Tenofovir disoproxil fumarate (TDF) is recommended for the prevention of perinatal transmission of the hepatitis B virus (HBV). This study aimed to systematically assess the efficacy and safety of TDF in pregnant women with chronic HBV and their infants.
Material and methodsDatabase searches were performed to identify studies blocking the mother-to-child transmission of the hepatitis B virus with tenofovir. The search included pregnant women with chronic HBV infection administered with TDF compared to the no treatment controls, and data from individual studies were pooled using RevMan v5.3 for meta-analysis.
ResultsSeven studies with a total of 911 patients met the inclusion criteria: 433 patients in the TDF group and 478 patients in the non-TDF group. The HBV mother-to-child transmission rate in the tenofovir group was effectively reduced compared to the control group (RR: 0.18, 95% CI: 0.08-0.40). HBV-DNA positivity was also significantly low in infants from TDF group (RR: 0.17, 95% CI: 0.10-0.30) and the TDF treatment resulted in significantly higher anti-HBs production (RR: 1.11, 95% CI: 1.04-1.18). Similarly, maternal HBV-DNA was suppression was significantly high in the TDF group (RR: 34.16, 95% CI: 16.40-71.13). Women treated with TDF and their infants did not result in serious adverse events that are statistically different as compared to the women who did not receive any treatment.
ConclusionTreatment of HBV infected pregnant women with TDF can effectively and safely prevent the perinatal transmission of chronic hepatitis B.
El fumarato de disoproxilo de tenofovir (FDT) se recomienda para la prevención de la transmisión perinatal del virus de la hepatitis B (VHB). El objetivo de este estudio fue evaluar de manera sistemática la eficacia y la seguridad del FDT en las mujeres embarazadas con VHB crónico y en sus hijos lactantes.
Material y métodosSe realizaron búsquedas en las bases de datos para identificar los estudios sobre el bloqueo de la transmisión del virus de la hepatitis B de madre a hijo con el tenofovir. La búsqueda incluyó a mujeres embarazadas con infección crónica por el VHB que recibieron FDT en comparación con controles sin tratamiento, y los datos de los estudios individuales se agruparon mediante el uso de RevMan v5.3 para el metaanálisis.
ResultadosLos criterios de inclusión se cumplieron en siete estudios con un total de 911 pacientes: 433 pacientes en el grupo de FDT y 478 pacientes en el grupo sin FDT. La tasa de transmisión de madre a hijo del VHB en el grupo de tenofovir se redujo efectivamente en comparación con el grupo de control (RR: 0,18, IC del 95 %: 0,08-0,40). Los resultados positivos del ADN del VHB también fueron significativamente bajos en los infantes del grupo de FDT (RR: 0,17, IC del 95 %: 0,10-0,30) y el tratamiento con FDT dio como resultado una producción significativamente mayor de anti-HB (RR: 1,11, IC del 95 %: 1,04-1,18). Del mismo modo, la supresión del ADN del VHB materno fue significativamente alta en el grupo del FDT (RR: 34,16, IC del 95 %: 16,40-71,13). El tratamiento de las mujeres con FDT y sus hijos lactantes no provocó acontecimientos adversos graves en ellas ni en sus hijos que fueran estadísticamente diferentes de los de las mujeres que no recibieron ningún tratamiento.
ConclusiónEl tratamiento con FDT de las mujeres embarazadas infectadas por el VHB puede prevenir de manera eficaz y segura la transmisión perinatal de la hepatitis B crónica.
Hepatitis B virus (HBV) infection remains a major global health problem. According to the 2017 World Health Organization's Global Hepatitis Report, 257 million people worldwide suffered from chronic hepatitis B infection, while 887 thousand people died of hepatitis B-related diseases in 2015 alone.1 Perinatal or mother-to-child transmission (MTCT) is the common route of transmission of chronic HPV infection, and also maintains the high incidence of chronic hepatitis B worldwide. Without any intervention, the risk of infecting with HBV increases dramatically to as high as 40-90% in the infants who born to women that test positive for hepatitis B surface antigen (HBsAg).2,3 Use of combined postpartum passive immunization with the hepatitis B viral immunoglobulin (HBIG) and active immunization with the HepB, reduces further the risk of MTCT.4 However, 10-30% of the newborns still cannot achieve effective HBV immunoprophylaxis,and especially for infants born to pregnant women with serum HBV-DNA levels greater than 2 x 105-107 IU/ml).5
More and more studies have shown that antiviral drugs, such as lamivudine (LAM), tenofovir disoproxil fumarate (TDF) and telbivudine (LDT), can effectively prevent HBV viral replication in pregnant women infected with CHB at a high viral load, which is the feasible way to reduce MTCT.6–10 The 2018 AASLD guidelines for the management of chronic HBV infection proposed for TDF administration to pregnant women with HBV infection in the third trimester.11 Besides, tenofovir was classified as category B by the US Food and Drug Administration (FDA) pregnancy risk classification system. Therefore, tenofovir is the recommended antiviral drug in the third trimester of pregnancy. In recent years, many studies have reported the efficacy and safety of TDF in preventing the perinatal transmission of HBV from mother to child, but the conclusions of each study are not consistent. Chen et al.12 studied 12 months of follow-up and found that MTCT still occurs in infants born to women with a high HBV viral load or with HBeAg (p=0.1423). However, Pan et al.4 concluded that TDF effectively reduced the HBV mother-to-child transmission rate (p=0.01). The sample size of different studies maybe the reason for the possible discrepancy. Therefore, we performed a meta-analysis to pool results on the effect of oral TDF therapy on MTCT prevention using HBsAg seropositivity, HBV-DNA suppression and anti-HBs production as indicators of efficacy. Maternal ALT elevation, serious adverse events in both mothers and infants are used as indicators of TDF safety. The estimates will add to the understanding of the benefits and risk of TDF use in the prevention of in utero HBV transmission.
