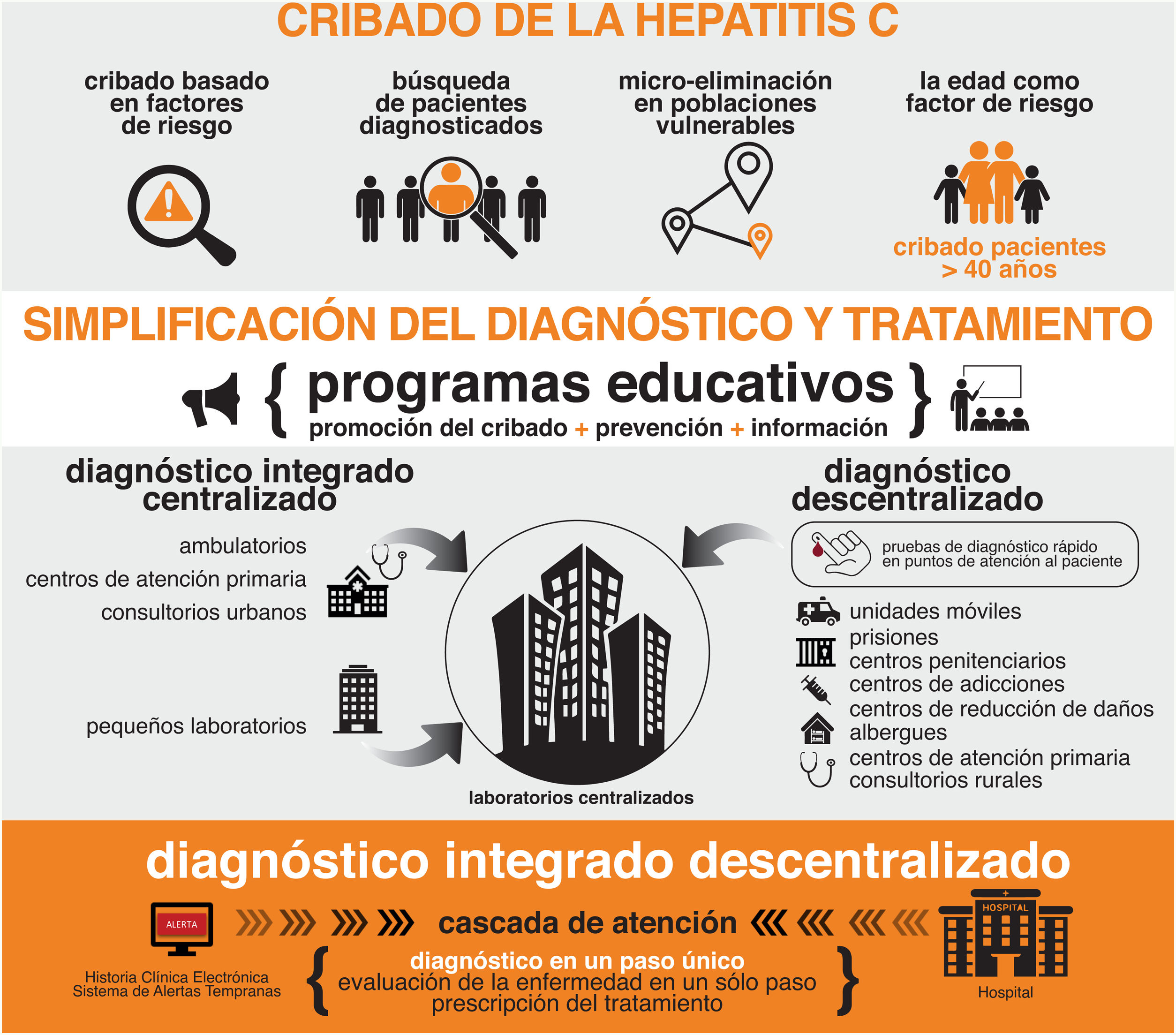
The Spanish Association for the Study of the Liver (AEEH) is convinced that the elimination of hepatitis C virus (HCV) in Spain is possible as long as we are able to use the resources and tools necessary for it. This document reflects the position of the AEEH regarding the elimination of HCV, establishing a wide range of recommendations that can be grouped into five categories: 1) Screening of HCV according to age, of the existence of classic acquisition risk factors of infection, active search of previously diagnosed patients and development of micro-elimination strategies in vulnerable populations, 2) Simplification of HCV diagnosis (one-step diagnosis and diagnosis at the point of patient care), 3) Simplification of patient treatment and improvement of care circuits, 4) Health policy measures and, finally, 5) Establishment of HCV elimination indicators.
La Asociación Española para el Estudio del Hígado (AEEH) está convencida de que la eliminación de la hepatitis C en España es posible siempre y cuando seamos capaces de emplear los recursos y las herramientas necesarias para la misma. Este documento refleja la posición de la AEEH respecto a la eliminación del VHC, estableciendo una amplia serie de recomendaciones que se pueden agrupar en cinco categorías: 1) Cribado del VHC en función de la edad, de la existencia de factores de riesgo clásicos de adquisición de la infección, búsqueda activa de pacientes diagnosticados con anterioridad y desarrollo de estrategias de micro-eliminación en poblaciones vulnerables, 2) Simplificación del diagnóstico del VHC (diagnóstico en un solo paso y diagnóstico en el punto de atención del paciente), 3) Simplificación del tratamiento de los pacientes y mejora de los circuitos asistenciales, 4) Medidas de política sanitaria y, finalmente, 5) Establecimiento de indicadores de eliminación del VHC.
