The association between inflammatory bowel disease (IBD) and primary sclerosing cholangitis should be considered a distinct clinical entity. This association involves genetic abnormalities, epidemiological factors (more common in men, with no a geographical pattern) and, commonly, subclinical inflammation, predominance of the right colon (endoscopic and histological), backwash ileitis and rectal sparing. Furthermore, there is an increased risk of colorectal cancer and cholangiocarcinoma. The aim of this review is to show how IBD influences the progression of this entity, transplantation requirements and recurrence. We also discuss the current evidence on the use of biological therapy in this group of patients.
La asociación entre enfermedad inflamatoria intestinal y colangitis esclerosante primaria debe ser considerada una entidad distinta. Su asociación involucra alteraciones genéticas, epidemiológicas (mayor frecuencia en varones y sin una clara distribución geográfica) y un cuadro clínico en el que destaca con mayor frecuencia un compromiso inflamatorio subclínico, el predominio de colon derecho (demostrado por endoscopia e histología), la presencia de ileítis por reflujo y la ausencia de compromiso rectal. A su vez, existe un mayor riesgo de cáncer de colon y colangiocarcinoma. El objetivo de esta revisión es mostrar como la enfermedad inflamatoria intestinal influye en su evolución, en los requerimientos de trasplante y en la recurrencia. A su vez, señalar la evidencia actual sobre el uso de la terapia biológica en este grupo de pacientes.
Primary sclerosing cholangitis (PSC) is defined as a chronic cholestatic liver disease characterised by lesions in the intra and/or extrahepatic bile duct.1 Symptoms are progressive and include fatigue, right upper quadrant pain and pruritus. It can cause cholestasis (elevation of alkaline phosphatases and gamma-glutamyltransferase) which, if it persists, can lead to deficiency in fat-soluble vitamins, autonomic dysfunction and sleep disorders. Histological examination has shown us that PSC can lead to fibrosclerotic stenosis and destruction of the bile ducts.2 As PSC progresses, the risks are liver cirrhosis and development of cholangiocarcinoma (CCA) and/or colorectal cancer (CRC).3
There is a significant association between PSC and inflammatory bowel disease (IBD), and it is here that we have focused our attention. PSC in IBD is considered an extraintestinal manifestation,4 which can even precede the development of gastrointestinal symptoms.5
