LncRNA-DANCR is involved in inflammation and acts as a major contributor to colon cancer. The effects and mechanism of LncRNA-DANCR were first investigated in a DSS-induced colitis model in vivo and vitro.
Material and methodsSprague-Dawley rats were given DSS to induce the colitis model. TNF-α, IL-1β, IL-6 levels and expression of intestinal adhesion proteins ZO-1 and MUC2 in colon tissues and DSS-induced NCM460 cells were measured using corresponding kits. A hematoxylin and eosin (H&E) staining assay was performed to evaluate colon tissue pathology conditions. Protein expression levels in DSS-induced NCM460 cells were evaluated by Western blotting, and cell apoptosis was detected using a TUNEL assay. Gene levels in DSS-induced NCM460 cells were evaluated by PCR. The StarBase online tool was used to predict the LncRNA-DANCR target. The LncRNA-DANCR target was verified using a luciferase reporter assay.
ResultsLncRNA-DANCR was up-regulated in DSS-induced groups of rats. TNF-α, IL-1β and IL-6 expression was significantly increased in DSS-induced groups of rats and cells. Zo-1 and MUC2 expression levels were decreased in DSS-induced groups of rats. Silencing LncRNA-DANCR reduced inflammation, cell apoptosis and up-regulated ZO-1, MUC2 and Claudin-1 in DSS-induced cells. MiR-125b-5p was the downstream LncRNA-DANCR target. All LncRNA-DANCR effects in the colitis model were reversed by the miR-125b-5p inhibitor.
ConclusionLncRNA-DANCR/miR-125b-5p, which may act as a regulatory axis in inflammation, apoptosis and barrier function dysregulation, can provide an essential reference for the development of new drugs in colitis treatment.
LncRNA-DANCR está involucrado en la inflamación y es uno de los mayores contribuyentes al cáncer de colon. Los efectos y el mecanismo de LncRNA-DANCR se investigaron por primera vez en el modelo de colitis inducido por DSS in vivo e in vitro.
Material y métodosLas ratas Sprague-Dawley recibieron DSS para inducir el modelo de colitis. Se midieron el nivel de TNF-α, IL-1, IL-6 y la expresión de proteínas de adhesión intestinal ZO-1 y MUC2 en los tejidos del colon y las células NCM460 inducidas por DSS utilizando los kits correspondientes. Se realizó un ensayo de tinción de hematoxilina y eosina (HE) para la evaluación de las condiciones patológicas del tejido del colon. El nivel de expresión de proteína en las células NCM460 inducidas por DSS se evaluó a través de Western blotting y se detectó apoptosis celular mediante el uso de un ensayo de TUNEL. El nivel genético en las células NCM460 inducidas por DSS se evaluó mediante un ensayo de PCR. La base estelar en línea se aplicó para predecir el objetivo de LncRNA-DANCR. El objetivo de LncRNA-DANCR fue verificado a través del ensayo Luciferase Reporter.
ResultadosLncRNA-DANCR fue regulado en grupos inducidos por DSS en ratas. La expresión de TNF-α, IL-1 e IL-6 se incrementó significativamente en grupos inducidos por DSS en ratas y células. El nivel de expresión de Zo-1 y MUC2 disminuyó en los grupos inducidos por DSS en ratas. Silenciar LncRNA-DANCR redujo la inflamación, la apoptosis celular y el ZO-1, MUC2 y Claudin-1 regulados en células inducidas por DSS. MiR-125b-5p era el siguiente objetivo de lncRNA-DANCR. Todos los efectos de LncRNA-DANCR en el modelo de colitis fueron revertidos por el inhibidor miR-125b-5p.
ConclusiónEl LncRNA-DANCR/miR-125b-5p, que puede ser un eje regulador en la inflamación, la apoptosis y la desregulación de la función de barrera, puede proporcionar una referencia vital para el desarrollo de nuevos fármacos en el tratamiento de la colitis.
Inflammatory bowel disease (IBD) as the common disease in the digestive system can be classified into Crohn's Disease (CD) and Ulcerative Colitis (UC), the incidences of which are rising across the world.1 The complicated pathogenesis of IBD is influenced by host genetic susceptibility, environment, microbial factors and other factors, leading to changes in mucosal barrier and immune system defects that can result in sustained inflammatory response.2,3
Inflammatory bowel disease with unknown etiology is a kind of refractory disease. The current therapeutic approaches mainly include drug treatment, immunomodulators, biologics and endoscopic therapy.4–6 The present treatments for IBD could only achieve the attenuative effects or prevent the worsening of pathological conditions, and the root cause of trouble cannot be eradicated.7 It is acknowledged that chronic intestinal inflammation could increase the risk of gastrointestinal cancer.8,9 Therefore, it is urgent to elucidate the pathogenesis of inflammatory bowel disease and develop effective therapeutic approaches.
Increasing evidence has confirmed that intestinal mucosal barrier dysfunction is considered is the critical event for IBD occurrence.10 The intestinal mucosal barrier is mainly composed of the basement membrane of the intestinal mucosa, the epithelial cell layer and the mucous layer on its surface. In the epithelial layer, tight intercellular connections between intestinal epithelial cells play the most important role in maintaining intestinal mucosal permeability. Intact function of tight junction proteins is crucial for maintaining intestinal mucosal permeability.11 Thus, it may be a promising strategy to find the critical regulator for tight junction proteins in epithelial layer.
LncRNAs with over 200 nucleotides in length served as the important regulator in gene expressions. Increasing studies have confirmed that lncRNAs play critical role in the modulation of inflammation and intestinal epithelial cell function and diseased intestinal barrier.12–14 Lnc-PlncRNA1 (prostate cancer-up-regulated long noncoding RNA 1, also known as CBR3-AS1) was reported to protect intestinal epithelial barrier through modulating tight junction proteins.15 LncRNA-DANCR (differentiation antagonizing non-protein coding RNA) plays an important role in a variety of tumors, and the level of LncRNA-DANCR is increased in patients with colorectal cancer.16 In addition, LncRNA-DANCR plays an important role in inflammation.17 Furthermore, in gastric cancer cells, LncRNA-DANCR could active β-catenin which is closely related to epithelial barrier functions.18,19 Thus, we speculate that LncRNA-DANCR may play a certain role in the inflammatory bowel disease. In this study, we first investigated the effects and mechanism of LncRNA-DANCR in inflammatory bowel disease.