Hepatitis C is a global public health problem. According to the latest published data, it affects more than 70 million people, which represents 1% of the world’s population.1,2 Recent studies carried out in Spain over the last 3 years estimate that the seroprevalence of the hepatitis C virus (HCV) antibody ranges between 0.8 and 1.2% of the adult population, while between 0.2 and 0.4% show an active HCV infection and, therefore, are at risk of developing liver cirrhosis in the medium to long term and the complications associated with it.3–6 A significant amount of these subjects have already been diagnosed and treated, although there is a relatively high number of patients who do not know they have hepatitis C, especially in patients more than 50 years old, as the Ministry's seroprevalence study shows.7 In summary, the final results of the survey conducted by the Ministry of Health,7 in a sample of 9,103 subject of the general population, are as follows: 9,002 were negative and 66 anti-HCV positive samples were detected (0.69%). Of these, HCV RNA was detected in 17 (0.17%). No confirmed case of hepatitis C was found in people younger than 20 years of age. Similarly, the prevalence of viremia was residual in people under 40 years of age. The prevalence of viremia increased in the cohorts from 40 to 49 (0.14%), from 50 to 59 (0.50%) and from 60 to 69 (0.34%), particularly in men. This prevalence, clearly lower than that demonstrated in previous studies, is probably a consequence of the enormous treatment effort made in our country over the last 4 years. Another very recent study, from Navarra, provides similar data.8,9
Despite the significant reduction in the number of infected patients since 2015, HCV infection continues to be a frequent and avoidable cause of liver cirrhosis and hepatocellular carcinoma. And despite the marked decrease in HCV as a cause of transplantation, the figure has stabilised over the last 2 years, with it being the cause of about 20% of transplants, probably due to the development of hepatocellular carcinoma on HCV cirrhosis, once it is eradicated.10 The World Health Organization (WHO), in line with other health institutions and governments, established in 2015 a global strategy in the viral hepatitis health sector with the aim of eliminating it as a public health problem by 2030.11 This strategy defines elimination as an 80% reduction in new HCV infections and a 65% reduction in HCV mortality. As a result of this global commitment, different governments have implemented specific action plans against hepatitis C. In the particular case of Spain, the National Strategic Plan for the Approach to Hepatitis C (PEAHC) in the National Health System12 established four lines of action: to quantify the magnitude of hepatitis C; to define access to treatment with direct antiviral agents (DAA); to establish coordination mechanisms to implement the strategy, and to promote progress in the knowledge of the prevention, diagnosis and treatment of hepatitis C in the National Health System (SNS) through actions in R&D&I. Since its beginning in April 2015 to May 2019, in Spain more than 130,000 patients with hepatitis C have been treated with DAA, which represents almost all of the patients diagnosed and monitored. To these patients, the thousands of patients treated with interferon-based regimens in the last two decades must be added, which probably means that about 150,000 patients have been treated.3 This plan, which is ground-breaking worldwide, has allowed Spain to be the focus of the countries that have advanced the most in terms of achieving the objectives set by the WHO for the elimination of hepatitis C in 2030.13 But despite these excellent data, it is estimated that amongst the general population seeking primary care, there are still 76,500 viraemic people. On the other hand, in Spain the diagnosis rate of this disease is not yet satisfactory and the number of adult subjects who are unaware that they are infected with HCV is estimated at around 22,500, to which we would also have to add all the patients who, despite being diagnosed, are not receiving treatment.7 This inadequate diagnosis rate may be due to the fact that there is an undetermined number of patients who do not access the health system because they belong to vulnerable social groups and are at risk of exclusion, such as the those sentenced to custodial and non-custodial sentences (already isolated before entering prison) or people who use drugs (PUD), and those who have access issues (immigrants). There are also those people with a fear of social stigmatisation who voluntarily stay out of the system. Finally, there are people who do not know the risk factors and, therefore, the possibility of being infected. They usually belong to age cohorts in which it has been shown that the prevalence of active infection is above average. These subjects can transmit the infection through risky practices, and a variable percentage may develop advanced liver fibrosis, cirrhosis and finally hepatocellular carcinoma. The identification of these subjects and their treatment is a good health investment, as evidenced by various studies conducted by members of the Spanish Association for the Study of the Liver (Asociación Española para el Estudio del Hígado - AEEH), although the studies have been conducted with incidences higher than the current ones.14–16
As for promoting early diagnosis in priority populations defined by the individual's membership of a vulnerable population, the current recommendation is to screen these groups. Several recent studies have shown that population screening associated with treatment is cost-effective in different age groups and in the general adult population.4,17,18 For this and for other reasons, both the AEEH and other scientific societies, such as the Spanish Society of Digestive Pathology, as well as the Alliance for the Elimination of Viral Hepatitis in Spain (Alianza para la Eliminación de las Hepatitis Víricas en España - AEHVE), advocate for the implementation of plans to establish strategies for screening and micro-elimination programmes for hepatitis C, which basically consist of segmenting and dividing the global elimination strategy focusing on different programmes aimed at primary sources of infection.