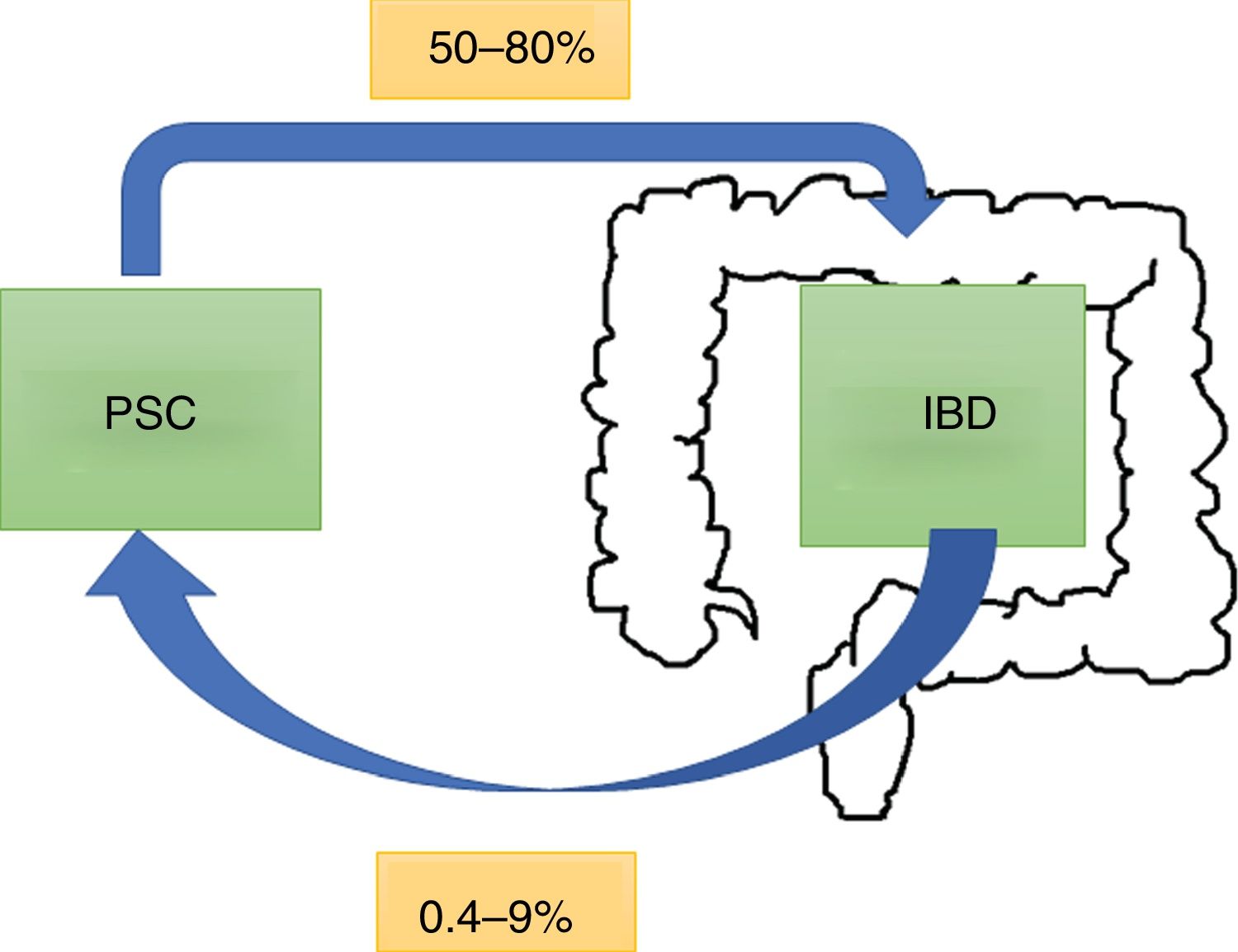
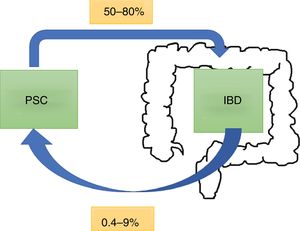
EpidemiologyPSC has an incidence and prevalence of 0–1.3 per 100,000 per year and 0–16 per 100,000 in North America and northern European countries, respectively.6 It tends to be more common in males. Worldwide, 50–80% of patients with PSC have concomitant IBD,7 with ulcerative colitis (UC) being more common than Crohn's disease (CD) or IBD unclassified.8 This rate can vary according to the criteria of the study, depending on whether the diagnosis is based on symptoms or colonoscopy and biopsy results.9 Of all patients with IBD, 0.8–8% of patients with UC and 0.4–9% of those with CD will develop PSC. In a Chilean cohort of 716 patients,10 only 1.4% had PSC, with the rates being 2% for UC, 1% for CD and no reported cases for IBD unclassified (Fig. 1).
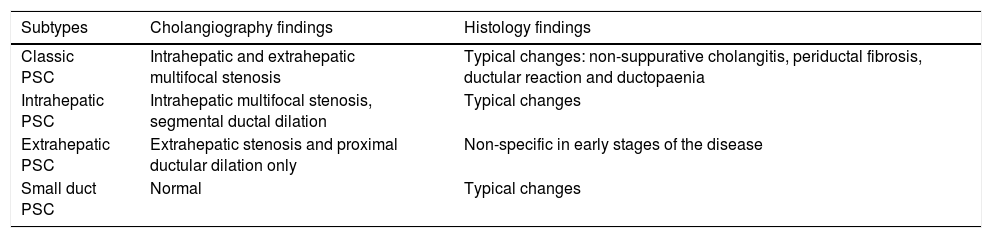
In a retrospective study11 which included 8231 patients with IBD, there were 222 patients with PSC and IBD (2.7%). In 40%, the intrahepatic bile ducts were affected, 58% had intra- and extrahepatic involvement, 8% had small duct involvement (defined by biopsy findings) and less than 2% had isolated extrahepatic involvement. PSC is classified according to which bile ducts are affected (Table 1).
Classification of primary sclerosing cholangitis (PSC).
| Subtypes | Cholangiography findings | Histology findings |
|---|---|---|
| Classic PSC | Intrahepatic and extrahepatic multifocal stenosis | Typical changes: non-suppurative cholangitis, periductal fibrosis, ductular reaction and ductopaenia |
| Intrahepatic PSC | Intrahepatic multifocal stenosis, segmental ductal dilation | Typical changes |
| Extrahepatic PSC | Extrahepatic stenosis and proximal ductular dilation only | Non-specific in early stages of the disease |
| Small duct PSC | Normal | Typical changes |
Miard et al.12 studied 390 patients having IBD-related intra-abdominal surgery. Two hundred and fifty-five had a liver biopsy, with 43 cases of PSC being detected. Of these, 29 (75.9%) had abnormal liver function tests at the time of surgery. In the group where no biopsy was performed (135 patients), PSC was found by imaging in four of the patients. In other words, the prevalence of PSC among those who had a biopsy was 16.7% compared to 2.9% among those who did not (p<0.001), with that being higher than reported in the literature.