Materials and methodsSearch StrategyA comprehensive search was performed in PubMed Database, Web of Science Database, Cochrane Library, MEDLINE CD-ROM Database, OVID Electronic Journal Full-Text database, EMBASE (embase.com) database, CALIS foreign language journal network and ClinicalTrials.gov, from the date of inception of each database until November 7, 2019. We further searched unpublished literatures using the http://www.opengrey.eu/ website. The search terms included: “tenofovir”, “TDF”, “Viread”, “tenofovir disoproxil fumarate”, “HBV”, “hepatitis B virus”, “chronic hepatitis B”, “intrauterine”, “maternity”, “mother”, “pregnancy”, “pregnant”, “perinatal transmission”, “vertical transmission” .Boolean operators ‘AND” and “OR” were used to merge and create search terms in PICO format. The search strategy is described in greater detail in Supplement Table 1.
Characteristics of the 7 included studies.
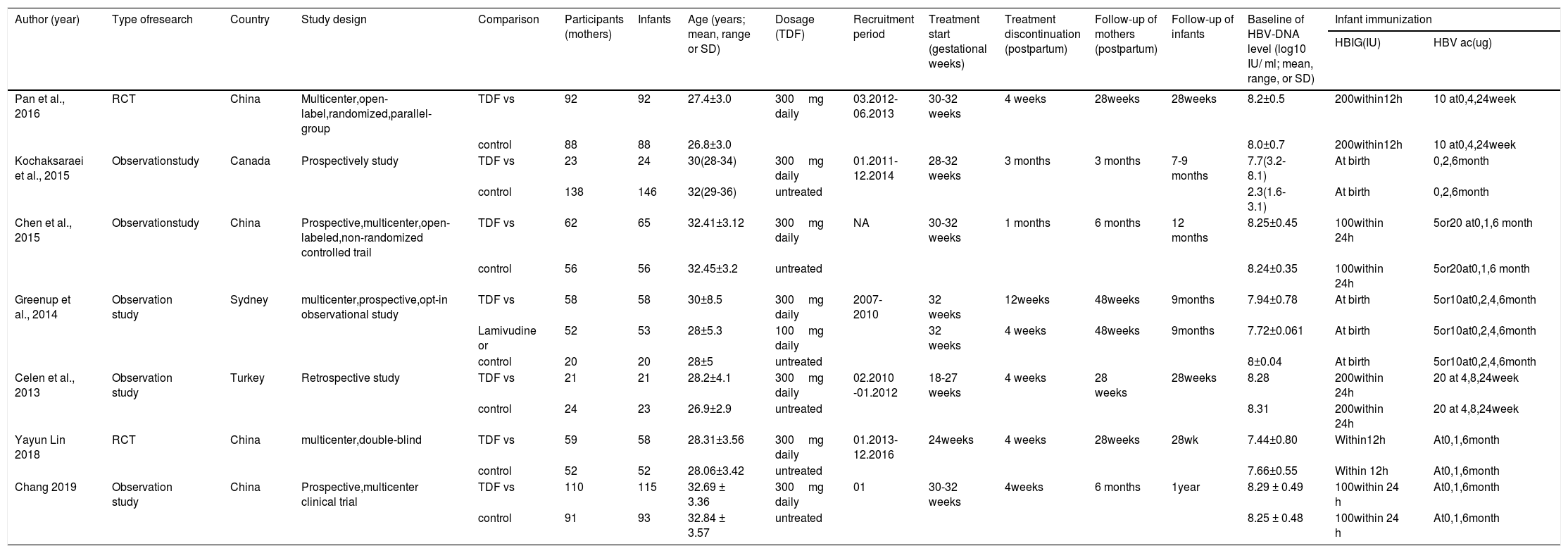
| Author (year) | Type ofresearch | Country | Study design | Comparison | Participants (mothers) | Infants | Age (years; mean, range or SD) | Dosage (TDF) | Recruitment period | Treatment start (gestational weeks) | Treatment discontinuation (postpartum) | Follow-up of mothers (postpartum) | Follow-up of infants | Baseline of HBV-DNA level (log10 IU/ ml; mean, range, or SD) | Infant immunization | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HBIG(IU) | HBV ac(ug) | |||||||||||||||
| Pan et al., 2016 | RCT | China | Multicenter,open-label,randomized,parallel-group | TDF vs | 92 | 92 | 27.4±3.0 | 300mg daily | 03.2012- 06.2013 | 30-32 weeks | 4 weeks | 28weeks | 28weeks | 8.2±0.5 | 200within12h | 10 at0,4,24week |
| control | 88 | 88 | 26.8±3.0 | 8.0±0.7 | 200within12h | 10 at0,4,24week | ||||||||||
| Kochaksaraei et al., 2015 | Observationstudy | Canada | Prospectively study | TDF vs | 23 | 24 | 30(28-34) | 300mg daily | 01.2011-12.2014 | 28-32 weeks | 3 months | 3 months | 7-9 months | 7.7(3.2-8.1) | At birth | 0,2,6month |
| control | 138 | 146 | 32(29-36) | untreated | 2.3(1.6-3.1) | At birth | 0,2,6month | |||||||||
| Chen et al., 2015 | Observationstudy | China | Prospective,multicenter,open-labeled,non-randomized controlled trail | TDF vs | 62 | 65 | 32.41±3.12 | 300mg daily | NA | 30-32 weeks | 1 months | 6 months | 12 months | 8.25±0.45 | 100within 24h | 5or20 at0,1,6 month |
| control | 56 | 56 | 32.45±3.2 | untreated | 8.24±0.35 | 100within 24h | 5or20at0,1,6 month | |||||||||