Finally, treatment is indicated in all infected patients.19–22 We know in detail the natural history of the disease and we know that proper treatment prevents progression to advanced stages and prevents transmission of the infection. Treatment achieves an individual benefit over the liver disease itself, decreases mortality from any cause,23 improves quality of life and prevents the development of extrahepatic manifestations.24,25 In cases with advanced fibrosis it prevents complications of cirrhosis,20 and in patients on the liver transplant waiting list it may even mean avoiding the procedure.26 In addition, it produces different collective benefits, such as reducing new infections or increasing the ease of access to liver transplantation for other patients.
For these reasons, the AEEH believes that the time has come to make a comprehensive plan to facilitate the elimination of hepatitis C (Table 1, Fig. 1). This plan is intended to be a framework in which all potentially useful initiatives for such elimination have a place. It is based on the following principles:
- –
Participation of a large group of professionals that ensures multi-disciplinarity.
- –
Based on the best available scientific evidence.
- –
Search for equity in all proposed measures.
- –
Inclusive, because it incorporates all local, regional, national and international measures that have proved useful for eliminating HCV and all elements of the healthcare chain.
- –
The search for all the tools that could lead to our ultimate goal: the elimination of hepatitis C as a public health problem in our country.
- –
Universal and executable plan by our health administrations.
Recommendations of the Spanish Association for the Study of the Liver for the elimination of the hepatitis C virus (HCV).
| Combinationof several HCV screening methods |
|---|
| Age-based HCV population screening |
| Screening based on risk factors for acquiring HCV infection |
| Active search for patients previously diagnosed but never treated or who have been treated, but have not been cured |
| Micro-elimination strategies in vulnerable populations |
| One-step diagnosis |
| Diagnosis at the place of patient care |
| Linking diagnosis to treatment. |
| Delegated dispensing |
| Development and use of HCV elimination markers |
| Strict epidemiological surveillance that allows the diagnosis of reinfections, the current aetiology of most incident cases |
| Increased awareness of the disease amongst health professionals and the general population |
The AEEH is firmly convinced that the elimination of hepatitis C in Spain is possible. But to achieve this elimination, it is essential to implement the following recommendations:
- –
Hepatitis C screening, with age as a risk factor for HCV infection. The results of the economic studies that evaluate the cost-effectiveness of screening recommend that it be carried out in the population aged 20 to 79 years. However, and since the majority of undiagnosed cases are concentrated in the 40–70 year segment, the AEEH recommends testing for HCV antibodies at least once in life in this age segment. Each health system should adjust the screening strategy to its local epidemiological information.
- –
To continue the active search for HCV in the population with risk factors both at the hospital and primary care levels.
- –
Programmes focused on micro-elimination simultaneously to the previous one, with the active search for HCV in vulnerable populations through all the assistance establishments that these people attend.
- –
One-step diagnosis in all NHS microbiology and/or clinical analysis laboratories.
- –
Adaptation of diagnostic techniques to the target population, including diagnostic means that facilitate access to a diagnosis and treatment for vulnerable groups at risk of social exclusion and who do not usually access conventional care establishments (primary and/or hospital care). Among these, use viremia at the point of patient care when the prevalence of viremia in the group to be screened is high, as well as an adequate link to the NHS care processes.
- –
Linking diagnosis to treatment. Equitable and universal access to screening and curative antiviral treatment.
- –
To expand coverage in harm reduction centres (for people who use drugs parenterally).
- –
To improve the continuity of care in the process of the global approach to the care of patients with hepatitis C.
- –
Delegated dispensation when this facilitates access to treatment in diagnosed patients.
- –
Development and use of HCV elimination indicators at the NHS level.
- –
Strict epidemiological surveillance that allows the diagnosis of reinfections, current origin of most of the incident cases, and that allows us to correctly distinguish the incident (acute) cases from the chronic (prevalent) cases, as recommended by the European Centre for Prevention and Disease Control (ECDC).
- –
Increased awareness of the disease amongst health professionals and the general population.
Following these recommendations will allow the identification and subsequent treatment of cases not diagnosed in our country, the diagnosis of reinfections, it will prevent the development of advanced liver diseases related to hepatitis C and, in short, it will contribute decisively to the elimination of this disease in the short term among our fellow citizens.
HVC screeningAge as a risk factor for HCV infectionScreening of a particular disease is a public health “service” in which members of a defined population are invited to answer questions or undergo tests to identify individuals who are more likely to obtain a benefit than harm —caused by successive tests or treatment— to reduce the risk of the disease that is being studied or its complications.25,26 HCV infection meets all the required criteria of a screening programme,27,28 in accordance with the criteria developed by our Ministry of Health29 about a decade ago and which we will divide into three categories:
- 1
Hepatitis C characteristics that support considering age as a risk factor for HCV infection:
- –
The disease is well defined and its natural history is known.
- –
The diagnosis of the infection has clear criteria.30 The determination of anti-HCV is simple, safe, fast, easy to perform, with high sensitivity, high specificity, high positive predictive value, well accepted by professionals and patients and with excellent cost-effectiveness.
- –
Treatment is extremely effective.
