evaluated. Results are presented with odds ratios (ORs) and 95% confidence intervals (CIs).
The diagnostic precision of both the EPAGE guidelines and the iFOBT for the prediction of CRC and SCL was determined by assessing the area under the receiver operating characteristic (ROC) curve. Finally, the number of endoscopies required (NER), defined as the number of people with certain characteristics who should undergo a colonoscopy to detect a case of CRC or SCL, was calculated.
ResultsDescriptive findingsDuring the study period, 1054 patients were referred for a diagnostic colonoscopy and therefore were potentially eligible candidates. Ultimately, 743 individuals were included in the analysis. Among the 743 patients, 428 (57.6%) were referred from primary care and 203 (42.4%) were referred from secondary or tertiary care. In total, 17 (2.3%) cases of CRC and 103 (13.9%) cases of SCL were detected, including 68 (9.2%) cases of AA and 6 (0.8%) cases of IBD.
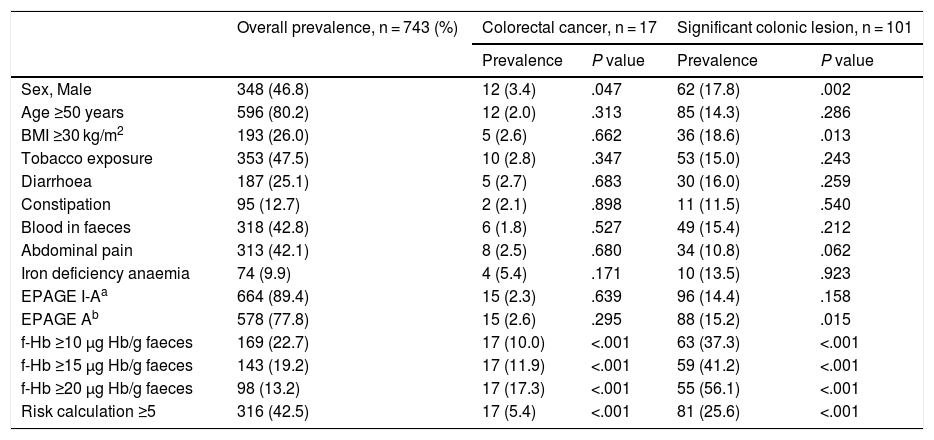
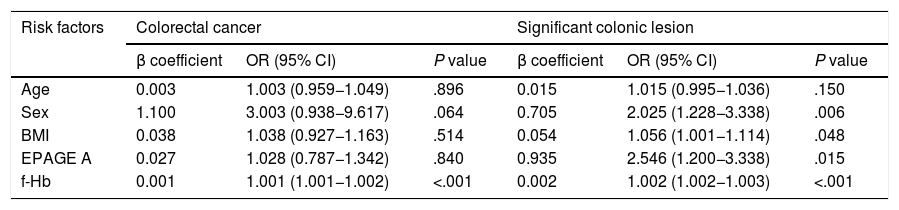
Univariate and multivariate analysis for prediction of colorectal cancer and significant colonic lesionFor the detection of CRC, the univariate analysis showed that male sex, a positive iFOBT (at any cut-off point) and a value ≥5 in the RC were risk factors for CRC with statistically significant differences. Neither EPAGE I-A nor EPAGE A was associated with CRC (Table 2). The multivariate analysis showed that the f-Hb value was an independent risk variable for CRC, adjusted for sex; BMI; age; and EPAGE, both entered as an EPAGE I-A and as an EPAGE A variable. Likewise, the value obtained in the RC was an independent risk variable for CRC, adjusted for EPAGE, both entered as an EPAGE I-A and as an EPAGE A variable (Table 3, Appendix B, Supplementary data).