Material and methodsThe establishment of inflammatory bowel disease modelThe collection and experimental methods of animal tissues are approved by animal care welfare committee of North China University of Science and Technology. Twenty Sprague-Dawley rats (170–180g) were obtained from North China University of Science and Technology. The rats were housed in a cage free of pathogens and were assigned into control group (n=10) and DSS group (n=10). For the control group, the rats were treated with normal drinking water. The 4% DSS (Biomedical, Solon, OH; no. 160110) was prepared in de-ionized water. The rats in DSS group were given 4% DSS for five days for inducing the colitis model. Colon tissues from rats in different groups were used for subsequent experiments.
Cell culture and treatmentAccording the previous study,20 we choose the human colon mucosal epithelial cell-line NCM460 (INCELL, San Antonio, TX, USA) for experiment. The cells were cultured in the Eagle's Minimum Essential Medium (EMEM) medium (#30-2003, ATCC) supplemented with 10% FBS (ATCC® 30-2020™) and 1% penicillin–streptomycin. The cells were maintained in an atmosphere of 5% CO2, at 37°C. The cells were then treated with 2% DSS for 24 to construct the cell model of inflammatory bowel disease.
Cell transfectionThe NCM460 cells with a density of 1×105cells/well were cultured in 6-well plates in the EMEM medium containing 10% FBS. After 2% DSS treatment for 24h, the medium above was changed into serum-free medium and the transfection was performed using SuperFectin II DNA transfection reagent (Invitrogen, China) according to the instructions of manufacturer. The ShRNA-NC, ShRNA-DANCR-1, ShRNA-DANCR-2, miR-NC, miR-125b-5p mimic, inhibitor-NC, miR-125b-5p inhibitor were all procured from GenePharma company.
Hematoxylin and eosin (HE) staining assayThe intestinal histopathology was evaluated via HE staining assay. 10% neutral buffered formalin was used for the fixation of colon samples and then the colon samples were embedded by paraffin. Then the embedded samples were cut into 5-μm thick sections transversely. Hematoxylin and eosin were then used for staining the cells.
qRT-PCRThe total RNA extraction was performed via using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and the DNA synthesis was performed via using the reverse transcription reagent Kit (Invitrogen). Thermo Script RT-PCR system (Invitrogen, Basel, Switzerland) was applied herein for qRT-PCR assay. The level of RNA level was calculated using 2−ΔΔCt methods. miR-125b-5p-F: 5′-GCUCCCUGAGACCCUAAC-3′; miR-125b-5p-R: 5′-CGAGCACAGAATTAATACGAC-3′; DANCR-F: 5′-AGTTCTTAGCGCAGGTTGAC-3′; DANCR-R: 5′-AAGGTGAACA TGAAGCACCT-3′; GAPDH-F: 5′- CCATGAGAAGTATGACAACAGC-3′; GAPDH-R: 5′-A TGGACTGTGGTCA TGAGTC-3′
Evaluation of TNF-α, IL-1β and IL-6 levelThe levels of TNF-α, IL-1βand IL-6 in cells and colon tissues were evaluated using the corresponding detection kits. Tumor necrosis factor (TNF)-α (cat. no. ADI-901-099; NeoBioscience Technology Co., Ltd.), interleukin (IL)-1β (cat. no. EHC002b.48; NeoBioscience Technology Co., Ltd.) and IL-6 (cat. no. ADI-901-033; NeoBioscience Technology Co., Ltd.). The cells after treatment were centrifuged and the supernatants in the different groups were submitted for detection according to the protocols of corresponding manufacture.
Western blotThe protein samples in the supernatants were obtained via lysis in RIPA buffer (Beyotime, P0013B) and centrifugation. The separation of proteins was performed on 10% SDA-PAGE. The separated proteins were then wet-transferred on the PVDF membrane. 5% non-fat milk was used for blockage of the membranes and then the membranes were incubated with the primary antibodies against Bcl2 (#ab182858, Abcam), Bax (#ab32503, Abcam), cleaved-caspase 3 (#ab49822, Abcam), cleaved-caspase 9 (#ab2324, Abcam), Pro-caspase3 (#ab32499, Abcam), Pro-caspase 9 (#ab202068, Abcam), ZO-1 (#ab96587, Abcam), MUC2 (#ab97386, Abcam), Claudin-1 (#ab15098, Abcam). Then, the membranes were incubated with secondary antibody (#ab150077, Abcam) for 2h, and Chemiluminescence kit (GE Healthcare, Chicago, IL, USA) was used for detecting the protein signal.
Measurement of cell apoptosisThe cell apoptosis was detected via TUNEL assay. One Step TUNEL Apoptosis Kit (Beyotime, Jiangsu, China) was used herein for the detection of cell apoptosis according to the instructions of the manufacturer. Briefly, the cells after treatment were set on glass slides. After being fixed and permeabilized, the cells were incubated with the tunnel working solution at 4°C overnight and counterstained with hematoxylin for 2min. After being washed with PBS, the slides were observed under the confocal Olympus FV-500 microscope.
Luciferase reporter assayThe DANCR gene containing the binding sites of miR-125b-5p was cloned into pGL3 vector (Promega, Madison, MI, USA). The Mut-DANCR containing the mutated binding sites of miR-125b-5p was generated by the Hanbio Biotechnology Co., Ltd. The MUT-or WT-DANCR vector was co-transfected into cells with miR-NC or miR-125b-5p mimic. The luciferase reporter assay system (Promega, Madison, WI, USA) was applied herein for the detection of the luciferase activity.
Statistical analysisAll the data herein were obtained for three repeated experiments and expressed as mean±SD. The statistical analysis was performed on GraphPad prism. The differences between groups were evaluated via one-way analysis of variance. p<0.05 was considered as statistically significant.
