Pneumatosis intestinalis (PI) is the presence of gas in the intestinal wall. It has multiple aetiologies, many of them benign but others with high mortality.1–4 Its location and radiological characteristics are indicative, but non-specific, and not pathognomonic for differentiating benign from life-threatening PI2,3; treatment and prognosis depend on the identified cause.
Establishing the cause of PI can be complex in patients with cancer, especially if there is a history of previous surgery, intra-abdominal stents, immunosuppression and chemotherapy.1 Several chemotherapy agents, including 5-fluorouracil (5-FU) have been associated with the development of PI,5–7 both in monotherapy and in combined regimens.
In addition to its indication as a first-line treatment in various solid tumours, 5-FU is also used with palliative intent in gastrointestinal and head and neck tumours.
We present the case of a patient with metastatic adenocarcinoma of the gastro-oesophageal junction on palliative chemotherapy with 5-FU, who presented secondary intestinal toxicity in the form of PI. We review the clinical and radiological characteristics of the condition, as well as its aetiopathogenesis and treatment.
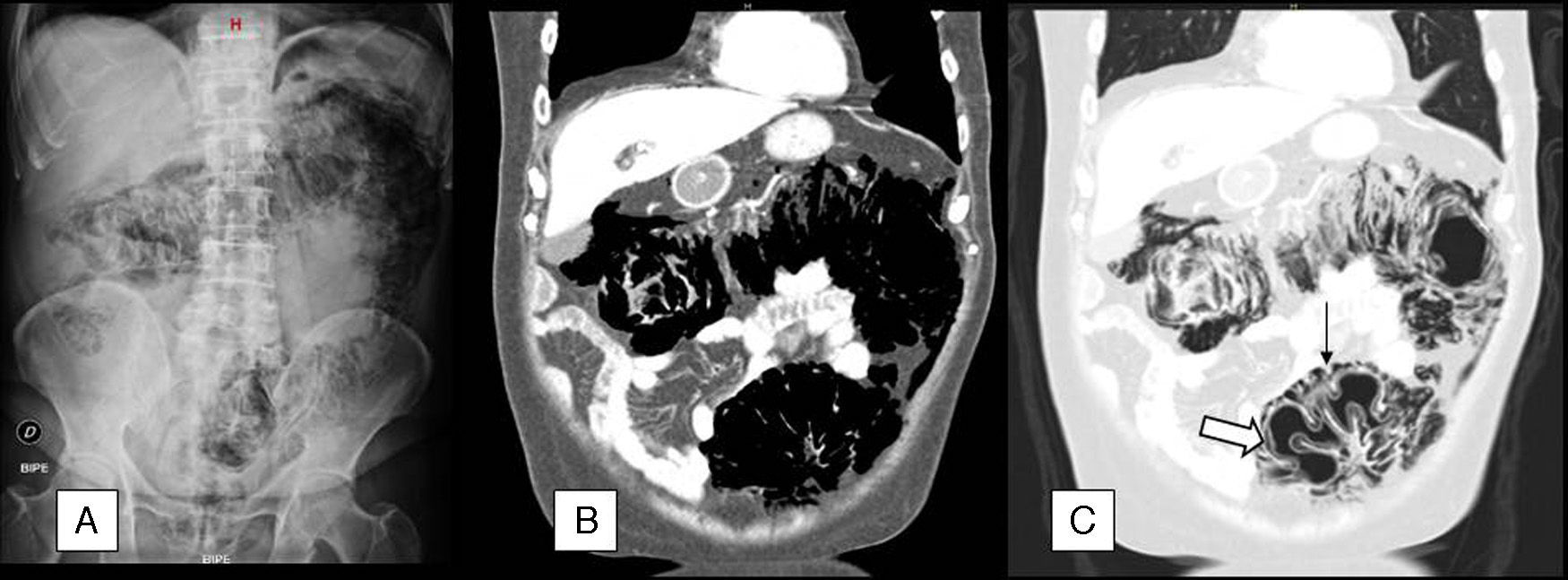
The case concerns a 65-year-old man with gastrostomy tube and a history of stage IV adenocarcinoma of the gastro-oesophageal junction (lung metastases) who was receiving palliative chemotherapy with a cisplatin (70mg/m2) and 5-FU (3000mg/m2) regimen every 21 days. After the fourth cycle of treatment, he presented to the emergency department for abdominal distension, colicky pain and diarrhoea (6 stools/day) with no mucus, blood or pus, which had commenced 4 days after completion of the 5-FU infusion. He was afebrile, and physical examination found: distended, non-tender abdomen with abundant bowel sounds and no signs of peritoneal irritation. Laboratory tests showed hypernatraemia, with sodium 148mEq/L, and hypokalaemia, with potassium 2.5mEq/L, with no evidence of sepsis or cytopenias. Radiological findings on the abdominal X-ray and computed tomography (CT) scan are described in Fig. 1.