Prevalence of colorectal cancer and significant colonic lesion according to risk variables.
| Overall prevalence, n = 743 (%) | Colorectal cancer, n = 17 | Significant colonic lesion, n = 101 | |||
|---|---|---|---|---|---|
| Prevalence | P value | Prevalence | P value | ||
| Sex, Male | 348 (46.8) | 12 (3.4) | .047 | 62 (17.8) | .002 |
| Age ≥50 years | 596 (80.2) | 12 (2.0) | .313 | 85 (14.3) | .286 |
| BMI ≥30 kg/m2 | 193 (26.0) | 5 (2.6) | .662 | 36 (18.6) | .013 |
| Tobacco exposure | 353 (47.5) | 10 (2.8) | .347 | 53 (15.0) | .243 |
| Diarrhoea | 187 (25.1) | 5 (2.7) | .683 | 30 (16.0) | .259 |
| Constipation | 95 (12.7) | 2 (2.1) | .898 | 11 (11.5) | .540 |
| Blood in faeces | 318 (42.8) | 6 (1.8) | .527 | 49 (15.4) | .212 |
| Abdominal pain | 313 (42.1) | 8 (2.5) | .680 | 34 (10.8) | .062 |
| Iron deficiency anaemia | 74 (9.9) | 4 (5.4) | .171 | 10 (13.5) | .923 |
| EPAGE I-Aa | 664 (89.4) | 15 (2.3) | .639 | 96 (14.4) | .158 |
| EPAGE Ab | 578 (77.8) | 15 (2.6) | .295 | 88 (15.2) | .015 |
| f-Hb ≥10 μg Hb/g faeces | 169 (22.7) | 17 (10.0) | <.001 | 63 (37.3) | <.001 |
| f-Hb ≥15 μg Hb/g faeces | 143 (19.2) | 17 (11.9) | <.001 | 59 (41.2) | <.001 |
| f-Hb ≥20 μg Hb/g faeces | 98 (13.2) | 17 (17.3) | <.001 | 55 (56.1) | <.001 |
| Risk calculation ≥5 | 316 (42.5) | 17 (5.4) | <.001 | 81 (25.6) | <.001 |
BMI: body mass index; f-Hb: faecal haemoglobin concentration.
A) Multivariate analysis for the detection of colorectal cancer and significant colonic lesion adjusted for an order considered appropriate according to EPAGE and faecal haemoglobin concentration.
| Risk factors | Colorectal cancer | Significant colonic lesion | ||||
|---|---|---|---|---|---|---|
| β coefficient | OR (95% CI) | P value | β coefficient | OR (95% CI) | P value | |
| Age | 0.003 | 1.003 (0.959−1.049) | .896 | 0.015 | 1.015 (0.995−1.036) | .150 |
| Sex | 1.100 | 3.003 (0.938−9.617) | .064 | 0.705 | 2.025 (1.228−3.338) | .006 |
| BMI | 0.038 | 1.038 (0.927−1.163) | .514 | 0.054 | 1.056 (1.001−1.114) | .048 |
| EPAGE A | 0.027 | 1.028 (0.787−1.342) | .840 | 0.935 | 2.546 (1.200−3.338) | .015 |
| f-Hb | 0.001 | 1.001 (1.001−1.002) | <.001 | 0.002 | 1.002 (1.002−1.003) | <.001 |
| B) Multivariate analysis for the detection of colorectal cancer and significant colonic lesion adjusted for an order considered appropriate according to EPAGE and the AN RC value | ||||||
|---|---|---|---|---|---|---|
| Risk factors | Colorectal cancer | Significant colonic lesion | ||||
| β coefficient | OR (95% CI) | P value | β coefficient | OR (95% CI) | P value | |
| BMI | 0.026 | 1.027 (0.917−1.149) | .648 | 0.046 | 1.048 (1.300−1.537) | .061 |
| EPAGE A | 0.388 | 1.474 (0.317−6.852) | .620 | 0.756 | 2.130 (1.067−4.252) | .032 |
| Risk calculation | 0.567 | 1.762 (1.383−2.2) | <.001 | 0.346 | 1.414 (1.300−1.537) | <.001 |
BMI: body mass index; EPAGE A: colonoscopy order considered appropriate; f-Hb: faecal haemoglobin concentration (quantitative value μg Hb/g faeces).