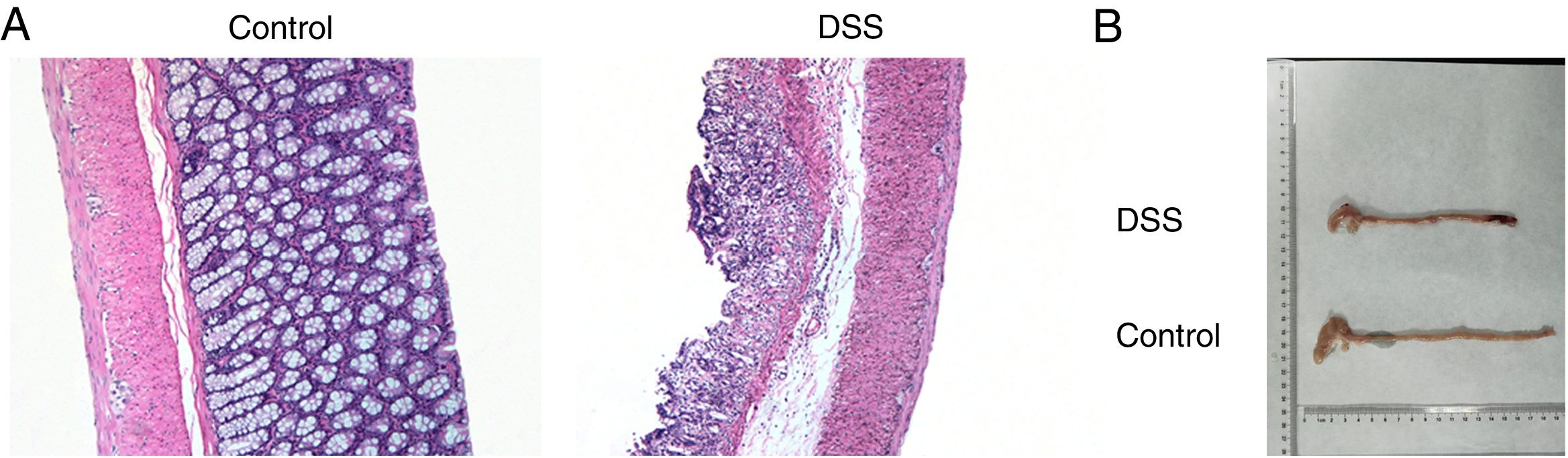
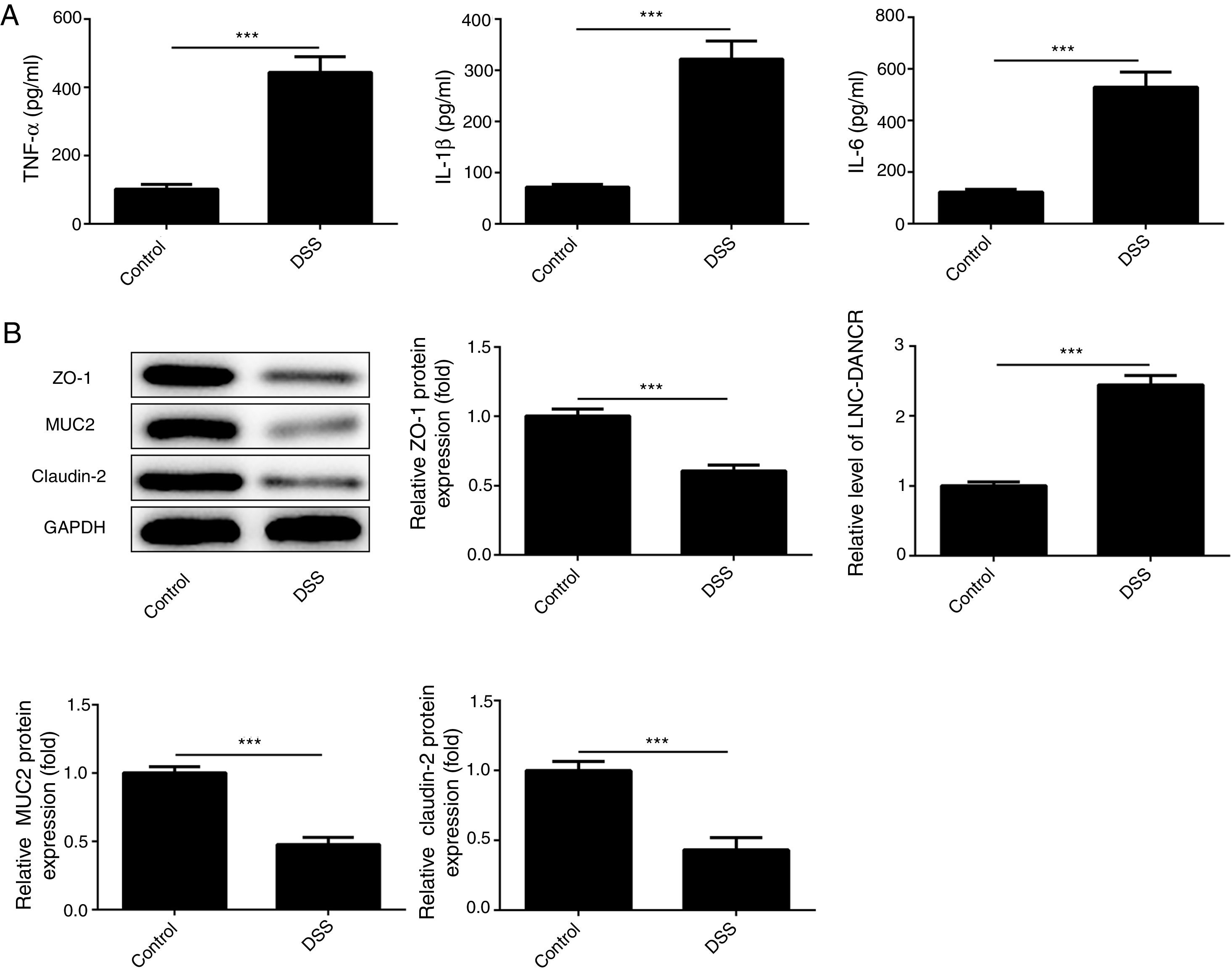
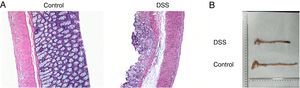
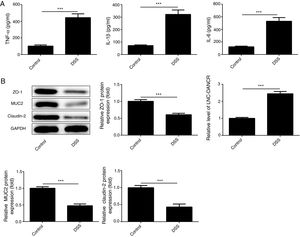
ResultsThe colon was severely injured by DSS and LncRNA-DANCR was up-regulated in DSS induced groups in ratsDuring the experiments, heavy diarrhea, reduced activity and severe mucus bloody stool were found in the DSS induced groups. As shown by the results from HE assay, goblet cell was arranged disorderly (Fig. 1A). The mucosa erosion and monocyte infiltration were observed in DSS induced groups. Moreover, the length of colon was shortened in DSS group (Fig. 1B), indicating that the colon was severely damaged in DSS induced group. Simultaneously, the inflammatory factors including TNF-α, IL-1β and IL-6 were increased significantly (Fig. 2A). The tight junction protein, ZO-1 which is crucial for maintaining intestinal mucosal permeability, was decreased by DSS (Fig. 2B). Furthermore, Mucoprotein2 (MUC2) as a vital defense protein was also down-regulated in DSS group. All the results demonstrated that the inflammatory bowel disease model was established successfully. The LncRNA-DANCR level was further evaluated herein. We found that the LncRNA-DANCR level was dramatically increased in DSS treated group.