It is likely that the prevalence of PSC will increase when using more sensitive diagnostic techniques. Lunder et al.13 found three times the number of cases of PSC performing magnetic resonance cholangiography in all patients with IBD under follow-up, with a rate of 7.5% compared to the 2.2% previously detected based on symptoms or abnormal liver function tests. Belle et al.14 showed that one in ten patients with abnormal liver function tests would have PSC, suggesting that diagnosis of PSC in IBD would increase if investigations were more targeted.7
PathogenesisMore than 22 related loci have been identified, most of them linked to the immune response, particularly in the HLA system. The HLA B8, DR3 (HLADRB1*0301) and DRw52a15 genes would give a higher risk of PSC, while others would be associated with a worse prognosis (DR2 and DR3) and others would be protective (HLA-DR4).16 The genetic overlap between PSC and IBD is lower than expected; only 5% of the 200 loci identified in IBD are associated with PSC.17 Moreover, it has been shown that the genetic association may be different depending on the type of IBD (UC r=0.29 and CD r=0.04), which differs from the correlation between the two IBD (r=0.56), suggesting that the combination PSC and IBD is a different disease.18
A recent study that included 3402 patients with PSC identified genetic variant rs853974, which may be associated with transplant-free survival.19 This gene was expressed in both murine cholangiocytes and human hepatic stellate cells, acting on pathways which would cause fibrosis. Despite these findings, known genetic factors would only explain about 10% of the disease, so there must be environmental risk factors to explain the unknown fraction.18
The basis of a possible immune-mediated pathogenesis is the presence of several autoantibodies: pANCA in 30–80%; hypergammaglobulinaemia in 30%; and elevated IgM in 40–50%.19 Doubts have been raised about this, however, given that there is a higher prevalence of PSC in males (unlike most immune-mediated diseases), and because of the lack of effectiveness of immunosuppressive drugs or a specific autoantibody.16
In view of the known association with IBD, different hypotheses have been proposed regarding the interaction between liver and intestine:
- 1.
Increase in intestinal permeability.20 The central components of the intestinal barrier are enterocytes which are tightly bound together by desmosomes and adhesion molecules. This barrier restricts the movement of microorganisms and substances from the intestinal lumen and acts as a line of defence. Chronic inflammation can compromise this line of defence, leading to an increase in bacterial translocation or bacterial products which then go straight to the portal system,21 contributing to the exaggerated inflammatory response of the cholangiocyte, and finally causing the inflammation to be translated into fibrosis or concentric rings with “onion skin” appearance.22
- 2.
Microbiome/bile acid interaction. There is bidirectional communication between the liver and the intestine, and bile acids (BA) and bioactive mediators are released from the bile duct into the systemic circulation.20 BA are molecules synthesised from cholesterol in the central hepatocytes. They are released into the biliary tract as glycine or taurine and reach the duodenum, where they enable emulsification and absorption of fats from the diet. About 95% are actively reabsorbed in the terminal ileum and transported back to the liver. The unconjugated 5% is transformed by the gut microbiota, passing passively to the portal circulation. At the same time, the intestine produces metabolites which go to the portal circulation; the intestine can thus influence liver function.
- 3.
The BA and the microbiota interact closely, with the BA exercising direct control over the microbiota. Binding to the farnesoid X receptor (FXR) or NR1H4 leads to the production of antimicrobial peptides which directly inhibit bacterial overgrowth. Cholestasis-related changes in BA have been detected which disrupt enzymatic activity, leading to alteration of the microbiota/dysbiosis.23
- 4.
Certain genes may generate greater susceptibility to damage induced by BA,1 such as the genes encoding the bile salt receptor at apical TGR5 (also known as GPBAR1) and the genes that encode the enzyme fucosyltransferase 2 (FUT2), generating stabilisation of glycocalyx, which protects cholangiocytes exposed to these salts with a concentration 10,000 times higher than in other cells. Alterations in these genes could be related to a greater predisposition to BA-induced damage.
- 5.
Chemokines and adhesion molecules common to the intestine and liver. Lymphocytes activated in the inflamed intestinal mucosa may enter the enterohepatic circulation and then be helped to remain there by chemokines and adhesion molecules from the intestine which would act as memory cells, perpetuating the inflammation.18 Various studies suggest that the IL-2 pathway may be activated in the development of PSC17 through the IL-2 receptor, promoting the T-cell-mediated inflammatory cascade which becomes evident in the liver and colon.