For the detection of SCL, the univariate analysis showed that male sex; BMI; EPAGE, both entered as an EPAGE I-A and as an EPAGE A variable; a positive iFOBT (at any cut-off point); and a value ≥5 in the RC were risk factors for SCL, with statistically significant differences (Table 2). The multivariate analysis showed that male sex; BMI; EPAGE, both entered as an EPAGE I-A and as an EPAGE A variable; and f-Hb were independent variables of SCL risk. Similarly, the value obtained in the RC was an independent risk variable for SCL adjusted for an appropriate order according to EPAGE (Table 3, Appendix B, Supplementary data).
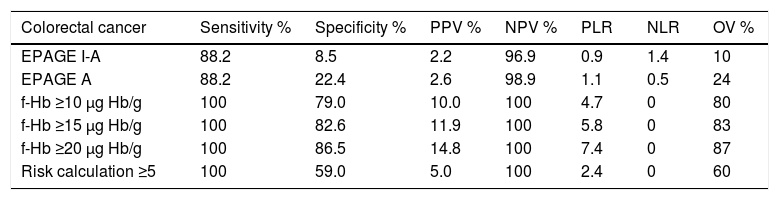
Diagnostic accuracy of EPAGE, faecal occult blood test and risk calculationThe diagnostic precision for the detection of CRC and SCL is shown in Table 4. The areas under the ROC curve for EPAGE for the detection of CRC and SCL were 0.61 (95% CI 0.49−0.75) and 0.55 (95% CI 0.49−0.61), respectively. The iFOBTs were 0.95 (95% CI 0.93−0.97) and 0.75 (95% CI 0.69−0.813), respectively, and the RCs were 0.90 (95% CI 0.87−0.93) and 0.78 (95% CI 0.73−0.83), respectively.
Diagnostic precision parameters of the EPAGE guidelines and of the value of the faecal occult blood test for the diagnosis of colorectal cancer and significant colonic lesion.
| Colorectal cancer | Sensitivity % | Specificity % | PPV % | NPV % | PLR | NLR | OV % |
|---|---|---|---|---|---|---|---|
| EPAGE I-A | 88.2 | 8.5 | 2.2 | 96.9 | 0.9 | 1.4 | 10 |
| EPAGE A | 88.2 | 22.4 | 2.6 | 98.9 | 1.1 | 0.5 | 24 |
| f-Hb ≥10 μg Hb/g | 100 | 79.0 | 10.0 | 100 | 4.7 | 0 | 80 |
| f-Hb ≥15 μg Hb/g | 100 | 82.6 | 11.9 | 100 | 5.8 | 0 | 83 |
| f-Hb ≥20 μg Hb/g | 100 | 86.5 | 14.8 | 100 | 7.4 | 0 | 87 |
| Risk calculation ≥5 | 100 | 59.0 | 5.0 | 100 | 2.4 | 0 | 60 |
| Significant colonic lesion | Sensitivity % | Specificity % | PPV % | NPV % | PLR | NLR | OV % |
|---|---|---|---|---|---|---|---|
| EPAGE I-A | 95.0 | 9.2 | 14.1 | 92.2 | 1.0 | 0.5 | 21 |
| EPAGE A | 87.1 | 23.7 | 15.2 | 92.1 | 1.1 | 0.5 | 32 |
| f-Hb ≥10 μg Hb/g | 62.3 | 83.5 | 37.2 | 93.4 | 3.7 | 0.4 | 81 |
| f-Hb ≥15 μg Hb/g | 58.4 | 83.0 | 41.2 | 93.0 | 4.4 | 0.5 | 83 |
| f-Hb ≥20 μg Hb/g | 54.4 | 90.6 | 47.8 | 92.7 | 5.8 | 0.5 | 86 |
| Risk calculation ≥5 | 80.0 | 63.0 | 26.0 | 95.0 | 2.2 | 0.3 | 66 |
EPAGE A: considers appropriate colonoscopy orders classified as appropriate by EPAGE; EPAGE I-A: considers appropriate colonoscopy orders classified as uncertain or appropriate by EPAGE; f-Hb: faecal haemoglobin concentration; NLR: negative likelihood ratio; NPV: negative predictive value; OV: overall value; PLR: positive likelihood ratio; PPV: positive predictive value.