- –
The evidence indicating that early diagnosis and treatment improve the evolution of the disease is overwhelming: they prevent the progression to cirrhosis, reduce transmission and practically nullify the incidence of hepatocellular carcinoma.31–33 Even late-stage diagnosis and treatment of the disease decreases liver mortality, overall mortality, the need for transplantation and the incidence of hepatocellular carcinoma (European Association for Study of the Liver [EASL], American Association for the Study of Liver Disease [AASLD]). In addition, it avoids or improves extrahepatic manifestations related to infection.34,35
- –
It is an important public health problem, as evidenced by its remarkable impact in terms of incidence, morbidity and mortality36 and predictive models of disease burden.
- –
The cost-effectiveness of screening in the adult population has been repeatedly demonstrated,37,38 although it is true that they have been carried out taking into account incidences that are higher than the current ones.
- –
The development of a vaccine against HCV is very unlikely, at least in the medium term.
- –
- 2
Characteristics of our health system that make it possible to carry out a diagnostic program based on the age of the population to be screened:
- –
Health care for all citizens with a universal character.
- –
Computerisation of medical records and development of clinical information systems and administrative records.
- –
There is no alternative to screening when it comes to diagnosis in an asymptomatic population who don’t have clear risk behaviours.
- –
Existence of proper care circuits that allow a standardised diagnosis, treatment and monitoring.
- –
It is a health opportunity for vulnerable groups with little access to the health system. HCV screening improves access to the diagnosis of people who are infected with hepatitis B virus (HBV), human immunodeficiency virus (HIV) and other infectious agents. It directly improves the health of the individual targeted by the programme and, indirectly, of the subjects that could be infected by such people. This situation occurs particularly in some vulnerable groups, such as PUD, inmates in prisons, men who have sex with men (MSM), the homeless, immigrants and people with serious psychiatric illnesses. However, these groups are not homogeneous and, especially in MSM, the risk is linked to drug use.39
- –
Given the extraordinary ease, simplicity and cost of determining HCV antibodies, the complete implementation of the programme can be carried out in a period no greater than 3-5 years, reaching total target population coverage in a period that avoids inequality in access.40
- –
It is a feasible programme within the NHS that must be accompanied by a health promotion information system.
- –
Screening as a tool to achieve elimination only works in countries where treatment has been approved for all patients, a situation present in our country.
- –
- 3
Foreseeable consequences in the event that this screening programme is not carried out:
- –
Diagnosis of hepatitis C in advanced stages of the disease. Current data recently reported in our country show that 20% of patients begin their treatment in advanced stages of the disease.41 In addition, there would be a diagnostic depletion, predicted for the year 2022. This would be the current —and especially future— cause of new diagnoses without screening being made in advanced stages of the disease.42,43
- –
A slow or very slow decrease in incidence due to infection by people not diagnosed or treated.
- –
The highly negative impact on groups such as PUD, where the incidence is very much in relation to prevalence (which should be reduced to avoid new cases).
- –
Finally, there would be a new increase in morbidity and mortality attributable to HCV in the coming years, with an increase in the number of admissions due to decompensated cirrhosis and hepatocellular carcinoma, associated with an increase in the need for liver transplantation in these patients.44
- –
Screening in subjects over 40 years of age should be combined with the active search for HCV infection in people with one or more of the following characteristics:
- –
In all patients with hypertransaminasemia or with acute or chronic liver disease. Ideally, it should be done in a reflex way, provided that HCV antibodies have not been determined before.
- –
Subjects with high risk of HCV infection parenterally and/or percutaneously:
- ◦
History of blood transfusion or blood products prior to 1992.
- ◦
History of surgery, invasive procedures or dental interventions before the use of single-use injection material (1980).
- ◦
Patients who have undergone haemodialysis at some time.
- ◦
People with a history of tattoos, piercings or procedures with sharp instruments (manicure, pedicure, acupuncture, etc.) performed without due security.
- ◦
Healthcare personnel after recognised accidental exposure to possibly infected biological material.
- ◦
People who share personal items, such as toothbrushes or razors.
- ◦
- –
People with a higher probability of infection due to a higher risk of infection through sex:
- ◦
Sexual partners and co-habitants of people infected with HCV.45
- ◦
People who have risky, unprotected sex, especially MSM.
- ◦
Sex workers.
- ◦
- –
New-borns of mothers infected with HCV, from 18 months.46
- –
Other groups in which HCV infection should be systematically ruled out:
- ◦
Subjects who have been interned in penitentiary institutions.
- ◦
Subjects sentenced to non-custodial sentences.
- ◦
Infected by HIV and/or HBV.
- ◦
Patients with sexually transmitted infections, particularly syphilis, gonococci, chlamydia and Mycoplasma genitalium infections, especially in anal location.
- ◦
Immigrants from high prevalence countries (Eastern Europe, specifically Romania, Central Asia and East Asia, Africa and the Middle East).47–49 The population in Spain of high prevalence countries (by convention > 3%) are: Romania, Ukraine, Russia and from sub-Saharan Africa. It is more appropriate to speak of South Asia (Pakistan), and as for the Middle East, only the Egyptian population is relevant in terms of prevalence, which in Spain there is none.
- ◦
People who use drugs by inhalation and share instruments.