Several studies up to now have tried to demonstrate the contribution of the microbiota in PSC.24 Microorganism receptors have been found in the cholangiocyte and the possibility of bacteriobilia or bacteraemia in the portal system, a promising response with the use of antibiotics,25 and even a favourable response to oral vancomycin in a small number of patients refractory to biological therapy who had PSC and IBD.26
The role of the microbiota in PSC has been supported by experimental studies in germ-free Mdr2(−/−) mice where, applying a model of acid toxicity in the biliary tract, it was demonstrated that the biliary disease would be more aggressive.27 It has also been shown that the microbiota in patients with PSC has less diversity.28,29 There is an increase in some subtypes, such as Veillonella genus, 4.8 times more common in PSC than in healthy individuals and 7.8 times compared to UC. In other words, the condition of the microbiome in these patients has more to do with their liver disease than their inflammatory disease.30 Bajer et al.31 showed recently that PSC and UC were associated with dysbiosis and were characterised by a reduction in bacterial diversity, with a change in composition. Rothia, Enterococcus, Streptococcus and Veillonella increased in all patients with PSC regardless of whether they had IBD or not, with a decrease in Adlercreutzia equolifaciens and Prevotella copri. The decrease in Phascolarctobacterium was associated with colon inflammation regardless of the IBD subtype.
The changes in the microbiota appear to be different according to the subtypes of IBD. We must, however, be careful about how we interpret these data, as the studies included limited numbers of patients and were able to use previously known diagnostic data, so we do not know whether they were primary changes or not.
Clinical scenariosThe majority of patients with PSC are asymptomatic at diagnosis, with the disease subsequently following a progressive course.32 When symptoms do occur, the most common are asthenia and pruritus. Over the course of the disease, 10–15% of patients may develop cholangitis.33 Blood tests show persistent elevation of alkaline phosphatases and gamma-glutamyl transpeptidase.
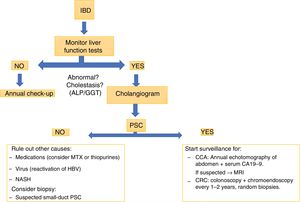
Magnetic resonance cholangiography is almost as sensitive as endoscopic retrograde cholangiopancreatography (ERCP) and involves less risk, so it would be the first test indicated where PSC was suspected. ERCP may be necessary to assess stenosis when investigating possible CCA, as it enables samples to be taken for cytology. New techniques, such as SpyGlass,34,35 may be useful in selected patients. Liver biopsy is not always required for the diagnosis of PSC; one exception is suspected PSC in the context of IBD with normal cholangiogram but abnormal liver function tests. These patients may have a small-duct variant of PSC.36
When small-duct PSC is diagnosed, a colonoscopy should be performed, with biopsies of the ileum and the various segments of the colon, even if the mucosa appears normal macroscopically.
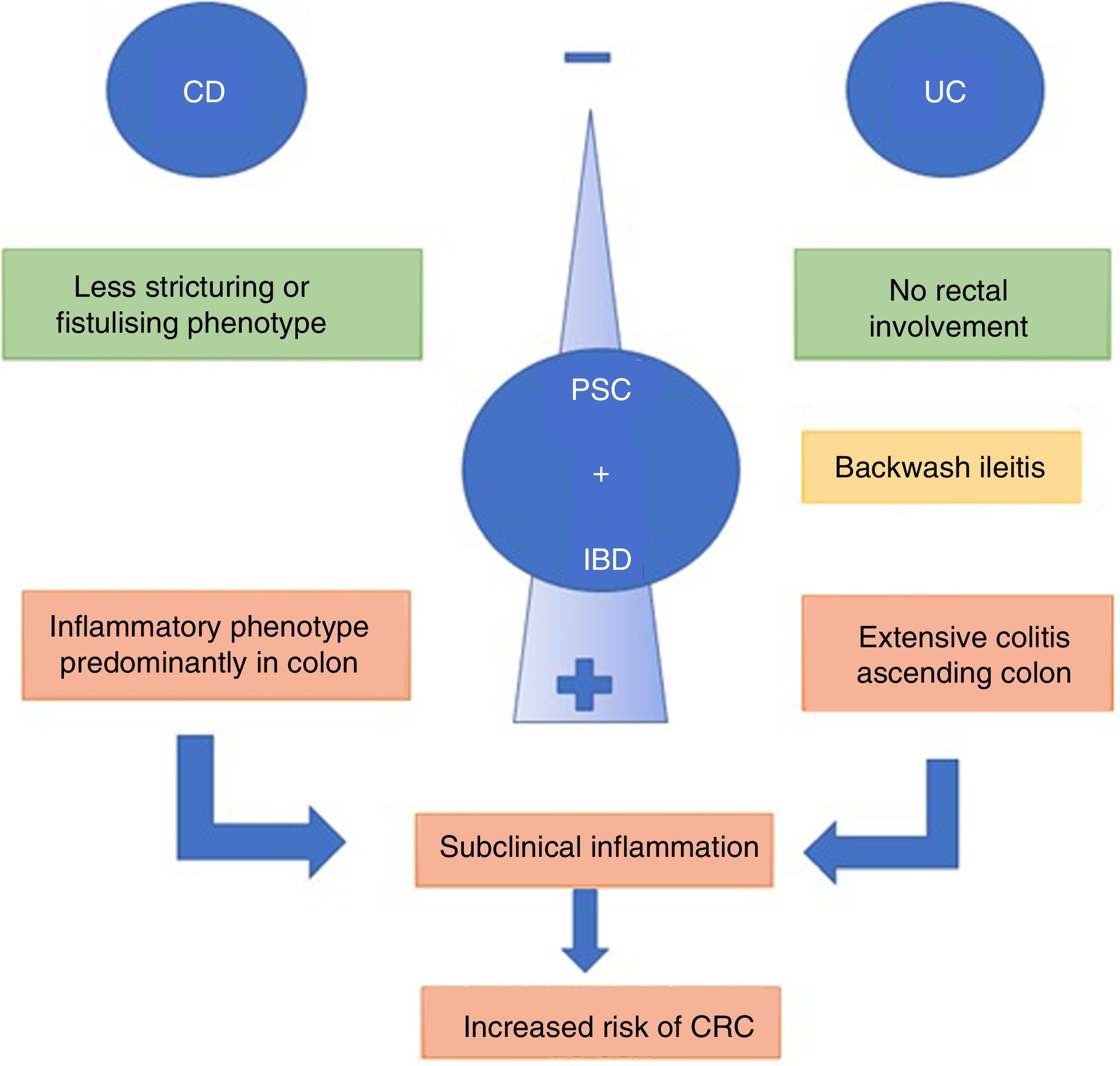