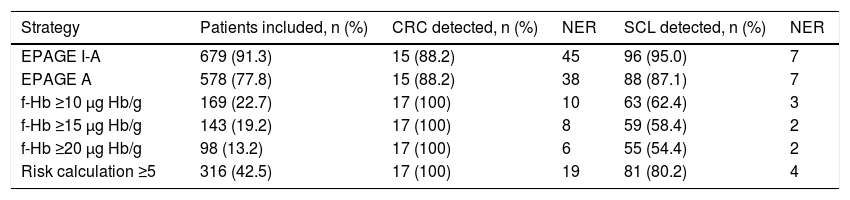
The percentages of patients whose order was classified as appropriate according to EPAGE I-A, EPAGE A and positive iFOBT with f-Hb values ≥10 μg, Hb/g, ≥15 μg Hb/g, ≥20 μg Hb/g were 91.3%, 77.8%, 22.7%, 19.2% and 13.2%, respectively. EPAGE I-A and EPAGE A detected 88.2% of cases of CRC. The iFOBT, at any of its cut-off points, identified 100% of cases of CRC. Table 5 shows the different management strategies in terms of number of selected patients and NER to detect a case of CRC and SCL.
Comparison of the different management strategies based on the number of patients included, colorectal cancer and significant colonic lesion detected, and number of endoscopies required to detect a lesion.
| Strategy | Patients included, n (%) | CRC detected, n (%) | NER | SCL detected, n (%) | NER |
|---|---|---|---|---|---|
| EPAGE I-A | 679 (91.3) | 15 (88.2) | 45 | 96 (95.0) | 7 |
| EPAGE A | 578 (77.8) | 15 (88.2) | 38 | 88 (87.1) | 7 |
| f-Hb ≥10 μg Hb/g | 169 (22.7) | 17 (100) | 10 | 63 (62.4) | 3 |
| f-Hb ≥15 μg Hb/g | 143 (19.2) | 17 (100) | 8 | 59 (58.4) | 2 |
| f-Hb ≥20 μg Hb/g | 98 (13.2) | 17 (100) | 6 | 55 (54.4) | 2 |
| Risk calculation ≥5 | 316 (42.5) | 17 (100) | 19 | 81 (80.2) | 4 |
CRC: colorectal cancer; EPAGE A: considers appropriate colonoscopy orders classified as appropriate by EPAGE; EPAGE I-A: considers appropriate colonoscopy orders classified as uncertain or appropriate by EPAGE; f-Hb: faecal haemoglobin concentration; NER: number of endoscopies required; SCL: significant colonic lesion.
Our study shows that f-Hb measured by an iFOBT is a tool for prioritising and determining the appropriateness of diagnostic colonoscopy for the detection of CRC that is superior to the EPAGE guidelines in patients for whom a diagnostic colonoscopy has been ordered.
The current pandemic caused by the SARS-CoV-2 virus has led to the shutdown of gastrointestinal endoscopy units, resulting in waiting lists that are difficult to manage using a system that, in addition, must resume its activity gradually. We therefore ask ourselves what is the best way to manage these waiting lists in terms of appropriateness and prioritisation. That is to say, it is important not only to select those patients who genuinely require an endoscopic examination, but also to identify the ones on the waiting list who will need an endoscopic examination on a priority basis.