The pathological conditions and colonic length in the study groups. (A) The results from HE assays, goblet cell was arranged disorderly. The mucosa erosion and monocyte infiltration were observed in DSS induced groups. (B) The length of colon was shortened in DSS group (data are presented as mean±SD, ***p<0.001, n=3).
The levels of inflammatory factors and protective proteins in the study groups. (A) The inflammatory factors including TNF-α, IL-1β and IL-6 were increased significantly. (B) The tight junction protein, ZO-1 was decreased by DSS. Furthermore, Mucoprotein2 (MUC2) as vital defense proteins was also down-regulated in DSS group. (C) LncRNA-DANCR level was significantly up-regulated in DSS-induced group (data are presented as mean±SD, **p<0.01, ***p<0.001, n=3).
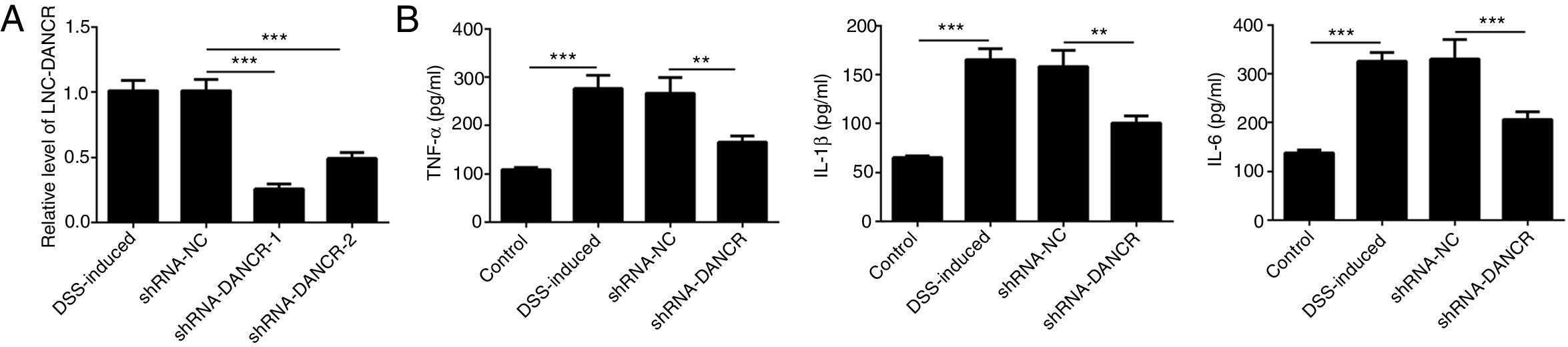
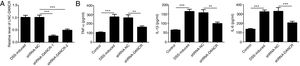
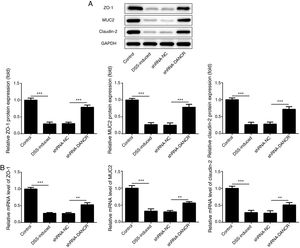
Since the LncRNA-DANCR level was significantly up-regulated in DSS-induced group, we speculated that LncRNA-DANCR may be an underlying therapeutic target for colitis. Thus, the role of silencing LncRNA-DANCR was further evaluated in colitis model. The LncRNA-DANCR level was reduced obviously by shRNA-DANCR-2 while more obviously by shRNA-DANCR-1 when compared with DSS-induced group (Fig. 3A). Thus, shRNA-DANCR-1 was used for silencing LncRNA-DANCR in the following experiments. Compared with control group, the levels of TNF-a, IL-1β and IL-6 were increased in DSS induced group and these inflammatory levels induced by DSS were further declined by shRNA-DANCR, suggesting that silencing LncRNA-DANCR has attenuative effects on inflammatory level induced by DSS (Fig. 3B). The protective proteins including ZO-1, MUC2 and Claudin-1, which play crucial roles in maintaining intestinal mucosal barrier, were also decreased by DSS in comparison with control group. The shRNA-DANCR further up-regulated the levels of these protective protein induced by DSS (Fig. 4A and B). All the results together confirmed that silencing LncRNA-DANCR, which results in decreased inflammatory levels and elevated protective protein level, is a promising strategy for colitis treatment.
The effects of LncRNA-DANCR knockdown in the colitis stimulated by DSS. (A) DANCR level was reduced obviously by shRNA-DANCR-2 and more obviously by shRNA-DANCR-1 when compared with DSS-induced group. (B) LncRNA-DANCR has attenuative effects on inflammatory level induced by DSS (data are presented as mean±SD, **p<0.01, ***p<0.001, n=3).
The effects of LncRNA-DANCR knockdown on levels of ZO-1, claudin-1 and MUC2 in in the colitis stimulated by DSS. The levels of ZO-1, claudin-1 and MUC2 evaluated by western blot (A) and PCR (B) in the different study groups. The results showed that the shRNA-DANCR further up-regulated the levels of ZO-1, MUC2 and Claudin-1, which play crucial roles in maintaining intestinal mucosal barrier induced by DSS (data are presented as mean±SD, *p<0.05, **p<0.01, ***p<0.001, n=3).
