The formation of fistulas from the pancreas to neighbouring organs as a complication of severe acute pancreatitis is well known, although rare.1 The most common such fistulas are pancreatic-colonic fistulas, which generally appear following pancreatic necrosectomy; it is very unusual for them to develop preoperatively.2,3 We present a case of severe acute pancreatitis with the complication of a spontaneous pancreatic-colonic fistula.
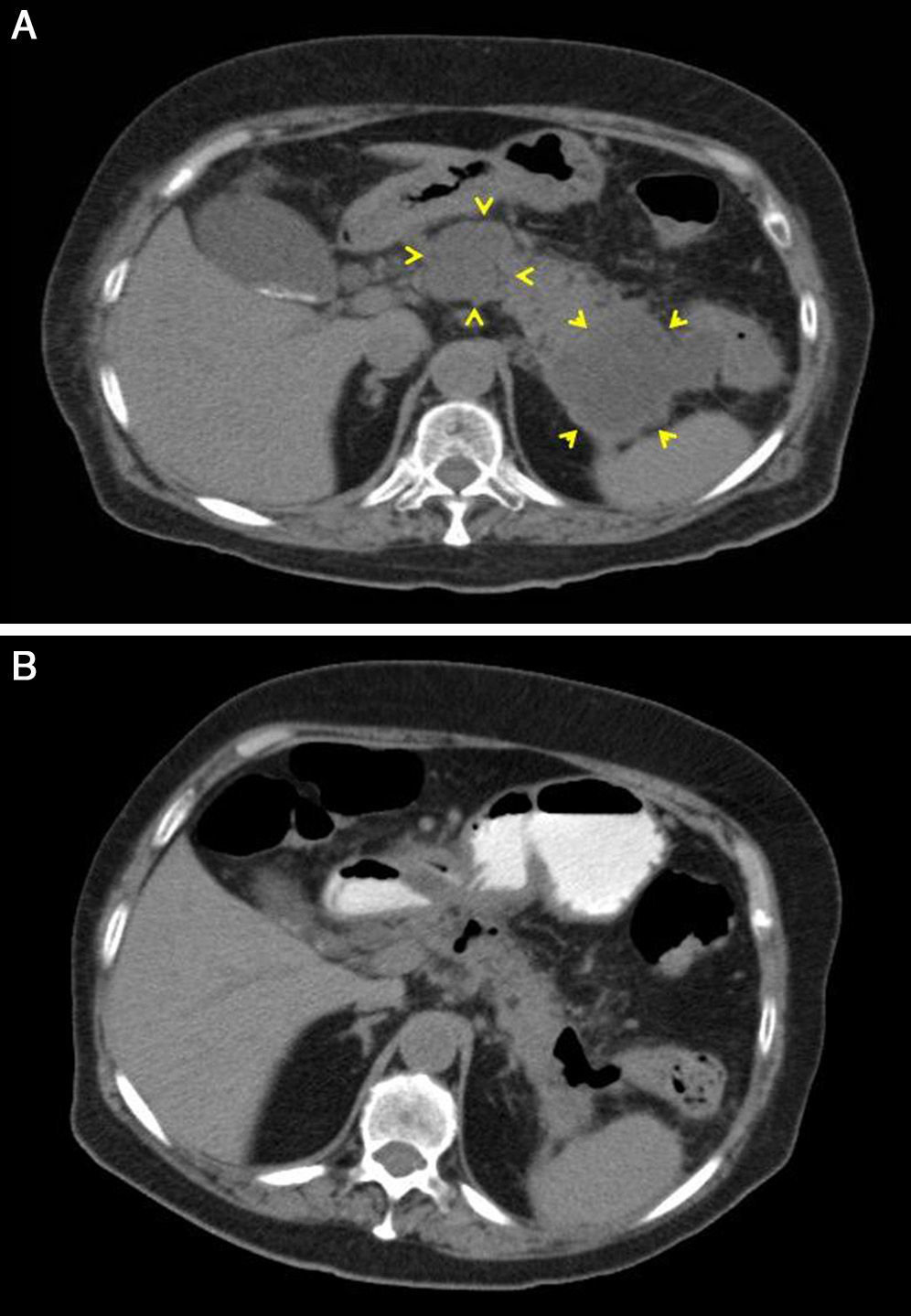
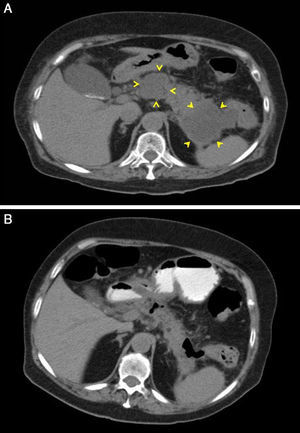
A 77-year-old woman with a personal history of chronic renal failure (CRF), ischaemic heart disease, pacemaker and mechanical aortic valve was admitted for a first episode of severe acute pancreatitis of biliary origin (cholelithiasis without cholecystitis on ultrasound). Computed tomography (CT) scans were performed, without IV contrast, initially because of exacerbation of the CRF and then because of patient refusal. Signs of septic shock 48hours after onset of symptoms were treated with piperacillin-tazobactam antibiotic. An urgent abdominal CT scan revealed blurring of the peripancreatic fat with no clear evidence of defined collections. On day 3, attempted endoscopic placement of a nasojejunal tube failed due to the presence of a duodenal stenosis with an inflamed appearance. Parenteral nutrition was started but was later complicated with bacteraemia caused by Staphylococcus epidermidis, for which vancomycin was added to the treatment. Owing to poor patient progress, another CT scan was performed 15 days later that showed 2 peripancreatic collections, one adjacent to the head and the other to the tail, measuring 50×37mm and 63×57mm, respectively (Fig. 1). Antibiotic cover and parenteral nutrition were maintained, with clinical and analytical improvement that continued after discontinuation of both. The patient was discharged 42 days after admission.