In terms of prioritisation, it is important to be aware that the performance of abdominal symptoms used to identify CRC is low. Overall, only 2% of patients with abdominal symptoms were diagnosed with CRC.9,10,17,18 Therefore, managing cases based solely on these symptoms can lead to missed or delayed diagnoses in people with significant lesions. Furthermore, no abdominal signs or symptoms have been associated with the presence of AA in the literature. The NICE clinical practice guidelines for the diagnosis of CRC use a combination of age-related symptoms, which increases the positive predictive value (PPV) of the individual symptoms.19,20 Likewise, there is evidence of the superiority of the iFOBT over clinical guidelines based on symptoms as a tool for the rapid diagnosis of CRC.16,21 Since 2018, based on the available scientific evidence, the NICE guidelines include the iFOBT as a prioritisation tool in the rapid diagnosis of CRC.20
Even though they are simple criteria, the use of warning symptoms or of the iFOBT is not widespread in daily practice in our setting. Moreover, in Spain, there is no consensus on which signs or symptoms should be considered preferential. In most cases, the physician decides on the prioritisation based on a subjective explanation of an order. Therefore, the situation created by COVID-19 has revealed our shortcomings in prioritising gastrointestinal endoscopies.
In terms of appropriateness, different scientific societies have developed guidelines to establish indications for colonoscopy. Specifically, EPAGE developed guidelines that determine the appropriateness of colonoscopy based on scientific evidence evaluated by an international expert committee. The SEED and the AEG have proposed the EPAGE guidelines as a strategy for establishing the appropriateness of colonoscopies at the present time.7 The applicability thereof was validated in our setting,11,12 with demonstration of a significant association between the correct indication for the examination and the finding of significant lesions (42 versus 21%, P < .001). From this study, it can be deduced that adjusting to EPAGE could better select patients who require a colonoscopy, although it shows limitations. On the one hand, more than half of the individuals evaluated as having an appropriate indication for a colonoscopy did not have any lesion, while up to 20% of colonoscopies identified as inappropriate revealed a significant lesion.11