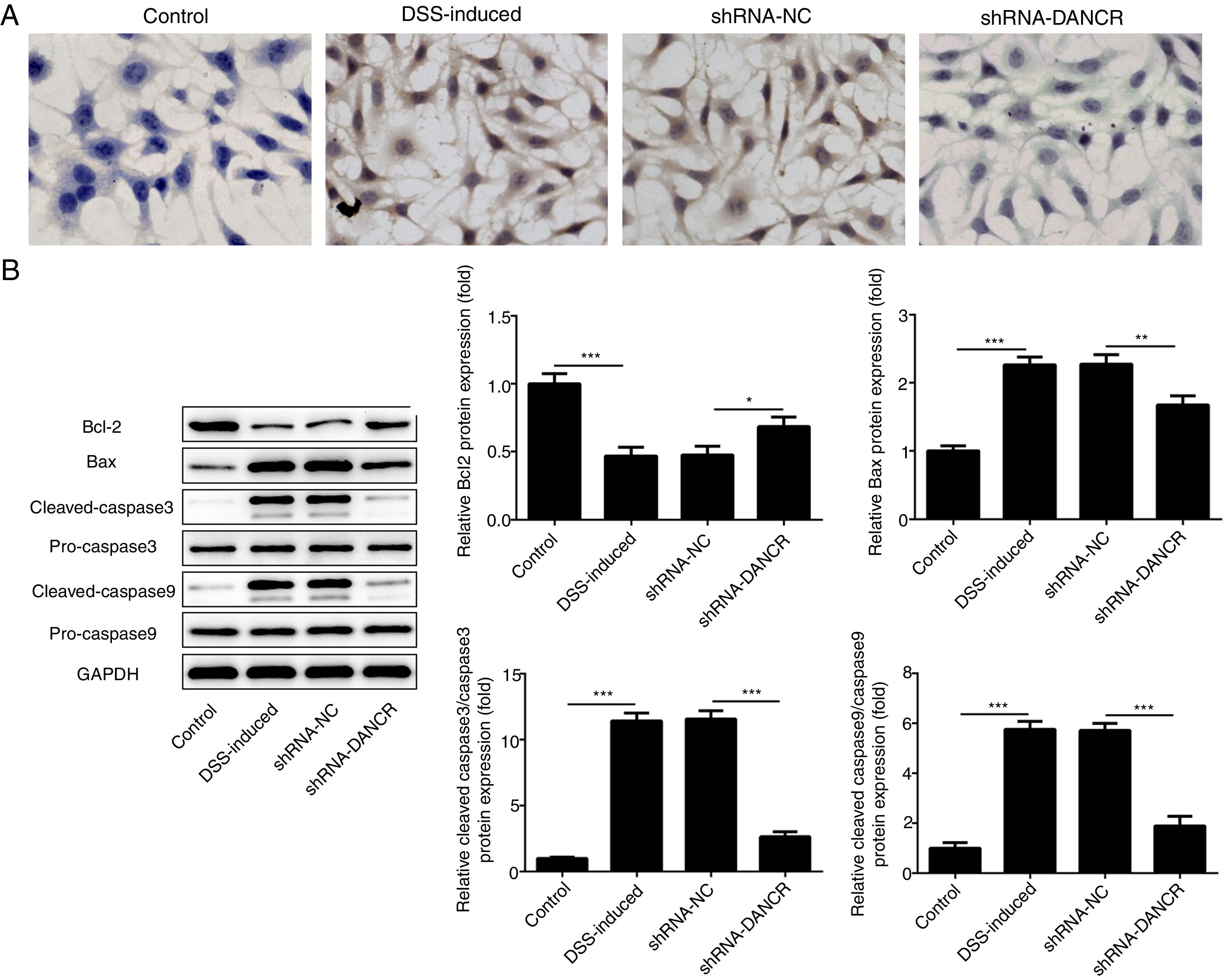
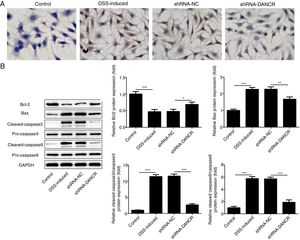
The increased cell apoptosis was found in DSS-induced group and shRNA-DANCR could reduce the cell apoptosis in DSS-induced group (Fig. 5A). Moreover, the anti-apoptotic protein bcl-2 was decreased and pro-apoptotic proteins, including Bax, cleaved-caspase 3 and cleaved-caspase 9, were up-regulated in DSS-induced cells (Fig. 5B). All these results suggested that the cell apoptosis was up-regulated in colitis model established via DSS. After silencing lncRNA-DANCR in DSS-induced cells, the cell apoptosis and apoptosis-related protein levels induced by DSS were reversed. All stated above supported that silencing LncRNA-DANCR has protective effects in colitis induced by DSS.
The effects of LncRNA-DANCR knockdown on cell apoptosis in the colitis stimulated by DSS. (A) The increased cell apoptosis was found in DSS induced group and shRNA-DANCR could reduce the cell apoptosis in DSS induced group. (B) Moreover, the anti-apoptosis protein, bcl-2 was decreased and pro-apoptosis proteins including Bax, cleaved-caspase 3 and cleaved-caspase 9 were up-regulated in DSS induced cells (data are presented as mean±SD, **p<0.01, ***p<0.001, n=3).