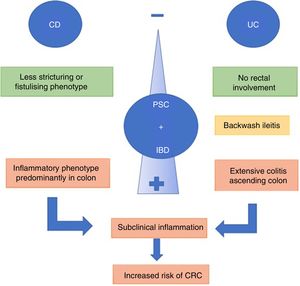
The combination of PSC and IBD is associated with a different IBD phenotype.37 Patients with UC and PSC tend to present with extensive colitis, backwash ileitis and preservation of the rectal mucosa (without inflammation), compared to patients with IBD without PSC.9 A recent review compared UC and PSC with extensive UC without PSC, finding that patients with clinically asymptomatic PSC and UC had greater histological activity in the right colon and less in the rectum.38
Backwash ileitis is inflammation of the ileum resulting from ileocaecal valve dysfunction, a situation more often found in extensive UC. De Vries et al.37 reported backwash ileitis in 16.7% of patients with PSC and UC compared to 2.5% in those with IBD only (Fig. 2).
When PSC is associated with CD, the course of the IBD tends to be more benign. The predominant phenotype is the colonic inflammatory type, which may or may not have terminal ileum involvement. The stricturing or fistulising phenotypes are less common.6 However, the combination of PSC and CD should not be considered harmless. O’Toole et al.39 showed that patients under the age of 65 with IBD at a tertiary hospital had a higher mortality rate compared to the general population, and PSC was considered an independent factor for death after the multivariate analysis. PSC was associated with the deaths of 9/58 patients (16%), with the rates being significant in both CD and UC (p=0.003 and p=0.03, respectively).
Primary sclerosing cholangitis and cancerPSC increases the risk of developing cancer. Ten years after diagnosis of PSC there is a 9% risk of CCA.40 A retrospective study by the Mayo Clinic,41 which included 399 patients with PSC and IBD, showed a 33% risk of developing CCA at ten years after diagnosis. The risk is not altered by a patient requiring a colectomy. The largest estimation of the risk of hepatobiliary cancer in PSC comes from an international multicentre study37 with a total of 7121 patients in 37 countries, in which the prevalence was 10%; CCA was the most prevalent, with 594 cases. This risk is greater if the PSC is associated with IBD.42 The recommendation of the European Crohn's and Colitis Organisation (ECCO)43 is an annual check-up with abdominal ultrasound and determination of CA19-9 in blood and, if PSC is suspected, a more targeted study, with magnetic resonance imaging or tomography.
Patients with IBD are significantly more likely to develop CRC, mainly due to the pro-neoplastic effect secondary to chronic intestinal inflammation.44 In turn, the relative risk of developing CRC is higher in patients with IBD and PSC compared to IBD alone (OR 4.79; 95% CI: 3.58–6.41). This risk remains even if the patient undergoes a liver transplant.45 Miard et al.12 demonstrated in a multivariate analysis that PSC increases the risk of CRC, with an OR 2.51 (1.01–5.8), to a similar degree to extensive colitis (OR 2.71). A comparative study46 of 273 patients with PSC (223 UC and 50 CD) found that patients with UC had a 56% greater risk of developing CRC than patients with CD.