In recent years, attention has been focused on the search for biomarkers that enable prediction of the presence of CRC or SCL among patients who show abdominal symptoms, the most studied one being the iFOBT.13,14,21–23 McDonald et al. evaluated whether the determination of f-Hb, measured through an iFOBT, could help decide which people with abdominal symptoms would benefit from a colonoscopy.22 The authors found that subjects with significant colorectal disease had a median f-Hb of 15 μg Hb/g faeces, significantly higher than that of participants without significant disease (P < .0001). Westwood et al. conducted a systematic review including nine studies that evaluated the f-Hb value in a symptomatic population. The authors concluded that an f-Hb ≥10 μg Hb/g faeces made it possible to correctly detect patients with CRC and avoid 75%–80% of colonoscopies in symptomatic individuals.24
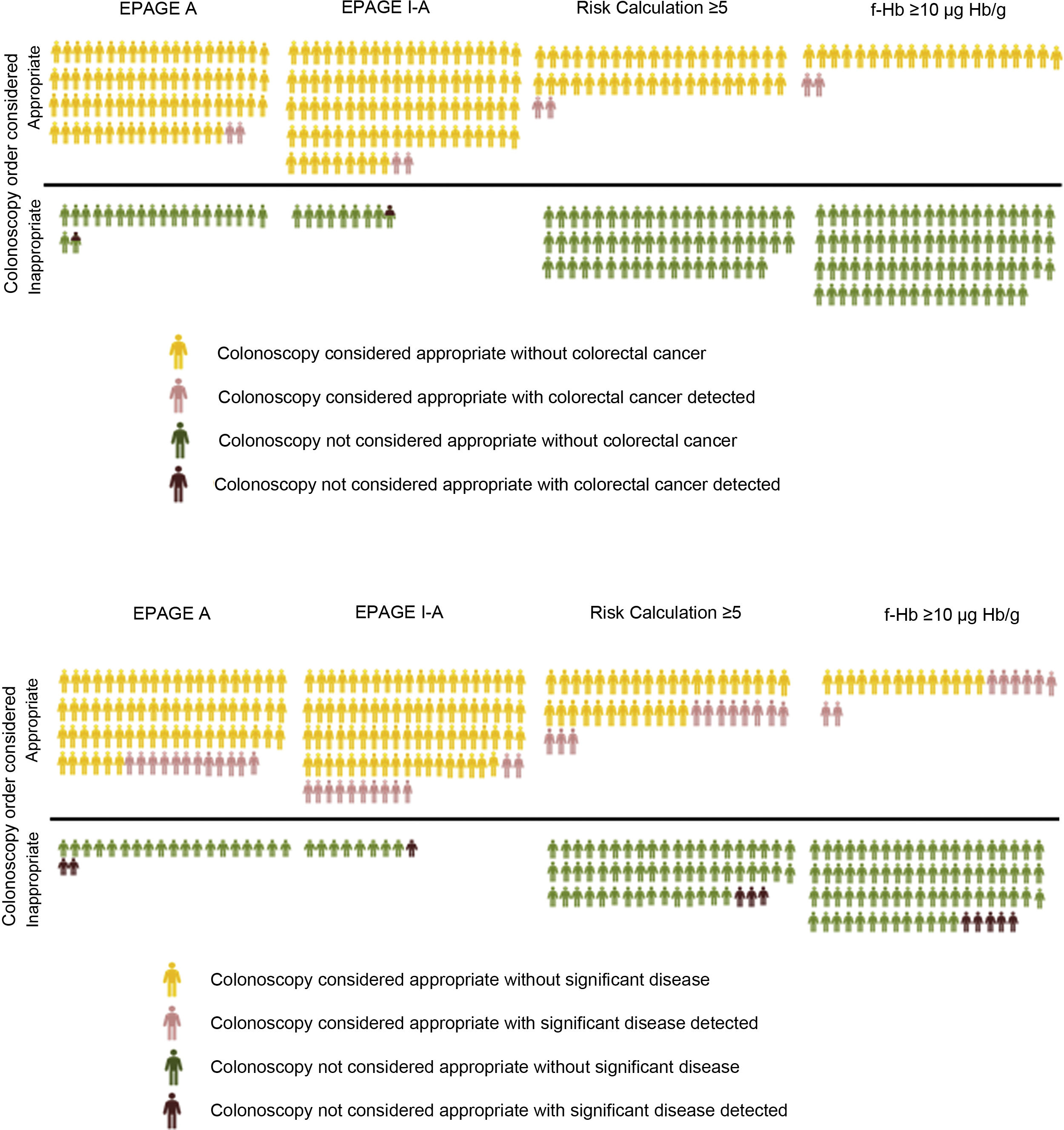
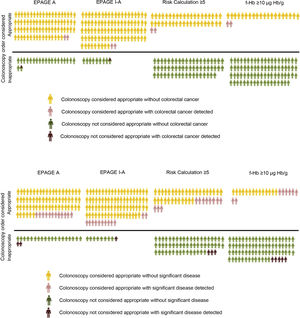
In our study, the value of the EPAGE guidelines was not associated with the diagnosis of CRC. However, the iFOBT was an independent risk variable for CRC diagnosis, with the area under the ROC curve clearly higher for f-Hb measured by the iFOBT compared to EPAGE (95.3 versus 61.8). Comparison of the EPAGE guidelines to a positive iFOBT, at its different cut-off points, as a colonoscopy waiting list management strategy reveals that the former deems practically all orders (92% of the total) uncertain or appropriate. However, two patients with CRC would be classified as inappropriate and therefore would not have been diagnosed. The iFOBT considers a lower percentage of orders (13%–22%) appropriate, classifying all orders for patients with CRC as appropriate. It should be noted that some individuals awaiting the test have undetected CRC. Therefore, efforts should be focused on identifying these patients. In this sense, the concept of NER can aid in identifying the best strategy (Table 5). If the EPAGE I-A or EPAGE A guidelines are used, 45 or 38 colonoscopies should be performed, respectively, to identify a case of CRC. If the iFOBT is used as a management strategy, six to 10 tests should be performed to detect a case of CRC. Fig. 1 visually represents the percentage of colonoscopy orders considered appropriate depending on the strategy used, as well as the numbers of cases of CRC and SCL detected with both appropriate and inappropriate orders.