| Greenup et al., 2014 | Observation study | Sydney | multicenter,prospective,opt-in observational study | TDF vs | 58 | 58 | 30±8.5 | 300mg daily | 2007-2010 | 32 weeks | 12weeks | 48weeks | 9months | 7.94±0.78 | At birth | 5or10at0,2,4,6month |
| Lamivudine or | 52 | 53 | 28±5.3 | 100mg daily | 32 weeks | 4 weeks | 48weeks | 9months | 7.72±0.061 | At birth | 5or10at0,2,4,6month | |||||
| control | 20 | 20 | 28±5 | untreated | 8±0.04 | At birth | 5or10at0,2,4,6month | |||||||||
| Celen et al., 2013 | Observation study | Turkey | Retrospective study | TDF vs | 21 | 21 | 28.2±4.1 | 300mg daily | 02.2010 -01.2012 | 18-27 weeks | 4 weeks | 28 weeks | 28weeks | 8.28 | 200within 24h | 20 at 4,8,24week |
| control | 24 | 23 | 26.9±2.9 | untreated | 8.31 | 200within 24h | 20 at 4,8,24week | |||||||||
| Yayun Lin 2018 | RCT | China | multicenter,double-blind | TDF vs | 59 | 58 | 28.31±3.56 | 300mg daily | 01.2013-12.2016 | 24weeks | 4 weeks | 28weeks | 28wk | 7.44±0.80 | Within12h | At0,1,6month |
| control | 52 | 52 | 28.06±3.42 | untreated | 7.66±0.55 | Within 12h | At0,1,6month | |||||||||
| Chang 2019 | Observation study | China | Prospective,multicenter clinical trial | TDF vs | 110 | 115 | 32.69 ± 3.36 | 300mg daily | 01 | 30-32 weeks | 4weeks | 6 months | 1year | 8.29 ± 0.49 | 100within 24 h | At0,1,6month |
| control | 91 | 93 | 32.84 ± 3.57 | untreated | 8.25 ± 0.48 | 100within 24 h | At0,1,6month | |||||||||
We included randomized controlled trials (RCTs) and cohort studies in this meta-analysis that enrolled pregnant women with chronic HBV infection (characterized by the presence of HBsAg for more than 6 months), with serum HBV-DNA levels>6log copies/ml before antiviral therapy. The mother-to-child transmission rate, HBV-DNA suppression, anti-HBs production, maternal and infant safety outcomes were the measure Indicators. The mother-to-child transmission rate as defined by infant HBsAg seropositivity at 6–12 months after birth. Pregnant women given oral TDF therapy were defined as treatment group. The comparison/control group involved pregnant women with chronic HBV but were not given any treatment or treated with placebo or treated with other type of antiviral therapy. Only studies published in English language were published.
