A 44-year-old male, native of Bolivia but living in Spain for the last 10 years without recent travels to his country, and with no history of interest, attended the emergency room complaining of rectal bleeding with hemodynamic instability. The patient also addressed a three-month period of diffuse abdominal pain (predominantly in left flank), dysmotility, dysthermia, night sweats and weight loss. Physical examination, including neurological examination, was unremarkable except for malnutrition and pain in the left flank's deep palpation without detecting masses, organomegaly or ascites.
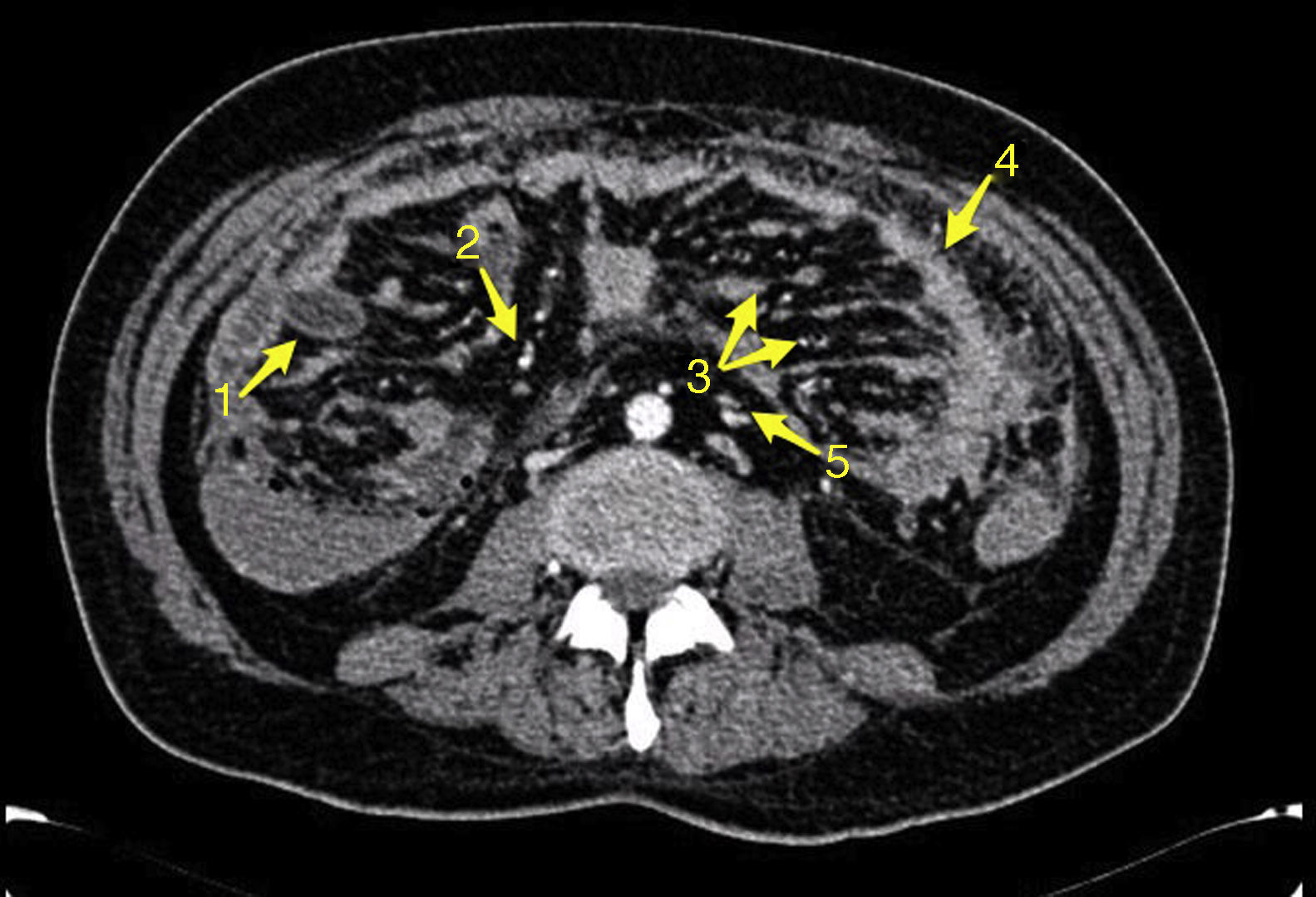
Tests revealed 7.6g/dL hemoglobin levels with ferropenia profile, 135mg/dL CRP, 60% Quick I. and severe malnutrition. The rest of the parameters, including WBC count, electrocardiogram and chest radiograph resulted without alterations. Colonoscopy was performed without finding mucosal lesions, though with hematic content in terminal ileum. Gastroscopy was normal. CT scan showed jejunal thickening with plenty intraluminal hematic content without actually observing bleeding points. In addition, a thickening of the omentum, multiple peritoneal implants, ascites, and lymphadenopathy were demonstrated. Double contrast CT and gastrointestinal transit were performed without new discoveries (Fig. 1).