A systematic review45 found that the risk of developing CRC was 3.24 (95% CI: 2.14–4.90) in patients with PSC and IBD compared to IBD without PSC. In addition to the inflammatory activity, the reduced absorption of BA due to cholestasis would lead to an increased amount of BA in the proximal colon, causing free radical-induced genomic instability, which directly damages the DNA. The length of time between the diagnosis of IBD and the development of dysplasia and CRC has not yet been established.37 However, low-grade dysplasia47 in patients with PSC and IBD is associated with a 30% likelihood of developing high-grade dysplasia or CRC itself within a year. These patients should be monitored more closely, even assessing the option of colectomy, as the presence of more advanced or multifocal lesions in the tissue samples has been reported.48
In view of the high association with CRC and IBD, all patients diagnosed with PSC should have an early colonoscopy, probably with chromoendoscopy.49 If this is normal, the endoscopy procedure should be repeated according to risk factors for CRC,50 and in five years if that study shows no IBD.37 If both PSC and IBD are present, an annual colonoscopy should be performed, ideally with chromoendoscopy and random biopsies.
Impact of biological and small-molecule therapy on primary sclerosing cholangitisBiological therapy has meant a great advance in the treatment of IBD. The FDA and the EMA have approved infliximab (IFX) and adalimumab (ADA), both anti-TNF-alpha monoclonal antibodies, and anti-integrin IgG1 monoclonal antibody vedolizumab (VDZ) for the treatment of moderate-to-severe CD and UC. At the same time, other therapies have been approved for specific types of IBD; certolizumab and ustekinumab in CD and golimumab in UC.51 Tofacitinib,52 a small oral molecule which inhibits JAK kinases, was recently shown to be effective in the induction and maintenance of remission in UC.
Elevated serum levels of tumour necrosis factor-alpha have been described in patients with IBD and PSC. However, studies have failed to demonstrate that IFX53,54 or ADA have any effect on inflammation of the bile ducts, while ADA only decreased alkaline phosphatase levels.55 This decrease may be related to a non-hepatic (bone) production, as some studies have shown that tumour necrosis factor can increase alkaline phosphatase levels in human chondrocytes.56
As previously mentioned, the pathogenesis of PSC is still not fully understood. However, one hypotheses is the role of the migration of activated lymphocytes from the intestinal mucosa to the liver causing fibrosis and focal inflammation in the bile ducts.57 Vedolizumab could play a role in the progression of evolution of PSC in patients with IBD by inhibiting integrin α4β7. However, studies have shown that vedolizumab does not have a great influence on the development of fibrosis and stenosis in the bile ducts.53,58
Although there is no contraindication for the use of golimumab, ustekinumab and tofacitinib in patients with IBD and PSC, there are no studies confirming their effectiveness in the progression of PSC.
One aspect of concern is the possible increase in infections when combining post-transplant immunosuppression with biological therapy. A recent systematic review59 which included patients on biological therapy who underwent transplantation vs non-transplant patients showed that there was no significant difference between the two groups (p=0.886). To date, there have been no randomised clinical trials assessing the immunosuppression typically used in transplantation in combination with biological treatment.
Colectomy in primary sclerosing cholangitis and inflammatory bowel diseaseThe indications for colectomy are varied, but major disease activity not controlled by drug therapy or the presence of cancer are the most common causes. In a retrospective review of 100 patients with PSC and IBD who underwent proctocolectomy with ileal pouch-anal anastomosis (IPAA), no mortality risk factor was found, not even the type of immunosuppression used.53 Although 30-day mortality was close to 50%, less than 20% required repeat surgery, with the only risk factor being the history of previous surgery (p=0.03). Another systematic review43 showed that pre-or peri-transplant colectomy would have a protective role for PSC. Other authors have shown a higher likelihood of graft loss among patients with a pouch compared to those who had a terminal ileostomy (2.8 vs 0.4 per 100 patient-years, p=0.005).60 The main causes of graft loss are hepatic artery thrombosis and the development of biliary stenosis. However, better quality studies are required before a colectomy can be recommended routinely to patients with IBD and PSC who need a liver transplant.
Does colectomy improve the course of primary sclerosing cholangitis?A retrospective study which included 2594 patients with PSC and IBD found that patients with IBD who underwent colectomy prior to the diagnosis of PSC had a 29% risk of dying or requiring a liver transplant due to PSC.53 In the multivariate analysis, the incidence of liver transplant in patients without colectomy after ten years of follow-up was 33%, vs 25% in those with prior colectomy (p=0.01).