Exclusion CriteriaExcluded studies were: (1) those published in a language other than English; (2) neither RCT nor cohort study designs; (3) repeated publication of the same findings; (4) unspecified/unclear subjects follow-up duration; (5) systematic review or meta-analysis; (6) patients who received steroids, chemotherapy/immunotherapy, liver transplantation or hemodialysis; (7) inability to access full-text in order to extract the required data and (8) low quality research.
Data ExtractionFor every study, data extraction was done in duplicate using a standardized form. Two reviewers independently and in duplicate screened the titles and abstracts from the literature and identified eligible papers based upon the inclusion and exclusion criteria, and then cross-checked the results. Conflicts were resolved through discussion or decided upon by a third reviewer. In addition to basic article information, we extracted information pertaining TDF dose, patient's recruitment period, gestational week at starting treatment, treatment duration, post-treatment follow-up duration for both mothers and infants, baseline HBV-DNA levels and infants immunization period.
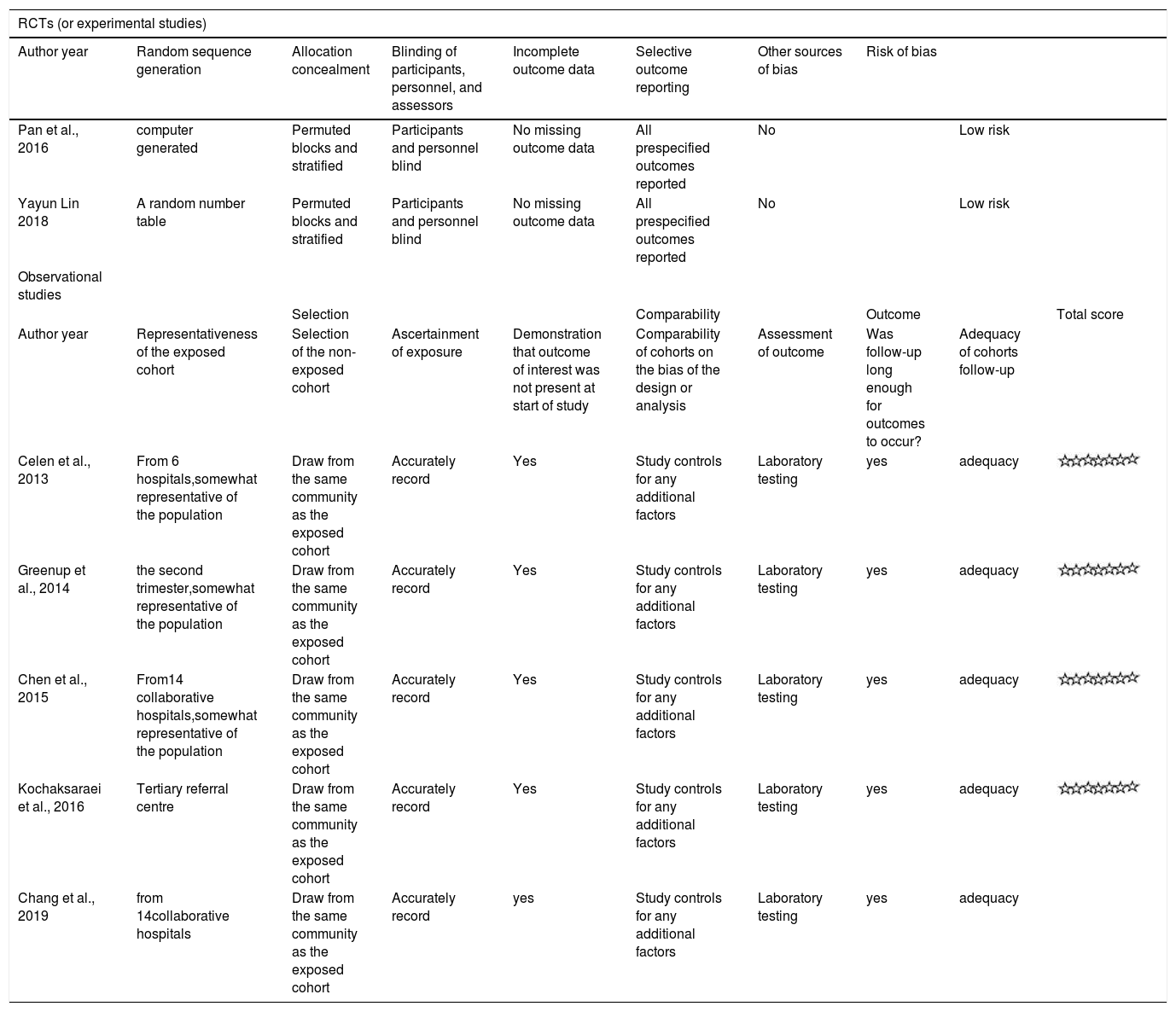
Risk of Bias AssessmentTwo reviewers independently assessed the bias risk of the RCTs using the Cochrane Reviewers’ Handbook 4.2.2 and the Newcastle-Ottawa Scale(NOS) for observational studies. RCTs with low risk and cohort studies with six stars and above on NOS were included in the analysis.
Statistical AnalysisWe calculated the risk ratios (RR) and 95% confidence intervals (CIs) using the binomial distribution for dichotomized outcomes. To measure the overall heterogeneity across the included studies, we used the I2 statistic and Q test. We planned to calculate the pooled effect size using the Mantel-Haenszel (M-H) fixed-effects model when I2<50% and p>0.05, and the random-effects model otherwise. The test level was set to ɑ=0.05. All statistical analyses were conducted using Review Manager 5.2 software from the Cochrane Library.
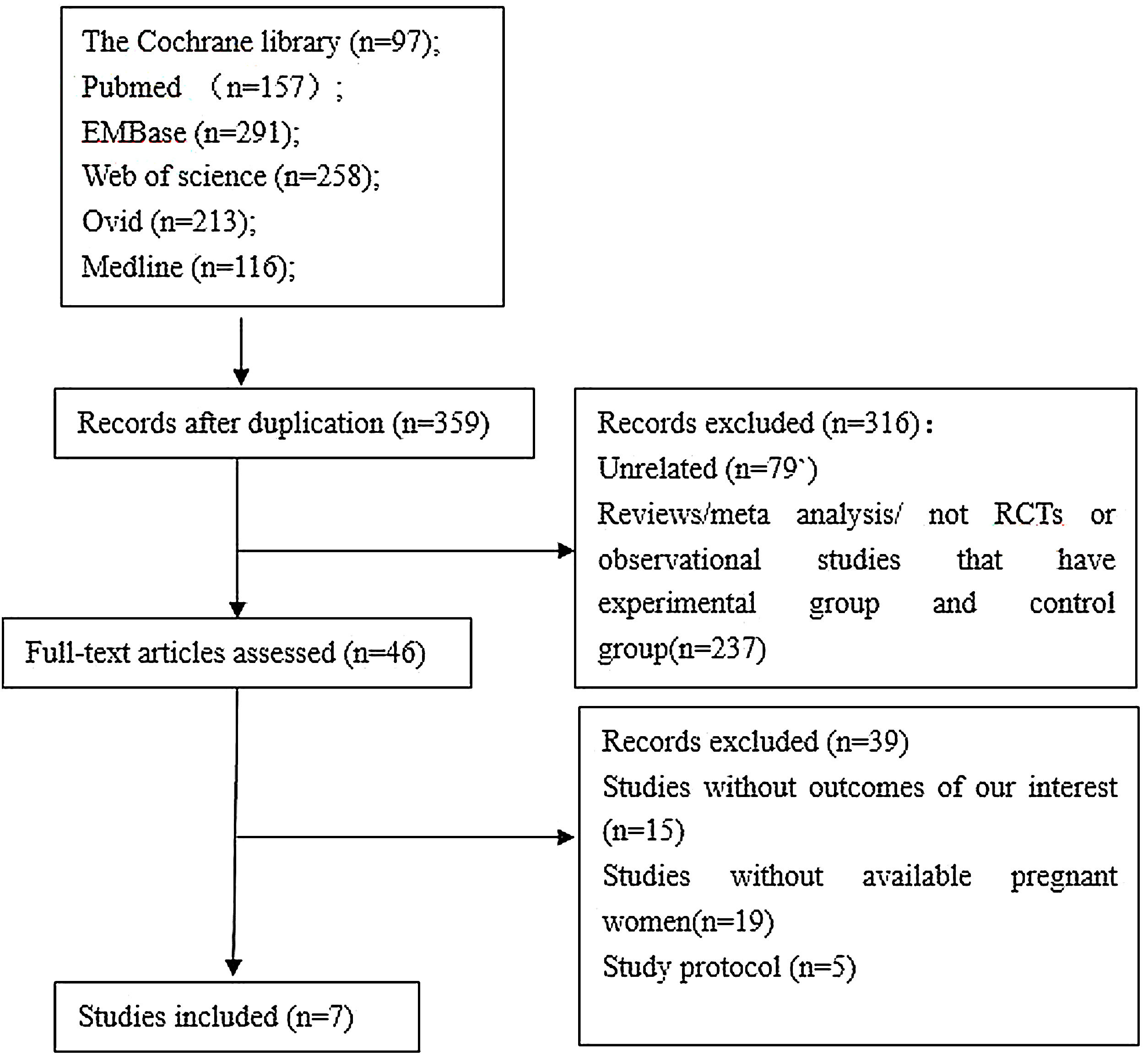
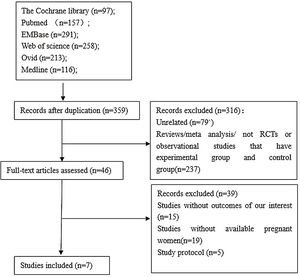
ResultsLiterature Search ResultsThe initial search process brought a total of 1132 references. After the screening of the titles and abstracts, 46 unique articles were retrieved. Screening the reference lists of selected studies identified no additional studies. After strictly applying the inclusion criteria by reading through the retrieved full texts,7 studies 2,4,12–16 were eligible for inclusion in the meta-analysis.The flow chart of the study selection process is presented in Supplemental Figure 1.