- ◦
The homeless.50
- ◦
The detection of these people with risk factors that use the health system could be enhanced through two strategies, mainly aimed at primary care professionals:
- –
Establish a system of warnings and/or alerts integrated into the electronic medical records regarding the need to test for HCV antibodies, based on clinical data related to the risk of HCV infection.
- –
Active search for people at risk of acquiring HCV infection from clinical information systems of electronic medical records.
An undetermined number of patients have been tested and diagnosed with hepatitis C (positive anti-HCV and/or positive HCV RNA) at some point in their life and, for different reasons, have not been treated and their follow-up within the health system has been lost. These patients are part of one of these two groups: a) patients who have anti-HCV antibodies (anti-HCV), who have not been tested for HCV RNA, so it is unknown if they are virulent, and b) patients who have been confirmed to be HCV RNA and who have not been treated or were not cured with interferon-based regimens or have not continued follow-up. The active search for these patients does not constitute a proper screening tool, but it contributes to the overall objective of diagnosing (and treating immediately after) as many patients as possible. These patients can be found in any part of our health system, both in primary care centres and in drug user care centres (DCC), and even in the hospital environment itself.50 Different strategies for the detection of these patients have been described:
- –
Review of cases with positive serology from microbiology information systems and/or central laboratories.51,52
- –
Review of the records of patients treated with DAA (and interferon-based) kept in our hospital pharmacies.
- –
"Conventional" systematic review of positive serology records in recent years.
- –
Use of systems based on artificial intelligence tools capable of identifying in different health records the potential existence of hepatitis C. This type of approach has been used in the communities of Galicia and Castilla y León.53
- –
Implementation of electronic alerts in medical records or in laboratory reports.50
- –
Although the data are not definitive, the seropositivity of patients admitted to psychiatric wards (acute and subacute) is close to 3%, so it seems reasonable to intentionally search for HCV infection in these patients.
To prevent this situation from occurring in the future it is essential:
- –
To develop a unique database of clinical information in different health systems.
- –
To increase surveillance, encourage mandatory reporting of HCV hepatitis cases and detection of cases in the healthcare environment.
- –
To increase the level of knowledge among health professionals about the need for early detection of this disease.
- –
To ensure that early diagnosis is accompanied by immediate access to treatment.
- –
To integrate the activity of early screening for hepatitis (HBV, HCV) and HIV into the portfolio of primary care services.
The concept of micro-elimination, recently revised,54 consists of segmenting the global strategy for the elimination of hepatitis C by focusing it on different programmes aimed at primary sources of infection (e.g., inmates in prisons, parenteral drug users, etc.). In our country there are multiple successful experiences of micro-elimination, both in prisons and in parenteral drug users with or without harm reduction programs.55 In these scenarios the following has proven useful for micro-elimination:
- –
Remote medicine in some prisons, both Spanish and international.56,57
- –
Decentralised diagnosis, whether in situ or not.
The AEEH recommends the combination of age-based population screening and the active search for people with risk factors for the acquisition of HCV and of patients previously diagnosed but never treated with micro-elimination strategies. Specifically:
- –
Implementing the carrying out of a single HCV antibody test in all adults between 40 and 70 years of age who have not been examined before.
- –
Implementing programmes for the active search of HCV in the population with risk factors both at hospital and primary care levels, as is currently done in some communities, such as Aragon and Catalonia.
- –
Active search of patients previously diagnosed but never treated or who have been treated, but have not been cured.
- –
Programmes focused on micro-elimination simultaneously to the previous one, through the active search for HCV in vulnerable populations in all the assistance establishments that these people attend.
Access to treatment for all patients with hepatitis C is a priority in any of the infection elimination plans. In this situation, the availability of tests for diagnosing active HCV infection in the microbiology and/or clinical analysis services is essential. Additionally, the high percentage of patients already treated and cured suggests that there should be access to agile information systems that allow this aspect to be identified, thus avoiding unnecessary and expensive repetitions, and also avoiding specialist consultation referrals of patients in whom the infection has already been cured and treated.
In the one-step diagnostic document (documento de diagnóstico en un solo paso (DUSP)) of hepatitis C jointly prepared by the AEEH and other societies, the markers of the hepatitis C virus can be observed, as well as the definition of active infection.58 The DUSP consists of the testing for viremia in all patients with a new serological diagnosis. After requesting a an HCV antibody test, and once it has been verified that it is the first time it had been requested and/or that a previous virological diagnosis has not been made, the viremia should be tested (without requiring a new clinical request). This DUSP is critical, since it reduces the access time for the start of treatment and, more importantly, prevents a significant loss of patients during the diagnostic process.59
But in addition to encouraging diagnosis in one step, the AEEH promotes decentralised and integrated community diagnosis. In Spain we are entering a new era with regard to HCV: the era of HCV elimination. The emergence of DAAs, which achieve cure rates above 97%, has made HCV elimination a real possibility. And to achieve this, it is necessary to diagnose and treat all patients. It is likely that many of the undiagnosed and/or untreated are part of groups of patients that avoid the system, as we mentioned earlier.60 In these groups it is essential to facilitate access to diagnosis.61 For this reason, in addition to the one-step diagnosis discussed above, it is necessary:
- –
To make diagnostic techniques more flexible and bring them closer to the patient, facilitating the implementation of methods such as dried blood spot (DBS) tests or diagnostic methods at the point of patient care.