Pouch in primary sclerosing cholangitis and inflammatory bowel diseaseThis is a group with a different phenotype compared to patients with IBD without PSC. These patients will have an increased risk of chronic pouchitis and inflammation at the afferent ileal loop and the pouch body.61 Given the greater risk of cancer in the pouch, these patients need to be in an endoscopic surveillance programme. Pouchoscopy should be performed every one to three years in this group of patients.62
Primary sclerosing cholangitis and inflammatory bowel disease: when will a liver transplant be required?One study which included 96 patients with PSC/UC63 showed that those who required a transplant more often had silent IBD, with fewer flare-ups and less need for immunosuppressants. In contrast, those who did not need a transplant had a greater need for intestinal surgery and a higher incidence of CRC. These data suggest that the severity of PSC could have a protective effect on UC activity.
In patients with IBD and PSC, the indication for liver transplant is based on the presence of liver failure, episodes of recurrent cholangitis, intractable pruritus or CCA (in very specific cases).64,65
Primary sclerosing cholangitis and inflammatory bowel disease after liver transplantationThere is conflicting evidence regarding IBD activity post-transplant. Some studies report that in at least 30% of these patients inflammatory bowel activity will worsen, with an increase in endoscopic and histological activity.66,67 Others have shown that IBD may even revert. Fattahi et al.68 found that out of 152 patients with PSC and IBD who underwent liver transplantation, the inflammatory activity did not change in 24 (15.8%), it decreased in 119 (78.3%) and it increased in nine (5.9%). The multivariate analysis showed that the use of ciclosporin (OR 0.14; 95% CI: 0.015–0.79) and the patient's pre-transplant weight (OR 0.81; 95% CI: 0.71–0.93) would have a protective effect.
In turn, de novo IBD (i.e. which develops post-transplant) is ten times more common in patients transplanted for PSC than in the general population, with the risk being 10–11% at 5 years and 14–30% at 10 years. De novo IBD is not only associated with transplants due to PSC, as post-transplant cases after kidney, heart or haematopoietic stem cell transplantation have also been described.69 Theories that might explain this risk are the loss of the protective effect of bile salts or being secondary to immunosuppression, especially due to the use of tacrolimus.70
However, the search for a differential diagnosis of diarrhoea is fundamental,71 considering infections, drugs (mycophenolate) and bacterial overgrowth. Investigations should include PCR for enteropathogens (including bacteria, viruses and parasites), including Clostridium difficile, inflammatory parameters such as complete blood count and CRP, and colonoscopy with biopsies.
What happens with immunosuppressive therapy?Initially, the preferred immunosuppressive drug was azathioprine72 in combination with another immunosuppressant. However, the most widely used immunosuppressants now are tacrolimus (which exerts strong suppression of the IL-2-producing T cells, preventing the regulatory response by that pathway45) and ciclosporin A.73 It was suggested that ciclosporin A may have twice the risk of recurrence, but this was not conclusive in the multivariate analysis.74
Post-transplant rejectionThe management of IBD in patients with liver transplantation represents a therapeutic challenge due to intercurrent flare-ups or possible associated comorbidities (especially infections) and contradictory effects between immunomodulatory therapy aimed at preventing rejection and other treatments aimed at preventing the IBD activity.64
Patients with PSC and IBD have a higher risk of acute cellular rejection after transplantation.23 We know that immunosuppression can lead to a reduction in immunosurveillance and the risk is higher if the IBD is active at the time of transplantation, if there is a short interval between the IBD symptoms and the transplant, if cytomegalovirus (CMV) is present in the donor organ or in the case of acute CMV infection or active smoking.75 However, these results come from case reports and case-control studies, and higher quality studies are necessary to properly establish this risk.
Recurrence of primary sclerosing cholangitis post-transplantIt has been established that by ten years post-transplant, the PSC may recur in 30–50% of patients,76 with that rate being higher in those with associated IBD.67 The risk is greater in patients with intestinal inflammatory activity, highlighting the need to achieve endoscopic cure targets. Lindstrom et al.77 recently established that having a pre- or peri-transplant colectomy would reduce the risk of recurrence of PSC by approximately 20%.
ConclusionPSC is associated with IBD in 50–80% of cases. These two conditions share a certain genetic susceptibility, some autoantibodies and bidirectional pathways through the intestine–liver axis, which perpetuates the inflammation.