Characteristics of the Included StudiesThe characteristics of the included studies are summarized in Table 1.The included studies were 5 observational2,12–15 and 2 RCTs,4,16 involving 425 patients in the treatment group and 469 in the control group. Four studies were conducted in China,4,12,13,16 1 in Canada,14 1 in Australia2 and 1 in Turkey.15 Pregnant mothers in TDF group,with a baseline HBV-DNA levels>6log copies/ml,were administered with 300mg of TDF daily for 1 to 3 months at 18 - 32 gestational weeks and followed up for 3 – 6 months postpartum. All infants in the included studies received hepatitis B immune globulin (HBIG) administration at birth and 3-dose HBV vaccination within 6 month after delivery. Infants were followed up for 7 – 12 months post HBV vaccination. Quality assessment score for each study is presented in Table 2. All observational studies scored 7 stars on the NOS and the RCTs were considered as having low risk of bias.
Risk of bias assessment for the included studies.
| RCTs (or experimental studies) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author year | Random sequence generation | Allocation concealment | Blinding of participants, personnel, and assessors | Incomplete outcome data | Selective outcome reporting | Other sources of bias | Risk of bias | ||
| Pan et al., 2016 | computer generated | Permuted blocks and stratified | Participants and personnel blind | No missing outcome data | All prespecified outcomes reported | No | Low risk | ||
| Yayun Lin 2018 | A random number table | Permuted blocks and stratified | Participants and personnel blind | No missing outcome data | All prespecified outcomes reported | No | Low risk | ||
| Observational studies | |||||||||
| Selection | Comparability | Outcome | Total score | ||||||
| Author year | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the bias of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur? | Adequacy of cohorts follow-up | |