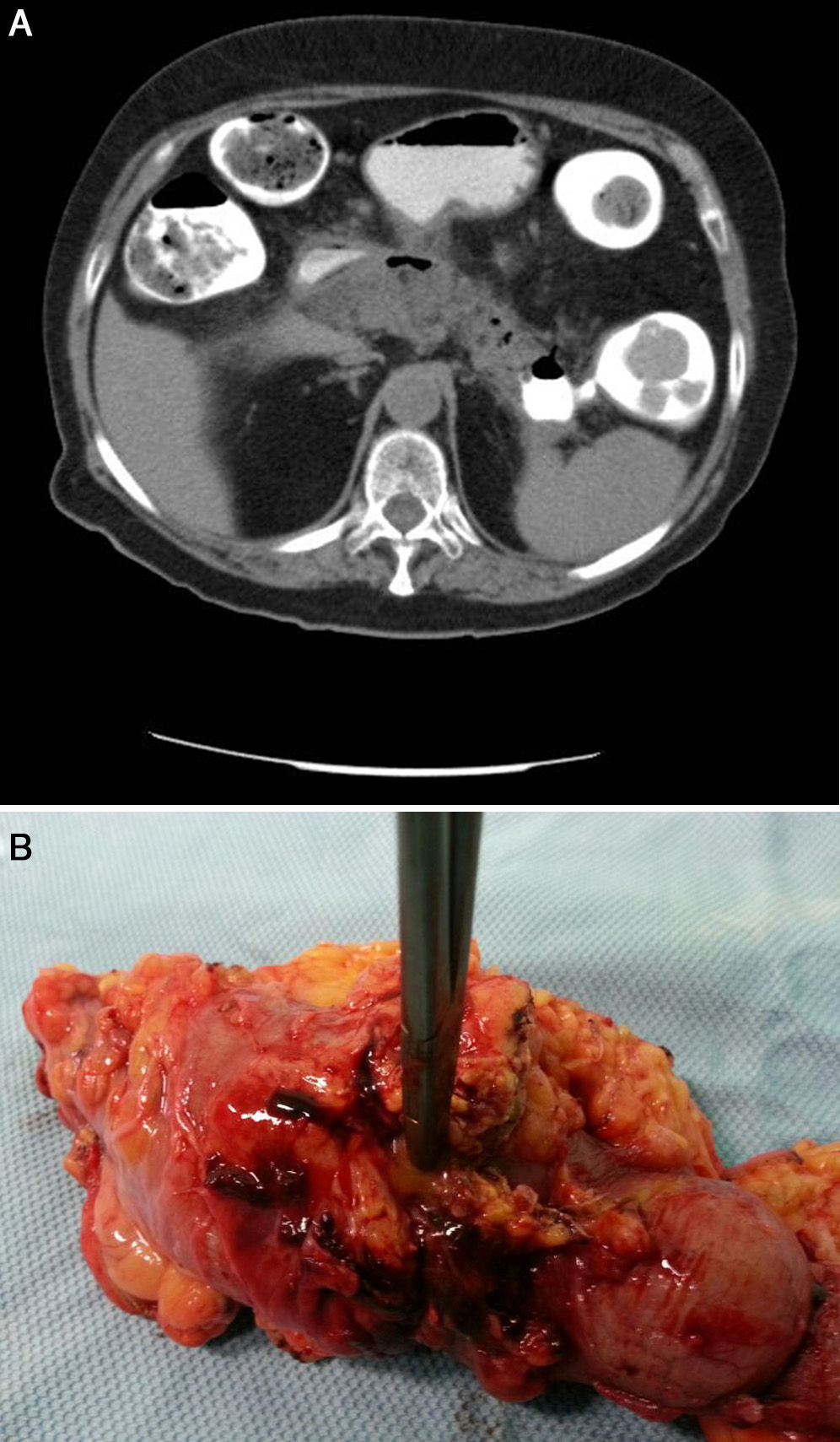
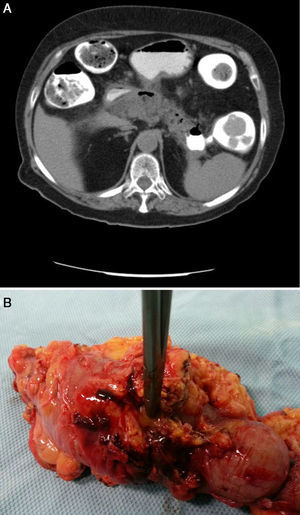
Twenty-four hours after discharge, the patient attended the emergency department for low-grade fever, vomiting and abdominal pain that fulfilled criteria for sepsis of probable abdominal origin. Empirical treatment was started with meropenem and an abdominal CT scan with oral contrast and enema revealed a decrease in the size of the peripancreatic collection of the head, with gas inside, suggestive of fistulization to the gastrointestinal tract (Fig. 1B). A CT scan of the colon confirmed the existence of a pancreatic-colonic fistula (Fig. 2A). Conservative treatment was commenced with nil by mouth and levofloxacin-metronidazole. The fistula persisted despite clinical and analytical improvement. Leukocytosis and acute phase reactants reappeared when antibiotic therapy was discontinued after 2 weeks. Given the low rate of spontaneous resolution of this type of fistula, we performed necrosectomy with segmented resection of the left colon and cholecystectomy (Fig. 2B). The patient required repeat surgery for suture dehiscence, for which a Hartmann reconstruction was performed, but eventually died of septic shock.
Pancreatic-enteric fistulas in the context of acute pancreatitis are well known, with an incidence of 3–10%.1,2 They can appear within 10–90 days after onset of pancreatitis. Most appear after necrosectomy, with preoperative presentation occurring only rarely.2 Pseudocysts/cysts tend to either rupture into the abdominal cavity or form a fistula in the gastrointestinal tract. The colon followed by the stomach and duodenum are the organs most affected.4,5
The main sign of fistulization is gastrointestinal bleeding (60% of patients), although the onset of vomiting or diarrhoea can also be indicators.4,6
Abdominal CT scans with contrast (oral, rectal or intravenous) and/or barium enema are generally used for diagnosis, despite having lower sensitivity compared to endoscopic retrograde cholangiopancreatography, traditionally considered the best diagnostic technique.5 The presence of air inside an area of pancreatic necrosis in a conventional CT scan can indicate either an infection of the pancreas or fistulization to the digestive tract. Endoscopy performed to investigate the location of the fistula opening to the gastrointestinal tract can be aided by the injection of a marker such as indigo carmine that will show the opening as a small erosion. However, few cases in the literature have been diagnosed using endoscopic techniques.7