(A) Abdominal X-ray in standing position, in which extraluminal gas density can be seen surrounding the entire colon, as well as a discrete amount of subphrenic pneumoperitoneum. (B) Coronal reconstruction of abdominal CT with intravenous contrast. The scan confirms the existence of large areas of gas density surrounding the different colon segments. C) The lung window allows us to more accurately determine the location of the gas, which is both inside (⇒) and outside (→) the colon wall.
A tentative diagnosis of PI probably secondary to 5-FU treatment was made, and the patient was admitted for intravenous fluid replacement, bowel rest and oxygen therapy 2L/min. He remained afebrile, with gradual improvement in the abdominal distension and diarrhoea and no signs of peritoneal irritation. Blood and stool cultures were negative. After 10 days of treatment, abdominal CT showed a significant decrease in the pneumo- and retropneumoperitoneum.
PI was first described in 1908,8 but has since been known by various names, and several possible causes have been reported.1–7
Although PI is probably a multifactorial condition, the bacterial and mechanical pathogenesis theories are the most widely accepted. The bacterial theory suggests that the gas-forming bacilli that colonise the intestine enter the mucosa due to increased mucosal permeability or disruption of the bowel wall, introducing gas into the wall. According to the mechanical theory, the presence of continuity solutions in the intestinal mucosa allows gas to enter the bowel wall, especially if there is an increase in the intraluminal pressure.9,10 In murine models, the cytotoxic effect of 5-FU on the cells of the intestinal mucosa reduces proliferation and increases cell apoptosis in the intestinal crypts; it also affects immunocompetent cells, enabling bacterial translocation from the lumen into the bowel walls and even into the bloodstream.10
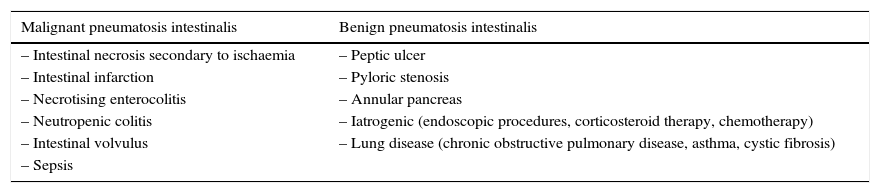
PI is a radiological sign and not a diagnosis in itself, and its radiological characteristics are indicative but not pathognomonic for differentiating between benign conditions and potentially serious diseases.1–4,8 This distinction can be especially complex in patients with cancer who, as mentioned, may have received treatments that have been associated with the development of PI. Pear1 classified some causes of PI according to their prognosis (Table 1).
Known causes of pneumatosis intestinalis.
| Malignant pneumatosis intestinalis | Benign pneumatosis intestinalis |
|---|---|
| – Intestinal necrosis secondary to ischaemia | – Peptic ulcer |
| – Intestinal infarction | – Pyloric stenosis |
| – Necrotising enterocolitis | – Annular pancreas |
| – Neutropenic colitis | – Iatrogenic (endoscopic procedures, corticosteroid therapy, chemotherapy) |
| – Intestinal volvulus | – Lung disease (chronic obstructive pulmonary disease, asthma, cystic fibrosis) |
| – Sepsis |
Source: Adapted from Pear BL.1
The absence of warning signs such as fever, signs of peritoneal irritation and laboratory findings of cytopenia and/or sepsis are probably the most important factors when assessing the possible causes and treatment of a patient with PI. Mimatsu et al.7 reported a case of 5-FU treatment-related PI that was symptomatically and radiologically similar to our patient. The main symptom was abdominal pain, with no fever or associated signs of peritoneal irritation. X-ray showed the presence of air in the intestinal wall and signs of pneumoperitoneum, while the CT scan showed the presence of PI with no signs of intestinal perforation. Blood and stool cultures were negative. Exploratory laparotomy was performed with no evidence of intestinal perforation. The patient was treated with empirical antibiotic therapy, oxygen, parenteral nutrition and gastrostomy placement to reduce the intraluminal pressure.
In cases of benign PI, such as the one presented, some authors have suggested that the treatment consists of bowel rest to reduce the intraluminal pressure and oxygen, since by increasing the partial pressure of oxygen in blood, the intramural gas would be displaced by simple diffusion.4,7
In summary, PI is a rare symptom. It has been related with several aetiologies, among them chemotherapy. Its radiological characteristics do not always allow the cause to be discerned. The patient's history and the clinical characteristics of the condition will point towards the most likely aetiology and determine the most appropriate therapeutic approach.
Please cite this article as: Vargas A, Pagés M, Buxó E. Neumatosis intestinal secundaria a quimioterapia con 5-fluorouracilo. Gastroenterol Hepatol. 2016;39:672–673.