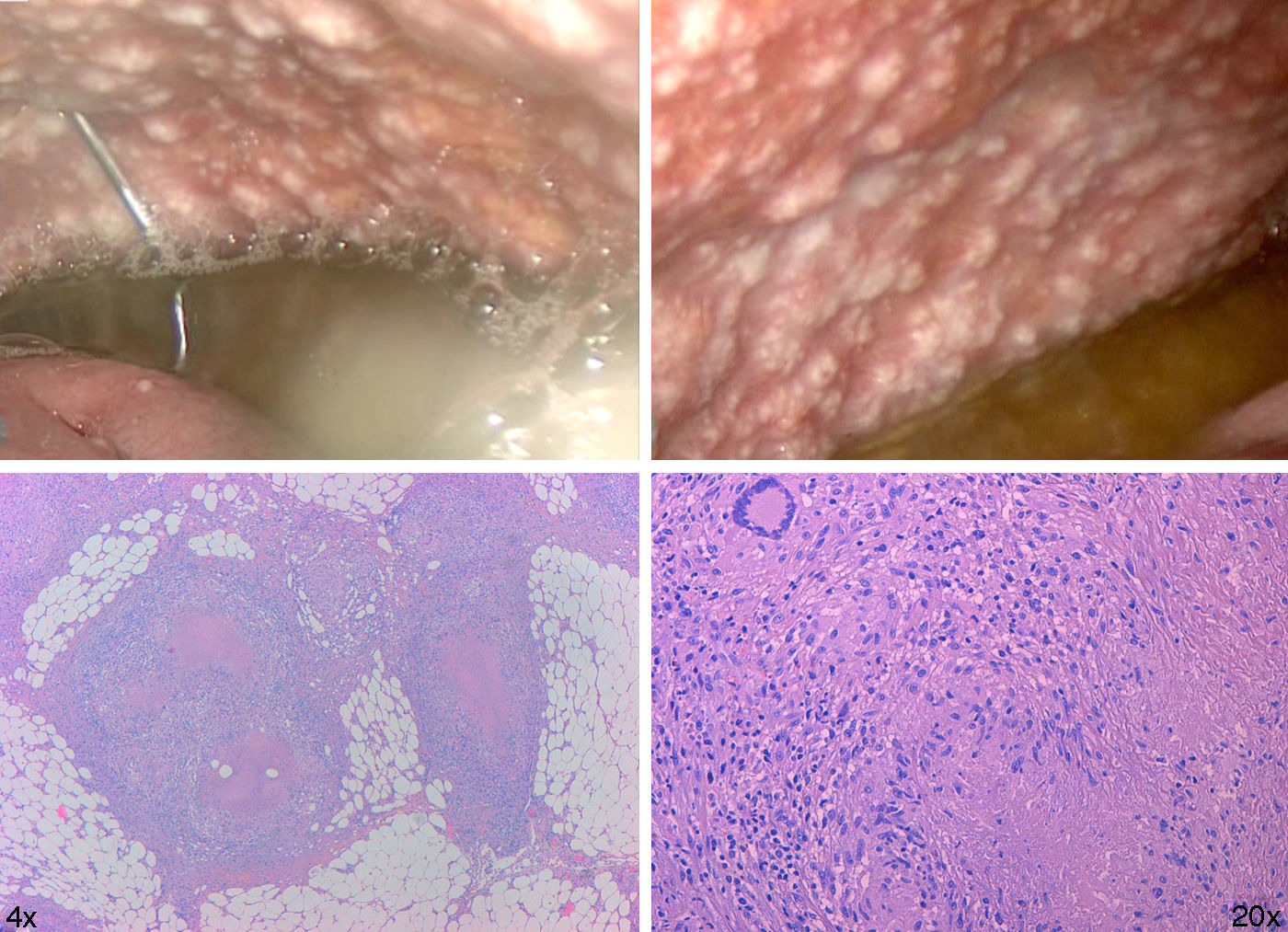
On admission the blood culture, HIV and Mantoux tests were negative. Early laparoscopy was performed, obtaining images that suggested miliary TB vs. peritoneal carcinomatosis (Fig. 2).