- –
To move forward from centralised diagnosis to diagnosis that is decentralised and integrated into the healthcare system.
We can define centralised care as that practiced in conventional hospital centres, where the patient is treated, HCV infection is diagnosed, liver disease stage is identified and treatment is initiated. On the contrary, decentralised care is characterised by a rapid diagnosis of HCV infection, rapid staging of liver disease and treatment is initiated immediately ("test and treat" strategy). The choice of one type of health care or another should be based on the prevalence of the infection, the type of population and the existence of administrative or geographical barriers.62 Both types of care have a clear difference: centralised diagnosis allows optimal epidemiological control; on the other hand, decentralised diagnosis increases accessibility and reduces losses. Without a doubt, if we want to eliminate hepatitis C, we must evolve towards integrated decentralised diagnosis, i.e., a combination of both types of technique that ultimately converge to simultaneously achieve excellent epidemiological control and facilitate accessibility. But centralised or decentralised diagnosis does not necessarily mean speedy diagnosis. In Spain we have the following potential diagnostic systems:
- –
Classic virological diagnostic tests.
- ◦
Antibody detection by enzyme immunoassay (EIA).
- ◦
RNA detection by molecular techniques (PCR or TMA).
- ◦
Detection and quantification of the HCV core antigen.
- ◦
Genotype determination and phylogenetic studies.
- ◦
- –
“Atypical” virological diagnostic tests. Diagnosis at the place of patient care Conceptually we can define it as a diagnostic test that is carried out very close to the patient's environment, which provides a result in a short space of time (usually < 1 h) and that allows health care to be offered to vulnerable patients who otherwise would not access the diagnosis and treatment of hepatitis C. The Spanish Society of Infectious Diseases and Clinical Microbiology (La Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC)) has recently prepared a document for the use at the Point of care (POC).63 Among these we can highlight:
- ◦
Dried blood spot test (DBS).64,65 Although it is not standardised, samples collected on a paper card allow the detection of antibodies, viral load and genotype and can be analysed in virtually any hospital system equipment. However, at this time this procedure is not standardised or approved by the manufacturers of viral load tests for use in their equipment.66,67
- ◦
Anti-HCV detection systems in capillary blood and/or saliva in approximately 20 min.68
- ◦
Rapid, individual, on-site HCV RNA detection systems, such as capillary blood based systems.69
- ◦
POC systems for determination of Core Ag.
- ◦
Any of the systems used for HCV detection should be followed by an effective communication policy. In this sense, computerised alert systems significantly increase effective diagnosis and referral rates.50
The AEEH recommends single-step diagnosis, but also the inclusion of the new decentralised diagnostic tests, whether they be integrated or not into the health system. Specifically:
- –
It is recommended to test for viremia in:
- ◦
All patients with their first positive anti-HCV serology
- ◦
In patients with resolved infection who maintain risk behaviours for reinfection. In these patients the repetition of anti-HCV testing should be avoided, with HCV RNA testing, with a minimum annual periodicity, being recommended.
- ◦
- –
In general, the determination of viral genotyping is not essential.
- –
Since the main barrier to micro-elimination is the diagnosis and evaluation of infected patients, the test that best suits the peculiarities of the health system, the population or the existence of different types of barriers (geographical, cultural, etc.) should be used.
- –
Another important barrier is the link to treatment centres.
- –
In an integrated decentralised model, the TGSS (Tesorería General de la Seguridad Social (Social Security General Treasury) is a good alternative.
- –
Having a system of alerts integrated into electronic medical records, regardless of the type of test used and the diagnostic system, facilitates access to treatment after diagnosis.
The treatment of hepatitis C is indicated in patients infected with HCV, regardless of the degree of liver fibrosis or the existence of different comorbidities. The purpose of this document is not to provide the reader with a guide to the treatment of patients with hepatitis C; in this regard, we recommend that you consult the AEEH hepatitis C treatment guide. In the era of elimination, it is not only essential to simplify the diagnosis of the infection, but also to address the treatment of the disease in a simple way for the patient, which prevents patients from “escaping”, and this can only be achieved by healthcare circuits of maximum simplicity. To achieve this simplicity we can opt for care in one of these two systems:
- –
High-resolution hospital consultations, in which patients are assessed for liver disease (fibrosis), are attended by the specialist and are prescribed and dispensed medication as quickly and efficiently as possible. In those hospitals without immediate access to transition elastography, the use of indirect fibrosis indices such as FIB-4 or others is an excellent alternative.70 The high resolution query can be defined as optimal in which the whole process is carried out in a single day. High-resolution consultations are important in patients with poor adherence to the health system and in those with advanced fibrosis.71,72
- –
Development of non-hospital care establishments and patient care points, especially for vulnerable populations, where the diagnosis is established quickly and treatment is started immediately.64 These strategies are especially indicated in immigrants (especially in rural areas), where there are geographical barriers, in the mentally ill, PUD, alcoholics, in high-turnover and low-stay prisons, sexual care clinics, extremely distant health centres or damage reduction centres.
The key aspects related to treatment that we must remember are:
- –
Treatment is indicated in all patients with an active HCV infection. A highly effective pangenotypic regimen is recommended, regardless of the degree of liver fibrosis.