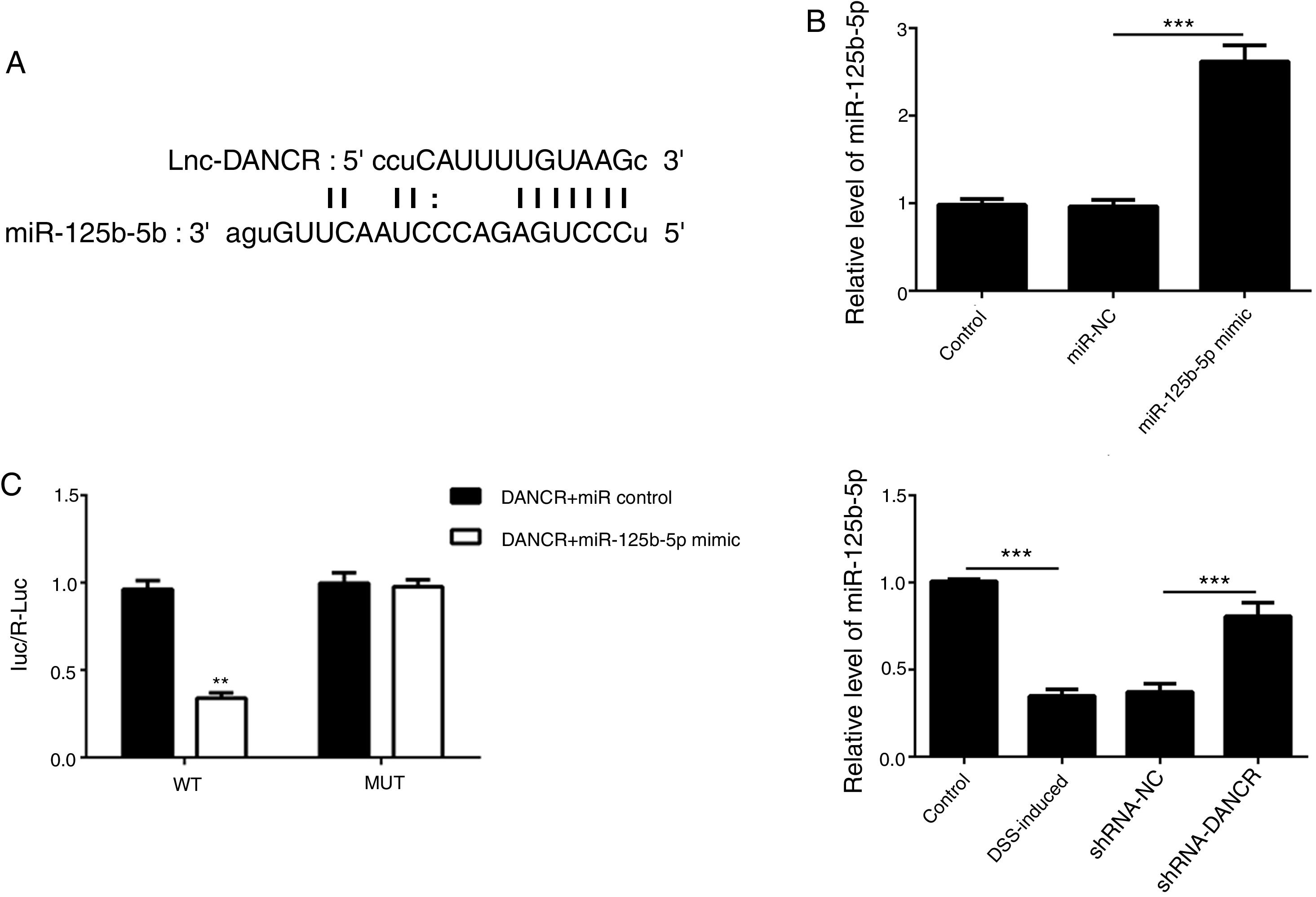
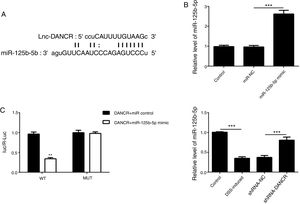
As predicted by Starbase website, miR-125b-5p is directly targeted by lncRNA-DANCR (Fig. 6A). In order to validate the binding between miR-125b-5p and lncRNA-DANCR, luciferase reporter assay was performed. As validated by PCR, the overexpression of miR-125b-5p was successful (Fig. 6B). The lowest luciferase activity was found in WT-DANCR+miR-125b-5p mimic group (Fig. 6C), demonstrating that miR-125b-5p was the downstream target of lncRNA-DANCR. The level of miR-125b-5p was further detected in DSS-induced cells. We found that miR-125b-5p was decreased significantly in DSS-induced cells, and after silencing lncRNA-DANCR, miR-125b-5p was up-regulated (Fig. 6D). We conjectured that silencing lncRNA-DANCR functioned via upregulation of miR-125b-5p in DSS-induced model.
MiR-125b-5p was validated as the target of LncRNA-DANCR. (A) As predicted by starbase website, miR-125b-5p is directly targeted by lncRNA-DANCR. (B) As validated by PCR, the overexpression of miR-125b-5p was achieved successfully. (C) The lowest luciferase activity was found in WT-DANCR+miR-125b-5p mimic group, demonstrating that miR-125b-5p was the downstream target of lncRNA-DANCR. (D) miR-125b-5p was decreased significantly in DSS induced cells and after silencing lncRNA-DANCR, the miR-125b-5p was up-regulated (data are presented as mean±SD, **p<0.01, ***p<0.001, n=3).
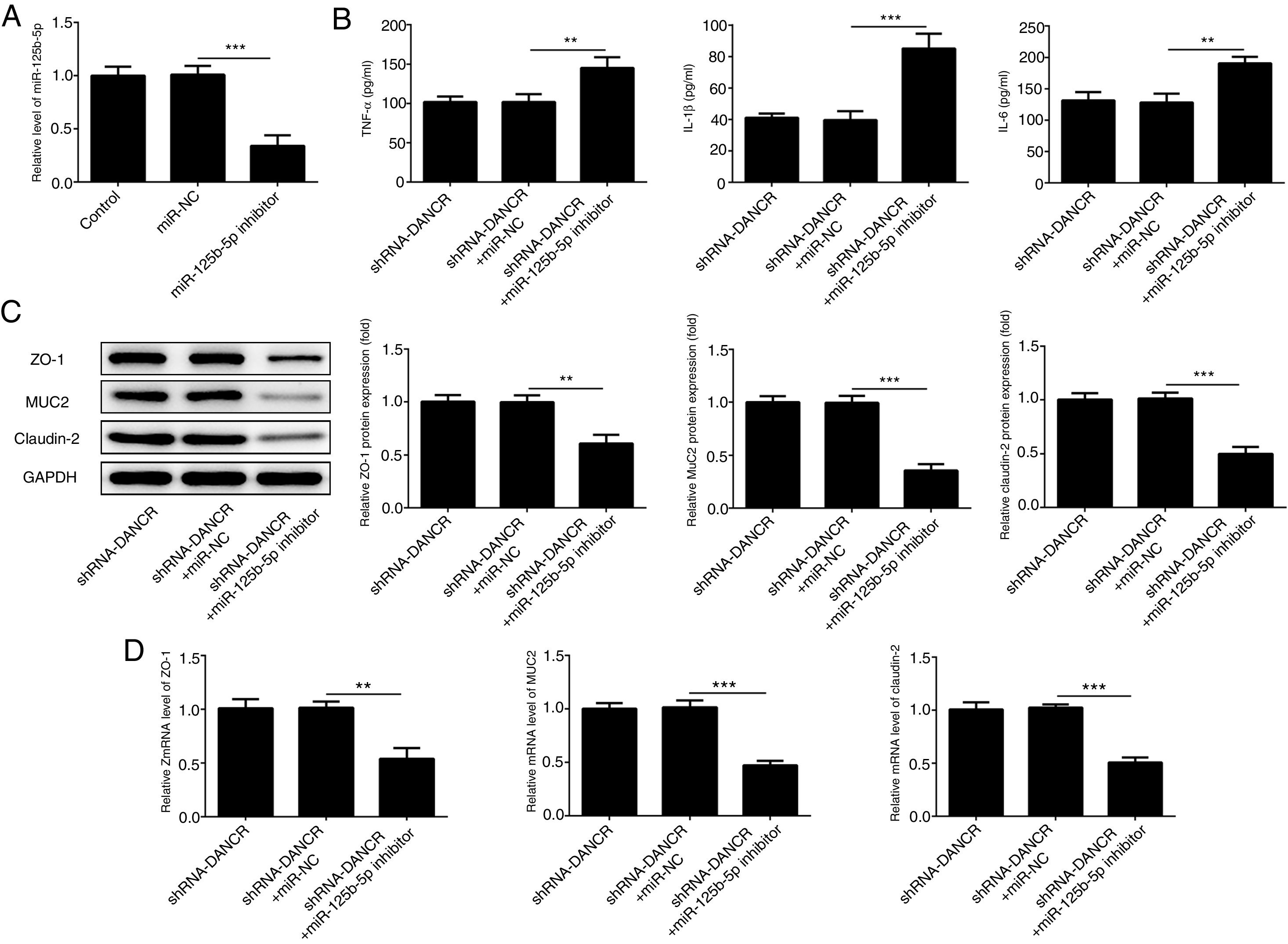
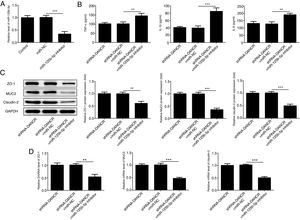