A) Percentage of colonoscopy orders considered appropriate based on the strategy used and the number of individuals with colorectal cancer detected in both appropriate and inappropriate orders. B) Percentage of colonoscopy orders considered appropriate based on the strategy used and the number of individuals with a significant colonic lesion detected in both appropriate and inappropriate orders.
In terms of appropriateness, it is not only the diagnosis of CRC but also the detection of SCL that is important. The aim is to identify patients for whom a colonoscopy will not provide added value in their diagnostic process. Although the EPAGE guidelines detect approximately 90% of SCLs, they do so at the expense of performing practically all examinations ordered (91%). If an appropriate colonoscopy order is considered to be one with a positive iFOBT, with f-Hb values ≥10 μg Hb/g, approximately 20% of patients would be selected. This strategy would detect 60% of SCLs, including 100% of those with CRC. The NERs for EPAGE and iFOBT for the detection of an SCL are seven and three, respectively.
The diagnostic precision of the iFOBT is limited when it comes to detecting advanced adenoma. Therefore, in a previous study, we designed an AN RC by means of a simple calculation based on three variables, shown in Table 1.16 The higher the score obtained, the greater the risk of the patient being diagnosed with AN in the endoscopic examination. In our study, the value obtained by this RC, which covered a range from 0 to 11, was an independent risk factor for both CRC and SCL. Based on the area under the curve, we found that a score greater than 5 in this calculation showed an optimal point between sensitivity and specificity for the detection of SCL. Therefore, a score greater than or equal to 5 in the RC means the possible cancellation of 58% of colonoscopy orders, detecting 100% of CRCs and 80% of SCLs. Furthermore, this numerical classification system would allow for ensuring not only the appropriateness of the examinations, but also their prioritisation, such that they could be performed first in the patients with the highest scores, who have a higher risk of CRC or SCL.
Our study has several limitations. First, it was conducted at a tertiary hospital, so there may be a selection bias. However, it is an open-access endoscopy unit and most of the orders come from the primary care setting. Second, we assessed the value of an iFOBT to determine the appropriateness of colonoscopy orders. In clinical practice, many of the patients referred for a diagnostic colonoscopy do not have an iFOBT result. However, the test is simple to perform, inexpensive and not subject to subjectivity as symptoms are, and its results can be available in 24–48 h. In the current context, we believe that the technical difficulties that may arise from ordering an iFOBT for patients on a colonoscopy waiting list are largely outweighed by its benefits when it comes to determining the appropriateness of and prioritising examinations. On the other hand, reliably determining EPAGE appropriateness, using the reason for ordering a colonoscopy, can be a complicated task that depends on the information received for the referral, which can be incomplete. Finally, having a quantitative result, such as that offered by the iFOBT or the RC, enables adoption of the optimal cut-off point depending on availability of endoscopy units.
In conclusion, the iFOBT is a method for prioritising and determining appropriateness that is superior to EPAGE for the detection of CRC. For the diagnosis of SCL, a simple calculation based on three variables (age, sex and iFOBT value) can aid in selecting patients who genuinely require a colonoscopy and prioritise those with higher risk. It is important to be aware that determining appropriateness or prioritising only by symptoms means assuming the loss of a non-negligible percentage of patients with CRC.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Our thanks to Ester Quilez for her administrative support and to Natividad Valera for her technical assistance in reading the faecal occult blood test.
Please cite this article as: Rodríguez-Alonso L, Rodríguez-Moranta F, Maisterra S, Botargues JM, Berrozpe A, Ruíz-Cerulla A, et al. EPAGE no es una estrategia eficaz para la gestión de colonoscopias durante la pandemia por COVID-19. Gastroenterol Hepatol. 2022;45:9–17.