- –
The duration of treatment should be between 8 and 12 weeks and the route of administration should be oral.
- –
Medical follow-up during the treatment is not necessary, except for patients at risk of complications due to advanced liver disease or patients in whom therapeutic compliance may be in doubt.
- –
The evaluation of liver disease, dispensing and prescription should ideally be done in a single visit.
- –
Since DAA prescription must be carried out in a hospital, we advocate that these diagnostic devices and on-site treatments be coordinated with prescribing hospitals and, through dispensation and delegated custody, the prescription can be carried out at the peripheral centre.
- –
It is necessary to establish adequate information systems and/or alerts that prevent the loss of continuity between diagnosis and treatment.
- –
Regarding the follow-up of these patients, it is important to differentiate between virological healing (this will be achieved in almost all patients) and the cure of the disease.
- ◦
Cured patients without fibrosis or with only mild fibrosis can be discharged once sustained virological response (SVR) is confirmed.
- ◦
Patients with advanced fibrosis and cirrhosis should be monitored indefinitely.
- ◦
- –
To consistently assess the incidence of reinfection, we must use a single definition of recurrence and reinfection. Academically it is not completely correct, and the most operational definition of reinfection is:
- ◦
Recurrence of infection: recurrence of viremia at any time between the start of treatment and up to 12 weeks after the end of the treatment (even without the need for genotyping before or after treatment).
- ◦
Reinfection: recurrence of viremia at any time after 12 weeks after the end of treatment (no need for genotyping before or after treatment).
- ◦
However, the above definition that differentiates between recurrence and time-based reinfection may not be fully adequate. In our environment, reinfection is relatively frequent between the end of treatment and 12 weeks post-treatment in people with risky behaviours.
- ◦
- –
In patients with a previous HCV infection who continue to be exposed to risk factors, annual testing of viral load is required as recommended by the clinical guidelines. In other words, screening for a potential HCV reinfection should not be done with anti-HCV testing but with molecular techniques (HCV-PCR).
- –
The possibility of testing for viremia should be provided to all out-of-hospital assistance contacts (primary care, community agents) with which these people interact.
- –
VHCWe must strengthen the multidisciplinary and transversal training of all the groups involved in the care of vulnerable populations at high risk of HCV infection.
- –
Knowledge of hepatitis C in primary care should be increased to ensure adequate patient management and the transmission of correct information to them. This training should address two aspects: patient care and avoiding transmission.
- –
Promoting the potential role of consulting nurses, who can contribute to patient follow-up once the specialist has indicated the treatment.
- –
In populations far from health centres (rural Spain), an alternative may be the coordination between pharmacies and primary care centres so that the administration is carried out there.
- –
Continuous training plans should be implemented for health professionals based on knowledge of this plan or other HCV elimination plans with two work areas: training and information.
- –
An integrated patient information plan must be made:
- ◦
Provide information, unified and agreed by the different pharmacy services, orally and in writing, about the prescribed treatment, as well as a direct contact details for the pharmacy service (telephone/email) for the dealing with queries.
- ◦
Promote patient training and education to encourage a motivated, committed, expert and active patient in terms of their self-care and the importance of complying with their treatment.
- ◦
Information about lifestyle and diet.
- ◦
Provide reliable information consultation websites.
- ◦
The AEEH recommends:
- –
Treatment is indicated in all patients infected with HCV.
- –
Delegated dispensing in vulnerable populations should be facilitated.
An elimination programme cannot be carried out without the support of healthcare institutions. In our opinion, the update of the PEAHC is essential, which must be made compatible with the regional plans that adapt that national plan to the reality of each autonomous community. In this sense, we already have the first regional plans aimed at the elimination of HCV. In this way, “regional micro-elimination” policies are being added in order to achieve “national elimination”: Cantabria Plan,73 Aragón Plan,74 Galicia Plan, Canary Islands Plan,75 Catalonia Plan,76 Madrid Plan,77 Murcia Plan and Navarra Plan. Likewise, the publication of the plans of different countries is periodic: Australia,78 Scotland,79 France,80 Egypt,81 Greece,82 Netherlands,83 Iceland,84 Switzerland,85 Thailand,86 United States,87 Rwanda,88 Mongolia,89 Pakistan90 or Georgia.91 Although we do not have updated results of its implementation, some work is being done on their modelling in order to implement them. For scientific societies, such as the AEEH, promoting these regional plans is very important. Among the different objectives of these plans are:
- –
To define and develop micro-elimination strategies in primary foci.
- –
To influence health education so as to avoid reinfections in vulnerable groups.
- –
To establish damage reduction programmes.
- –
To create new specific assistance programmes for prison inmates and people with non-custodial sentences.
- –
To advocate for healthcare responsibilities to pass from prisons and addiction treatment centres to the public health system, as established by law (Law 16/2003, of 28 May, on cohesion and quality of the National Health System; Official State Journal no. 128, of 29/05/2003).
- –
To establish new care routes in groups that are isolated from the health system (vulnerable groups).
- –
Creation of community centres or landmarks for screening to combat primary foci.
- –
To ensure homogeneity and territorial coordination in the approach to hepatitis C.
- –
To develop prevention policies that affect risk practices.