| Celen et al., 2013 | From 6 hospitals,somewhat representative of the population | Draw from the same community as the exposed cohort | Accurately record | Yes | Study controls for any additional factors | Laboratory testing | yes | adequacy | |
| Greenup et al., 2014 | the second trimester,somewhat representative of the population | Draw from the same community as the exposed cohort | Accurately record | Yes | Study controls for any additional factors | Laboratory testing | yes | adequacy | |
| Chen et al., 2015 | From14 collaborative hospitals,somewhat representative of the population | Draw from the same community as the exposed cohort | Accurately record | Yes | Study controls for any additional factors | Laboratory testing | yes | adequacy | |
| Kochaksaraei et al., 2016 | Tertiary referral centre | Draw from the same community as the exposed cohort | Accurately record | Yes | Study controls for any additional factors | Laboratory testing | yes | adequacy | |
| Chang et al., 2019 | from 14collaborative hospitals | Draw from the same community as the exposed cohort | Accurately record | yes | Study controls for any additional factors | Laboratory testing | yes | adequacy | |
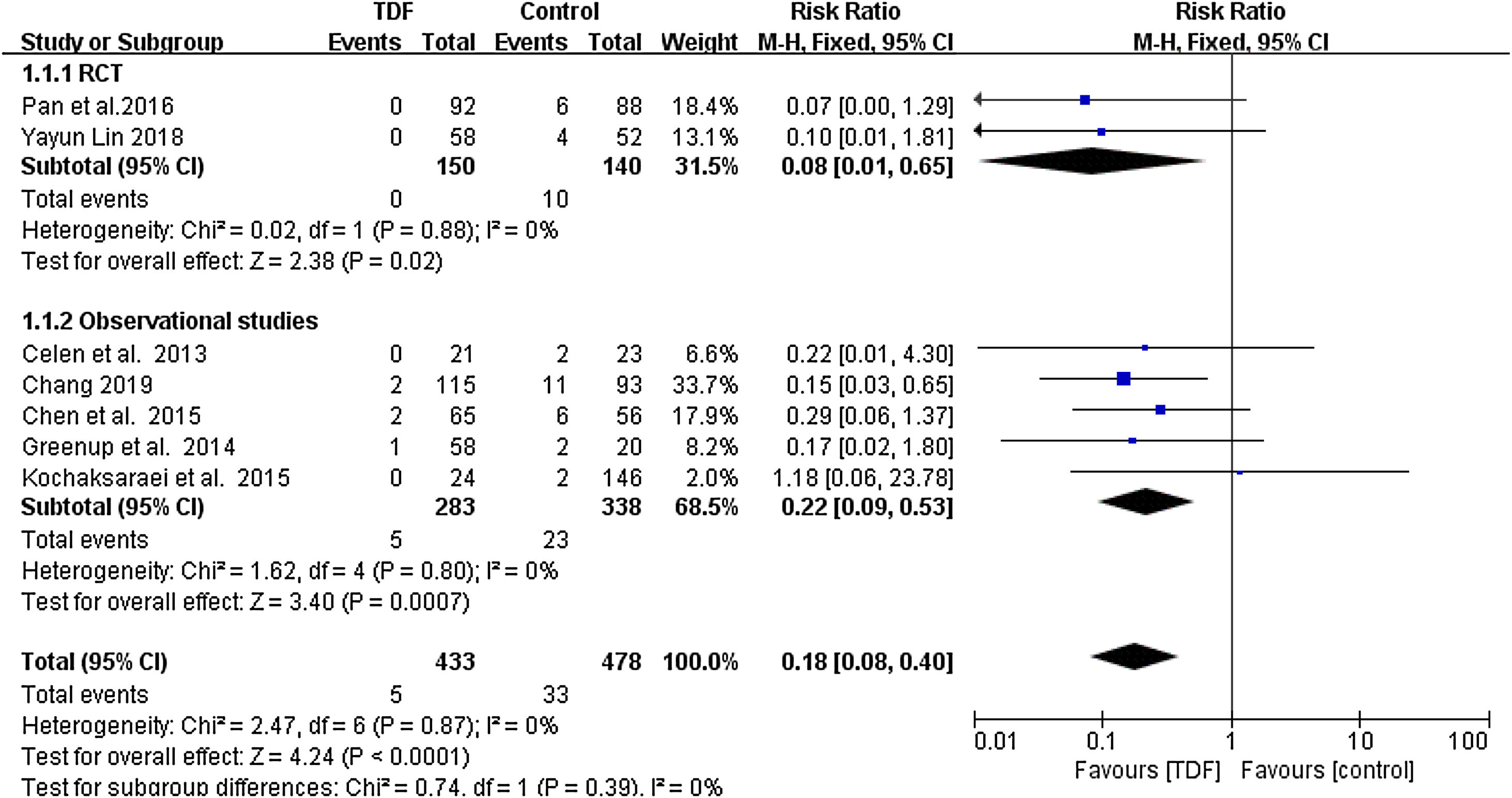
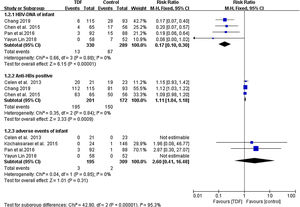
All included studies reported the HBsAg seropositivity in infants born to HBV mothers administered with TDF. The HBsAg seropositivity was assessed 6-12 months after birth. A total of 5 infants born to 433 HBV infected mothers administered with TDF were tested HBsAg positive, equivalent to 1.155% mother-to-child transmission rate. In the control group, 33 infants born to 478 HBV infected mothers in the control group were tested HBsAg positive, equivalent to 6.903% transmission rate. Meta-analysis resulted in a statistically significant estimate between the two groups in favor of TDF therapy (RR: 0.18, 95% CI: 0.08-0.40, p<0.01, I2=0%) (Figure 1).
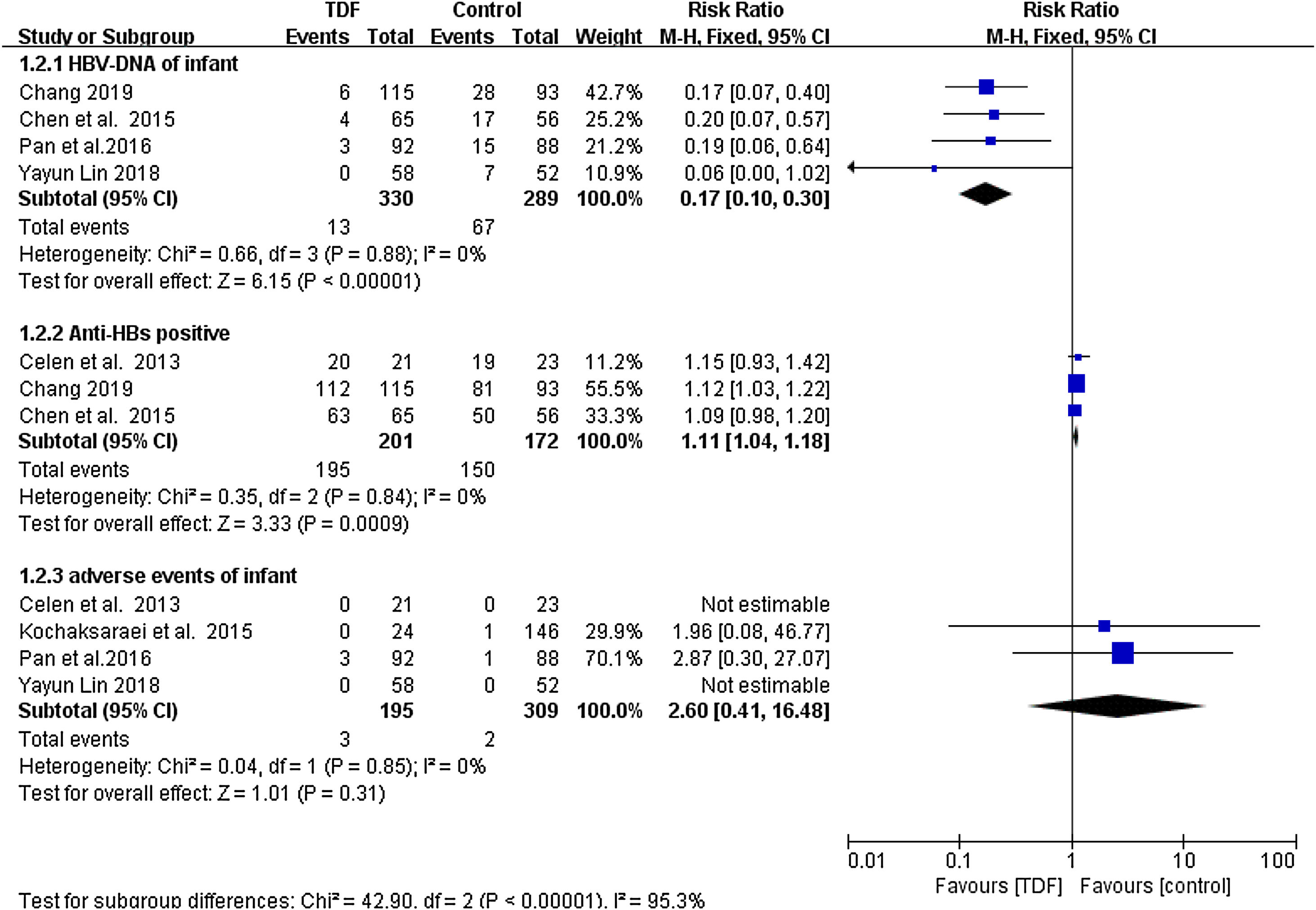