Most fistulas to the upper gastrointestinal tract can be treated conservatively (nil by mouth, antibiotics and nutritional support). Fistulas to the colon, which are associated with a mortality rate of 17–67%, rarely cure spontaneously and so are often treated surgically.8
Surgical treatment is based on (a) diversion by ileostomy or colostomy and (b) resection of the affected section of the colon.8 Depending on the associated complications, other interventions may include necrosectomy, cyst drainage or endoscopic placement of a pancreatic stent if there is interruption of the pancreatic duct. Several recent articles describe cases of pancreatic fistulas to the gastrointestinal tract in which endoscopic treatment was performed with good outcomes. Although the indications are not well established, this approach to management may prove effective as a less invasive alternative to surgery. The techniques used were as follows:
- -
Conventional endoclips. These are suitable for perforations <10mm, with no inflammation of the margins, but are not effective for larger perforations.7,9
- -
Over-the-scope clips. Made of a nitinol alloy with a memory effect and great elasticity, these allow a permanent force to be applied to seal the fistula opening, but require good previous drainage and the absence of abscesses.7
- -
Ligature bands, fibrin glue and endoloop (anecdotally). The bands are placed at the margins of the fistula opening to form pseudopolyps and cause inflammation that, with the glue, will seal the opening and the endoloop is applied to stabilize the margins.10
In conclusion, the review inspired by our case would indicate that although pancreatic-colonic fistulas, as a rare complication of severe acute pancreatitis with high mortality, are usually treated surgically, endoscopic treatment may be attempted in some selected cases.
Conflict of interestsThe authors declare that they have no conflict of interests.
Please cite this article as: Merino Rodríguez E, Borobia Sánchez R, Ramia Ángel JM, Rebolledo Olmedo S, de la Plaza Llamas R, Miquel Plaza J. Fístula pancreático-colónica espontánea en paciente con pancreatitis aguda grave. Gastroenterol Hepatol. 2016;39:221–223.