The miR-125b-5p inhibitor was used to determine whether lncRNA-DANCR functions via targeting miR-125b-5p. After transfection with miR-125b-5p inhibitor, the miR-125b-5p level was decreased significantly, confirming that the expression of miR-125b-5p was inhibited successfully (Fig. 7A). The levels of inflammatory factors and protective proteins including ZO-1, MUC2 and Claudin-1 in DSS-induced cells were reduced obviously by miR-125b-5p inhibitor (Fig. 7B–D), suggesting that the effects of silencing lncRNA-DANCR on inflammation and protective proteins were counteracted by miR-125b-5p inhibitor. Collectively, silencing lncRNA-DANCR inhibited inflammation and up-regulated protective protein levels in DSS-induced cells via targeting miR-125b-5p.
Silencing lncRNA-DANCR inhibited inflammation and up-regulated protective proteins level in DSS induced cells via targeting miR-125b-5p. (A) After transfection with miR-125b-5p inhibitor, the miR-125b-5p level was decreased significantly, confirming that the miR-125b-5p was inhibited successfully. The levels of inflammatory factors (B) and protective proteins including ZO-1, MUC2 and Claudin-1 in DSS induced cells were reduced obviously detected by western (C) and PCR (D) in the different study groups (data are presented as mean±SD, **p<0.01, ***p<0.001, n=3).
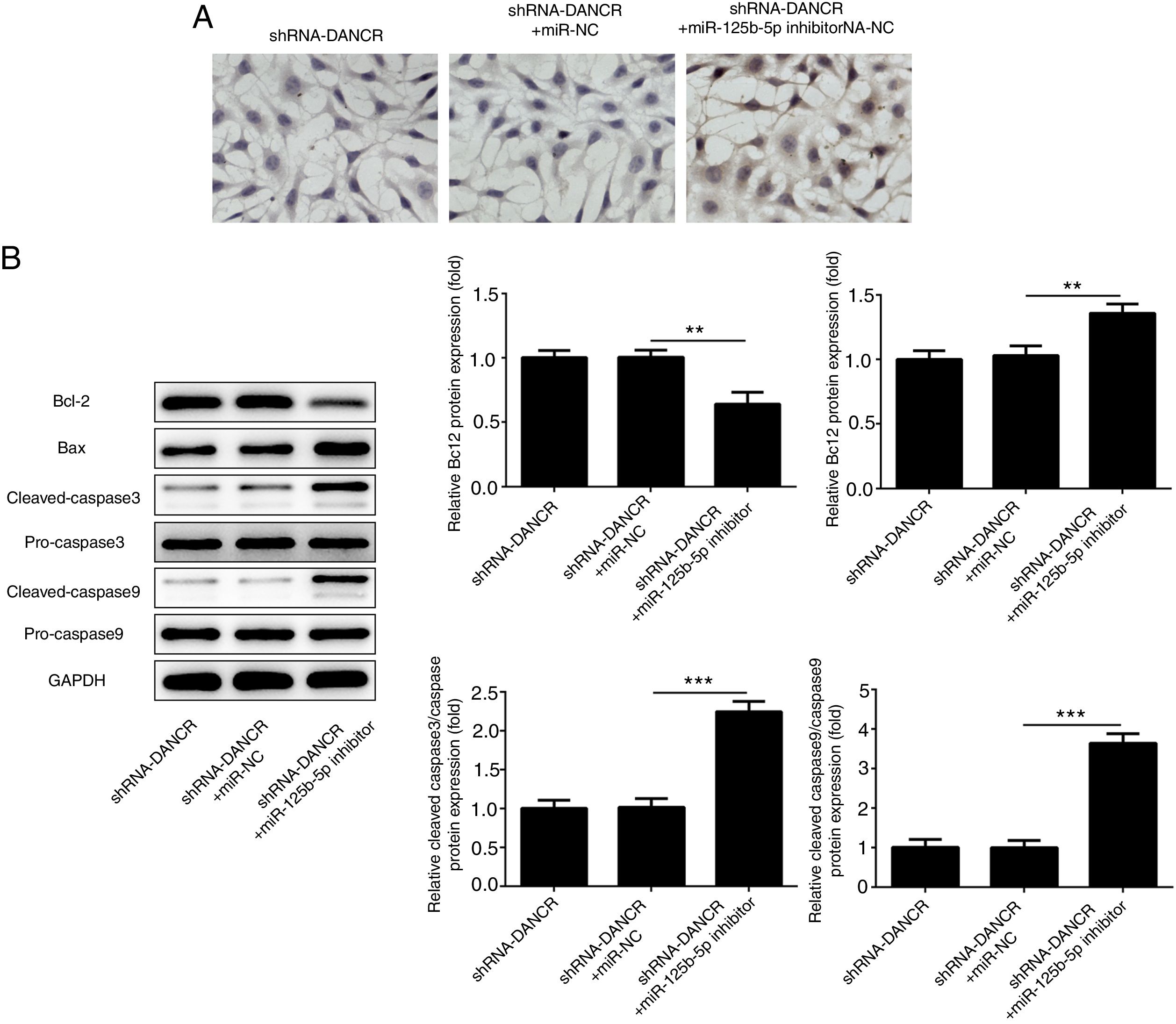
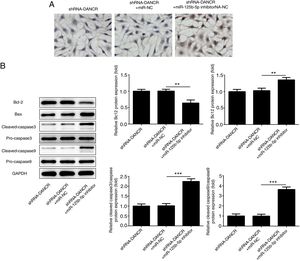
The cells were more severely injured in ShRNA-DANCR+miR-125b-5p inhibitor group than that in ShRNA-DANCR group (Fig. 8A). Simultaneously, the expression of bcl-2 was reduced and expressions of pro-apoptotic proteins were up-regulated in ShRNA-DANCR+miR-125b-5p inhibitor group, when compared with those in shRNA-DANCR group (Fig. 8B). The anti-apoptotic effects of lncRNA-DANCR were partly reversed by miR-125b-5p inhibitor, indicating that the inhibitory effects of silencing lncRNA-DANCR on cell apoptosis was realized via upregulation of miR-125b-5p.