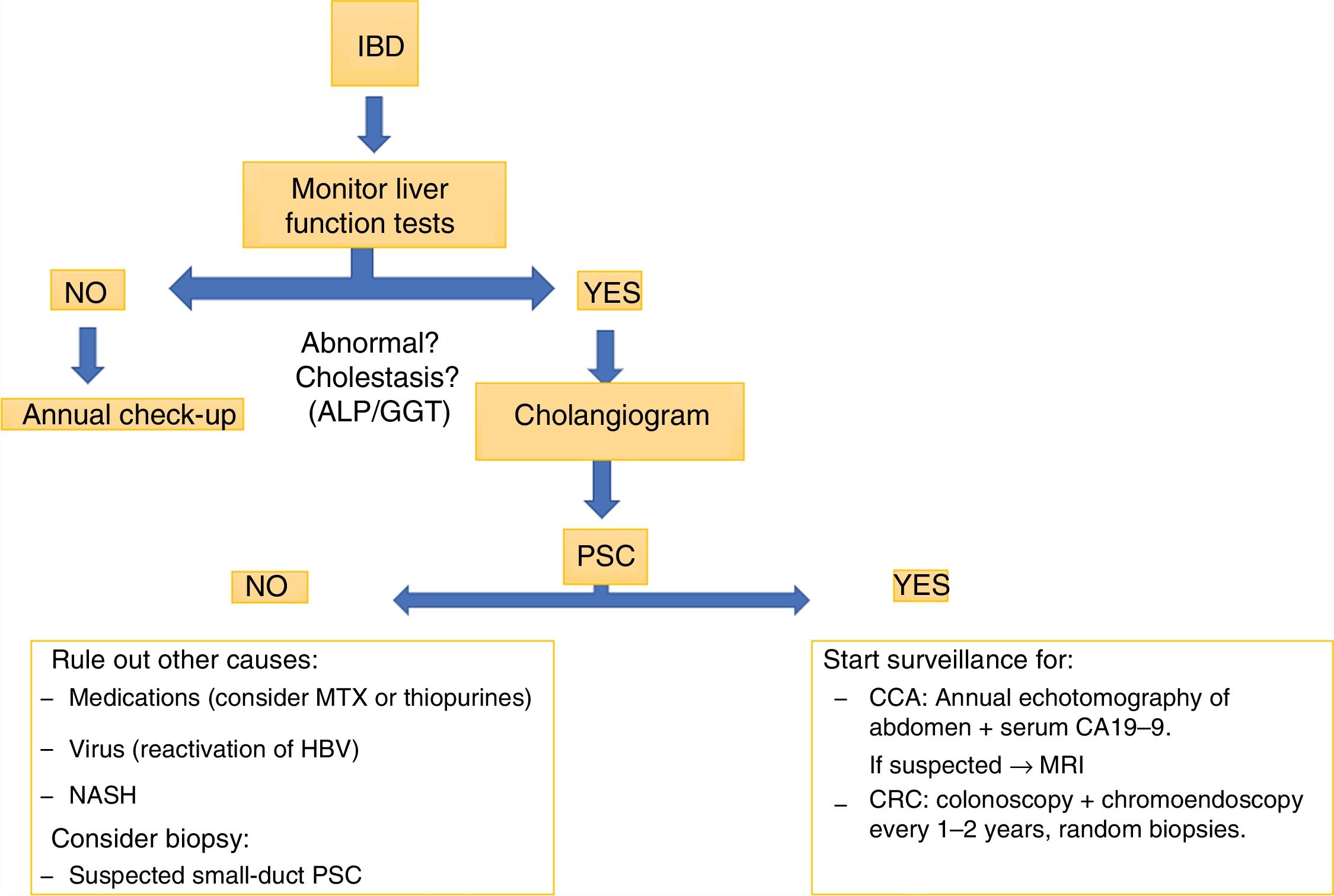
Patients with PSC should be routinely investigated for IBD. Patients with IBD should be investigated for PSC, at the very least when abnormalities are found in their liver function tests (Fig. 3). The clinical implications are related to the endoscopic finding/phenotype with extension of the disease with predominance in the right colon, backwash ileitis and preservation of the rectum, with a less symptomatic clinical presentation. However, the histology would show up more activity than that reported by the clinical findings, detecting an increased risk of CRC.
It is essential that we investigate whether or not the suppression of colonic inflammation through the optimisation of IBD therapy can change the natural course of PSC, bearing in mind the fact that PSC can even recur post-transplant, meaning that there is no curative treatment for this disease. We also need to determine whether or not this same control of the inflammation will reduce the cases of associated CRC. There is no doubt that more studies are required in order to generate a recommendation.
FundingNone.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Núñez F. P, Quera P. R, Gomollón F. Colangitis esclerosante primaria y enfermedad inflamatoria intestinal: interrelación intestino-hígado. Gastroenterol Hepatol. 2019;42:316–325.