As shown in Figure 2, Infants born to HBV infected mothers administered with TDF were significantly less likely to be tested HBV-DNA positive as compared to infants born to HBV mothers in the non-TDF group (RR: 0.17, 95% CI: 0.10-0.30, p<0.001,I2=0%). Three studies reported anti-HBs production in infants including 195 cases from 201 TDF mothers and 150 cases from 172 non-TDF mothers. Meta-analysis resulted in statistically significant estimates in favor of infants born to non-TDF mothers (RR: 1.11, 95% CI: 1.04-1.18, p<0.001, I2=0%). Four studies4,14–16 compared severe adverse events occurring in infants as an indicator of TDF safety. It was only in Pan et al4 where 3 infants born to TDF mothers developed severe adverse events of grade 3 or 4, with an incidence of 1.538%. In the non-TDF group, 2 infants were reported with adverse events, with an incidence of 0.647%. (Figure 2). Statistically, the was no enough evidence to support development of adverse events from the two intervention groups (RR: 2.60, 95% CI: 0.41-16.48, p=0.31, I2=0%).
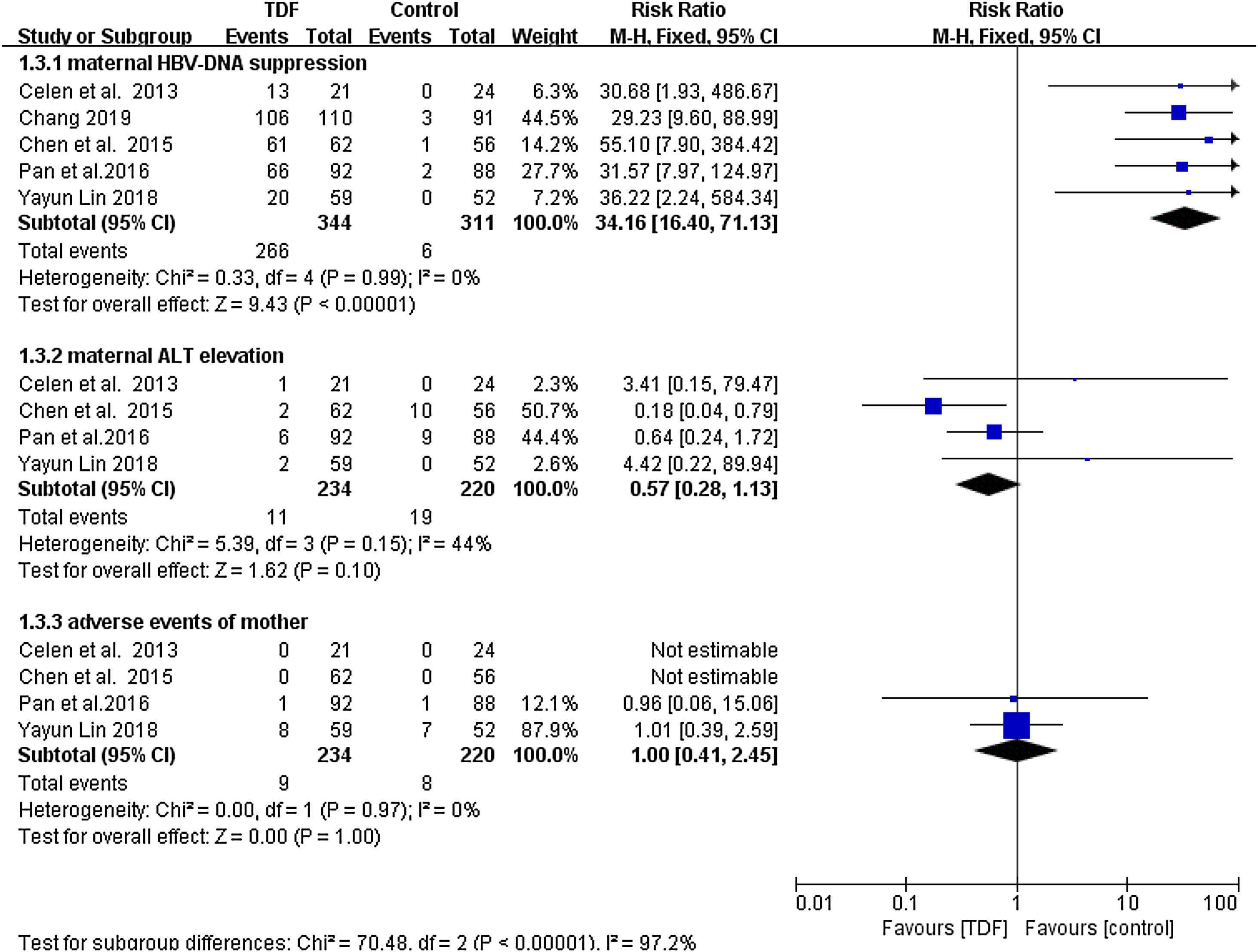
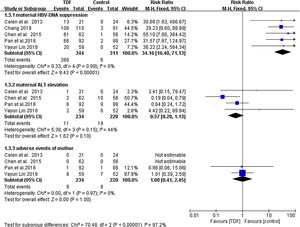
Maternal OutcomesMaternal HBV-DNA suppression was assessed at delivery in five studies,4,12,13,15,16 including a total of 655 mothers (344 TDF and 311 non-TDF). HBV-DNA suppression was successful in 266 TDF mothers (77.3%) and 6 non-TDF mothers (1.9%). The suppression was statistically significant in favor of TDF mothers (RR: 34.16, 95% CI:16.40-71.13, p<0.01, I2=0%). When compared to controls, pregnant women receiving tenofovir consistently had improved maternal ALT normalization at delivery; four articles4,12,15,16 described this outcome. The analysis found that the difference between the two groups was not statistically significant (RR: 0.57, 95% CI: 0.28-1.13,p=0.10, I2=44%). Miscarriage, stillbirth, and pre-eclampsia were reported as adverse events in both TDF and non-TDF mothers but statistically none of the interventions was superior to the other (RR: 1.00, 95% CI: 0.41-2.45, p=1.00, I2=0%) (Figure 3)
Publication biasWe were unable to elevate publication bias due to the small number of studies for each outcomes.