Histology showed abundant caseating granulomas. The Ziehl Neelsen stain and PCR for M. Tuberculosis in peritoneal fluid were negative. Due to these findings the patient was isolated until the negative result of sputum smear microscopy arrived. Pleural effusion was found in a second radiography with negative RCP for Mycobacteria.
Treatment was initiated with rifampicin, isoniazid, pyrazinamide and ethambutol in weight-adjusted doses, with resolution of symptoms. As an adverse effect the patient presented transient elevation of transaminases. After 8 weeks, sputum results arrived: both solid and liquid medium cultures were positive for Mycobacterium Tuberculosis sensitive to all TB drugs. Peritoneal fluid and urine cultures in both solid and liquid medium were negative. We had no peritoneum sample for culture. Currently our patient is progressing well with a 5kg weight gain and continues with rifampicin and isoniazid, intending to maintain the treatment for at least 12 months.
In this case gastrointestinal bleeding related to jejunal affectation and probably secondary to peritoneal extension, allowed the diagnosis of miliary tuberculosis.
Despite being a relatively old disease, tuberculosis remains nowadays the World's third leading cause of death by an infectious agent. According to the latest report of the World Health Organization,1 in 2013 nine million people developed TB and 1.5 million died from it. In recent years we are also witnessing another problem, the increasing incidence of multidrug-resistant TB (3.5% in new patients and 20.5% in previously treated). Although the initial target organ is the lung, tuberculosis is a systemic disease that can affect any organ.2 The fact that this can occur after the first contact with the organism, in a second time or never, has to do with various adaptive mechanisms of M. tuberculosis, but also and largely with host factors.3 In the case of intestinal tuberculosis, the organism reaches the abdominal organs through hematogenous dissemination, direct spread from adjacent tissues or, less commonly, through direct ingestion when near a TB focus (positive sputum-smear patients). It is worth noting that up to 25% of abdominal affectation presents itself with pulmonary tuberculosis, and that M. tuberculosis can implant anywhere in the abdominal cavity. This leads to varied and nonspecific clinical manifestations, making the diagnosis difficult.4 There are four main forms of abdominal TB5; lymphadenopathy, peritoneal, gastrointestinal and visceral, being the most common manifestations the first two or a combination of them. It is important to realize that only 30% of cases have fever and that the most common clinical sign is ascitis.6 For early diagnosis and treatment the biopsy is essential since it allows us to obtain histologic results, and most importantly, cultures and mycobacteria RCP (60–70% diagnosis sensitivity).7 However, as we have seen in our case, the profitability of ascites fluid culture and even its RCP is low (15% and 7% respectively). In gastrointestinal manifestation cases all this gains special importance when making the differential diagnosis with other processes such as Crohn8 disease or intestinal lymphomas. If, as in our case, endoscopy misses suspicious lesions’ biopsies, laparoscopy becomes an essential tool in the diagnosis of abdominal TB with high yield and minimal risks compared with the high morbidity and mortality rate in non-treated patients pending completion of the study.9
Conflict of interestThe authors declare no conflict of interest.
The authors want to thank the Emergency Department and the Microbiology Service of the Severo Ochoa University Hospital.