- –
To promote in the male homosexual community the adoption of safe sex practices and the performance of the HCV test within the approach for risk factors agreed in the regional plan.
- –
To collaborate with NGOs working in the field of MSM, programmes for the prevention of sexually transmitted diseases, hepatitis A virus (HAV), HBV, AIDS, syphilis, etc.
- –
To maintain the basic criteria for the selection of donors of blood and/or blood components.
- –
To strengthen and update, where appropriate, the recommendations to avoid biological risks in invasive diagnostic and therapeutic procedures, as well as post-exposure protocols for HCV due to occupational exposure.
- –
To review compliance with the control mechanisms of piercing, tattoo and micropigmentation establishments.
- –
To develop interventions that allow reducing population risk.
- –
To increase knowledge about hepatitis C in the general population, through information campaigns using posters, brochures, media, social networks, etc., that simply and clearly bring the knowledge and message to the population about what hepatitis C is and how it is transmitted and that convey, above all, the message that treatment is effective today and hepatitis C is a curable disease.
- –
To have educational resources available as guides for different types of users: patients, associations, work groups, etc.
These interventions require very dynamic methodologies based on the interaction and interdisciplinary work of patient associations, health personnel and the media, to ensure that the message reaches as many people as possible, ensuring that the messages are accessible from a cultural and linguistic point of view for the different target populations. At this point, collaboration with social workers and sectors (NGOs) that know the idiosyncrasies of the different risk populations and specific populations is very important in order to be able to approach and work with them.
- –
Design of specific material that serves as the basis for conducting workshops in the health and community field, as well as for carrying out activities in the field of sexual-affective education programmes in the school environment.
- –
To design actions to reduce the risk in the health system. Despite the implementation of safety measures in the healthcare environment, the incidence of new cases of hepatitis C continues in this environment, attributed to unsafe practices or inadequate infection control.
- –
The action plan to protect patients and workers within the healthcare environment should include:
- ◦
To improve monitoring and detection of cases produced in the healthcare environment.
- ◦
To reduce the risk associated with improper handling of disposable instruments and syringes by reducing exposure to percutaneous instruments and updating guidelines for the management of HCV exposure in the healthcare environment.
- ◦
To provide the necessary education in the most important aspects of this infection for its adequate control in acute, chronic health centres and in outpatient control.
- ◦
Safe handling and disposal of sharp objects and contaminated waste.
- ◦
Analysis of donated blood.
- ◦
Training of health personnel: hand washing and use of gloves, including hand preparation for surgery; safe cleaning of medical and surgical equipment.
- ◦
The overall objective of HCV elimination requires the development of different indicators that allow the health outcomes of the different interventions that are being carried out to be assessed, both at the level of screening and micro-elimination, or diagnosis and treatment. Among them the following could be done:
Participation indicators- –
Target population of each programme (macro and micro-elimination)
- –
Detection rate of anti-HCV/HCV-PCR.
- –
Bi-annual/tri-annual incidence of HCV. Repeat prevalence studies every 2-3 years.
- –
Infection diagnosis/One-step infection diagnosis ratio
- –
Diagnosis/Non-centralised diagnosis ratio.
- –
Indicators of the impact of diagnosis and treatment in the medium term: number of patients diagnosed with hepatocellular carcinoma, number of hospital admissions due to HCV, number of transplants due to HCV, mortality due to HCV.
- –
Percentage of patients with prior known/unknown infection.
- –
Percentage of patients with mild/advanced disease.
- –
Extrahepatic disease.
- –
Co-infections: HIV, HBV.
- –
Form of presentation of the disease: early or late. If it is late: decompensation and type and hepatocellular carcinoma.
- –
Ratio of those diagnosed/treated.
- –
Ratio of treatments established in the first consultation/treatments established.
- –
Treatments used.
- –
SVR.
The AEEH recommends:
- –
The use of outcome indicators is critical for the evaluation of HCV elimination.
- –
The superiority of one over the other has not been demonstrated, so it is desirable to use at least one marker from each of the categories indicated above.
Coordinator: Javier Crespo.
Editorial Group: Agustín Albillos, María Buti, José Luis Calleja, Javier García-Samaniego, Manuel Hernández-Guerra, Trinidad Serrano, Juan Turnes.
Reviewing Group: Enrique Acín García, Juan Berenguer, Marina Berenguer, Joan Colom, Inmaculada Fernández, Conrado Fernández Rodríguez, Xavier Forns, Federico García, Rafael Granados, Jeffrey Lazarus, José María Molero, Esther Molina, Fernando Pérez Escanilla, Juan Antonio Pineda, Manuel Rodríguez, Manuel Romero, Carlos Roncero, Pablo Saiz de la Hoya, Gloria Sánchez Antolín.
Please cite this article as: Crespo J, Albillos A, Buti M, Calleja JL, Garcia-Samaniego J, Hernández-Guerra M, et al. Eliminación de la hepatitis C. Documento de posicionamiento de la Asociación Española para el Estudio del Hígado (AEEH). Gastroenterol Hepatol. 2019;42:579–592.
This article is published simultaneously in [Gastroenterología y Hepatología - https://doi.org/10.1016/j.gastre.2019.09.002] and [Revista Española de Enfermedades Digestivas - https://doi.org/10.17235/reed.2019.6700/2019], with the consent of the authors and editors.