Silencing lncRNA-DANCR alleviated cell apoptosis via targeting up-regulation of miR-125b-5p. (A) The cells were more severely injured in ShRNA-DANCR+miR-125b-5p inhibitor group than that in ShRNA-DANCR group. (B) Simultaneously, the bcl-2 was reduced and pro-apoptosis related proteins were up-regulated in ShRNA-DANCR+miR-125b-5p inhibitor group, when compared with that in shRNA-DANCR group (data are presented as mean±SD, **p<0.01, ***p<0.001, n=3).
The intestinal epithelium acts as the vital defense barrier against harmful microorganisms in colon.21 The defense function of intestinal epithelium barrier is mediated via the connections of protein complex between adjacent cells, wherein protein complex includes adherent junction (AJ) and tight junction (TJ) proteins.22 Intestinal barrier dysregulation will result in decline of host defense barrier and the onset and progression of colitis. Thus, the protein complex, which connects and seals the adjacent cells, is crucial for maintaining the intestinal epithelium barrier. Simultaneously, it is known to all that the alleviation of inflammation is essential for colitis therapy. Thus, screening the key regulator of protein complex and inflammation is beneficial for developing novel and efficient therapeutics. Many researchers have confirmed that lncRNA DANCR is a vital regulator in many diseases.23–26 Additionally, LncRNA-DANCR is also involved in inflammation and acts as avital contributor to colon cancer.17,27 While the role of LncRNA-DANCR in inflammatory bowel disease has not been reported. In the present research, we found that lncRNA DANCR was high expressed in the model of colitis and silencing lncRNA DANCR exerted inhibitory effects on inflammation and it also up-regulated the expression levels of protective proteins via targeting miR-125b-5p.
We firstly established the colitis model in vivo using DSS. Consistent with the previous studies,28–30 mucosa erosion and monocyte infiltration occurred in the colitis model group. Additionally, the inflammatory level was elevated. MUC2 that is secreted by goblet cells is the primary component in mucus layer, which functions as a vital barrier defense invasion of pathogens.31,32 ZO-1 and Claudin-1 are the major components in tight junctions, playing a crucial role in maintaining the mucosal barrier.33 In the present research, the protective protein including ZO-1, MUC2 and Claudin-1 were decreased dramatically in colitis model groups. These results demonstrated that the colitis model was established successfully.
After that, we found that lncRNA-DANCR was overexpressed in colitis model group and lncRNA-DANCR may be an underlying therapeutic target. This speculation was further explored in cells induced by DSS. Consistent with the results in colitis model in vivo, the inflammatory factors were increased and protective proteins including ZO-1, MUC2 and Claudin-1 were declined significantly in cell model of colitis, which was also in accordance with the previous reports.20,34,35 As expected, after silencing lncRNA-DANCR, the levels of cell apoptosis and inflammation were both reduced obviously. The ZO-1, MUC2 and Claudin-1 were all elevated by silencing lncRNA-DANCR. This strongly supports that lncRNA-DANCR is a promising therapeutic target.
It is known to all that lncRNA usually functions via targeting certain miRNAs. To investigate the mechanism thoroughly, we investigated the downstream target of lncRNA-DANCR via online Starbase prediction and luciferase reporter assay. We found that miR-125b-5p was directly targeted by lncRNA-DANCR. MiRNA-125b-5p also functions as a vital regulator in a variety of cancers.36–40 Additionally, microRNA-125b-5p has been confirmed as the vital regulator on the inflammatory state in macrophages by sponging B7-H4.41 Simultaneously, microRNA-125b-5p is reported to be involved in barrier function dysregulation via regulation of tight junction proteins.42 Evidently, miRNA-125b-5p, which is associated with inflammation and barrier function dysregulation, is a vital regulator in colitis. In this study, miRNA-125b-5p was down-regulated significantly in DSS-induced cells, while it was elevated in lncRNA-DANCR knockdown group. The effects of silencing lncRNA-DANCR on inflammation, cell apoptosis, and protective proteins were reversed by microRNA-125b-5p inhibitor. This result confirmed that miRNA-125b-5p acts as the target molecular in the downstream of lncRNA-DANCR. All these findings support that lncRNA-DANCR functions in colitis via targeting miRNA-125b-5p. LncRNA-DANCR/miRNA-125b-5p regulatory axis, which is closely related to inflammation and barrier function dysregulation, is a vital therapeutic target in colitis.
ConclusionLncRNA-DANCR/miRNA-125b-5p was found as a key axis in inflammation and barrier function dysregulation in this study. We also found that silencing lncRNA-DANCR have protective effects on colitis induced by DSS via downregulation of inflammation and elevation of MUC2, ZO-1 and Claudin-1 through upregulation of microRNA-125b-5p. This finding provides new strategy for development of drugs in colitis therapy, laying vital foundations for future research.
Conflict of interestThe authors declare that they have no conflict of interest.
This study was financed by Hebei Natural Science Fund (H2019209241).