DiscussionPregnancy in patients with HBV infection is seriously harmful to the health of mothers and children. Acute maternal HBV infection have been associated with a higher incidence of low birth weight and prematurity. In early pregnancy, it is associated with a 10% perinatal transmission rate.17 Chronic maternal HBV infection is associated with increased risk of pregnancy-induced hypertension, cesarean delivery, preterm birth and macrosomia.18 Up to 85% infants born to HBeAg-positive women develops chronic infection.19 The transmission risk is a little lower in HBeAg-negative mothers, as about 10% of infants develops the infection.20 The current standard active-passive immunoprophylaxis with HBIG and HBV vaccination prevents transmission in approximately 95%, yet 8%-30% of mothers with high levels of viremia can still transmit HBV infection.20 In addition to higher maternal viremia, immunoprophylaxis failure is attributed to intrauterine infection, or mutations of the HBV surface protein in association to vaccine.21 Tenofovir have been approved by United States Food and Drug Administration for the preventing of mother-to-infant transmission due to its HBV inhibition ability and higher barrier to resistance.22 In recent years, many studies have evaluated the safety and effectiveness of TDF in preventing MTCT of HBV,22–24 and our analysis added the latest evidence to scientifically delineate the advantage effect and potential risks associated with TDF use in pregnant women with high HBV viral loads in order to provide more reliable evidence for clinical guidance.
In our meta-analysis, TDF significantly reduced HBsAg seropositivity (RR: 0.18, 95% CI: 0.08-0.40, p<0.01, I2=0%), HBV DNA positivity (RR: 0.17, 95% CI: 0.10-0.30, p<0.01,I2=0%) and increased anti-HBs production (RR: 1.11, 95% CI: 1.04-1.18, p<0.001, I2=0%) in infants born to HBV infected women. Similarly, 77.3% of TDF mothers had significant suppression of maternal HBV-DNA compared to only 1.9% in non-TDF mothers. Antiviral drug treatment with TDF is effective for blocking the chronic hepatitis B transmission between mother and child, which were in consistent with the other studies.22,24 The two studies have demonstrated that the use of tenofovir was effective in reducing mother-to-child transmission in pregnant women with high viral loads, which could significantly lower the risk of infant HBsAg positive by 75% and 79%, respectively.
In this meta-analysis, maternal ALT elevation and serious adverse events in both mothers and infants are used as indicators of TDF safety. Synthesis results revealed that the therapy did not have serious adverse events to mothers and infants as compared to controls. The result was in consistent with an excellent systematic review which included 26 studies and found that there were no safety issues with TDF use in maternal and fetal outcomes.25 But studies by ChenJZ and colleagues noted that TDF induced more drug-related adverse events as compared with controls group,24 possibly because their meta-analysis have selected adverse events of grade 1 or 2 not grade 3 or 4 of mothers and infants as a indicator of safety. Serum ALT levels severe elevation in the pregnant women receive antiviral drug treatment with TDF are uncommon but occur, and may produce post-partum flares if ALT rise to five or more times the upper limit of normal.26,27 In this meta analysis, we found that there was no evidence of significant maternal ALT elevation as compared to non-TDF mothers(RR: 0.57, 95% CI: 0.28-1.13,p=0.10, I2=44%). Therefore, TDF appear to be effective and safe in the prevention of perinatal HBV transmission. However, the number of included studies is inadequate, therefore these results need further verification.
The limitations encountered in this meta-analysis includes. First, only 7 studies were eligible, and majority of the studies are observational (5 studies), only 2 studies were RCTs. The quality of evidence from observational studies is always lower than that of RCTs. Second, most studies were conducted in China making an irrational presentation of ethnic variations of response to therapy. Third, 3 of the included studies with a relatively small sample size in TDF group or untreated group, which may more likely to overestimate the treatment effect. Beside, the language of the literature included in this study was limited to English. The lack of a large amount of literature in other languages and the lack of unpublished literature may result in certain biases. All of the restrictions remind us the conclusions of this study still should be treated with caution when popularized.
In summary, the use of TDF to pregnant women with HBV infection is effective in the prevention of MTCT of the HBV. In addition, the therapy did not result into serious adverse events different from those occurring in the non-TDF group.
Conflict of interestThe authors have declared that no conflict of interest exists.