Acute hepatitis C virus (AHC) infection is increasingly common among HIV+ men who have sex with men (MSM). Until 2017, the guidelines recommended therapy with pegylated-interferon plus ribavirin with a mild sustained virological response (SVR). This prompted many patients to reject that treatment, at that time, waiting to be treated with better and safer options with new Direct-Acting-Antivirals (DAA).
ObjectivesAssess the efficacy and safety of Elbasvir/Grazoprevir to treat recent chronic hepatitis C infection, genotype 1 or 4, in HIV+ MSM patients.
MethodsProspective, open-labeled, two center, pilot study. SVR is analyzed for treatment with Elbasvir/Grazoprevir (8 weeks in GT1b or 12 in GT1a or GT4) in patients with a recent chronic HCV infection, defined as HCV infection lasting less than 4 years and mild liver fibrosis (liver stiffness <8kPa).
ResultsForty-eight patients were included (May 2017–March 2018): 2 GT1b, 24 GT1a and 22 GT4. HCV-RNA>800000UI in 63% and medium liver stiffness 4.9kPa. The SVR was 98%, one patient failed due to poor adherence. 67% of patients had adverse effects, but only 16% treatment related. The most frequent side effects were gastrointestinal (19%), related with the central nervous system (18%), respiratory (16%) and systemic symptoms (15%).
During one year of follow-up post-therapy, 4 AHC and 18 patients with sexually transmitted diseases (STD) were diagnosed.
ConclusionsTreatment with Elbasvir/Grazoprevir in this scenario is highly effective and safe. Patients with risky sexual practices must remain linked to the medical care system to detect new STD and HCV reinfection.
La infección aguda por el virus de la hepatitis C (HCA) es cada vez más frecuente entre los hombres VIH+ que mantienen relaciones sexuales con hombres (HSH). Hasta 2017, las directrices recomendaban el tratamiento con interferón pegilado más ribavirina con una respuesta virológica sostenida (RVS) leve. Esto llevó a muchos pacientes a rechazar dicho tratamiento en ese momento, a la espera de recibir tratamiento con opciones mejores y más seguras con los nuevos antivirales de acción directa (AAD).
ObjetivosEvaluar la eficacia y la seguridad de elbasvir/grazoprevir para tratar la infección por hepatitis C crónica reciente, genotipo 1 o 4, en pacientes HSH VIH+.
MétodosEstudio preliminar, prospectivo, abierto y realizado en 2 centros. Se evalúa la RVS para el tratamiento con elbasvir/grazoprevir (8 semanas en GT1b o 12 en GT1a o GT4) en pacientes con una infección por VHC crónica reciente, definida como una infección por VHC que dura menos de 4 años y fibrosis hepática leve (rigidez hepática <8kPa).
ResultadosSe incluyeron 48 pacientes (mayo de 2017-marzo de 2018): 2 en GT1b, 24 en GT1a y 22 en GT4. ARN-VHC>800.000UI en el 63% y rigidez hepática media de 4,9Kpa. La RVS fue del 98%; un paciente fracasó debido a un cumplimiento terapéutico deficiente. El 67% de los pacientes presentó efectos adversos, pero solo el 16% estuvo relacionado con el tratamiento. Los efectos secundarios más frecuentes fueron síntomas gastrointestinales (19%), relacionados con el sistema nervioso central (18%), respiratorios (16%) y sistémicos (15%). Durante un año de seguimiento postratamiento se diagnosticaron 4 HCA y 18 pacientes con enfermedades de transmisión sexual (ETS).
ConclusionesEl tratamiento con elbasvir/grazoprevir en este contexto es muy eficaz y seguro. Los pacientes con prácticas sexuales de riesgo deben permanecer vinculados al sistema de asistencia médica para detectar nuevas ETS y reinfecciones por VHC.
An increase in acute hepatitis C (AHC) infection among HIV-positive men who have sex with men (MSM) has been reported in the last decade in different counties around the world1 including our territory.2,3
Transmission rates among HIV-infected men who have unprotected sex with men are high, particularly among those who engage in risk sexual practices that increase mucosal trauma and blood exposure.4 Spontaneous clearance in this scenario is low,5 around 15%.
At the time to design the present study, the European AIDS Treatment Network (NEAT) AHC consensus panel recommended therapy with pegylated interferon and ribavirin (P/R) to the patients who do not spontaneously reduce HCV-RNA more than 2 log at week 4 or who remain HCV-RNA positive at week 12 after diagnosis.6 Sustained virological response (SVR=negative HCV-RNA 12 weeks after end of treatment) was achieved in 70% with P/R therapy in those regardless of HCV genotype.7
This rate is clearly lower than the currently achieved with the HCV Direct-Acting Antiviral (DAA) combinations. The anticipated availability of these new drugs leaded to decrease the P/R treatment in acute hepatitis C waiting for a shorter, well-tolerated and highly successful DAA-based therapy that soon would be approved in our country.
At the beginning, DAA in Spain was only approved and reimbursed to treat chronic HCV infected patients with advanced fibrosis (Metavir score>2).8 This situation became an issue for AHC patients with early fibrosis stages, that remained untreated, viremic and at high risk of HCV transmission.
The present study focuses in a group of patients who were excluded from the initial indications of DAA therapies in our country: HIV/HCV co-infected patients with a recent chronic hepatitis C infection and low fibrosis (F0-F1). We analyze the efficacy and safety to treat them with Elbasvir/Grazoprevir; a DAA co-formulated combination approved by the US Food and Drug Administration in 2016 for the genotype 1 or 4 of HCV9 with demonstrated high efficacy and a good tolerability in HIV patients with chronic HCV infection.10–12
MethodsStudy design and participation centersThe study was a phase IV, open-label, prospective, single-arm trial performed in the specialized HIV units of two Spanish hospitals (Hospital Clínic from Barcelona and Fundación Jiménez-Díaz from Madrid).
The study was conducted in accordance with the principles of Good Clinical Practice. The institutional ethics committee from participating centers approved the study. All patients provided a written informed consent before entering the study. The clinical trial is registered in EudraCT (number 2016-001536-36).
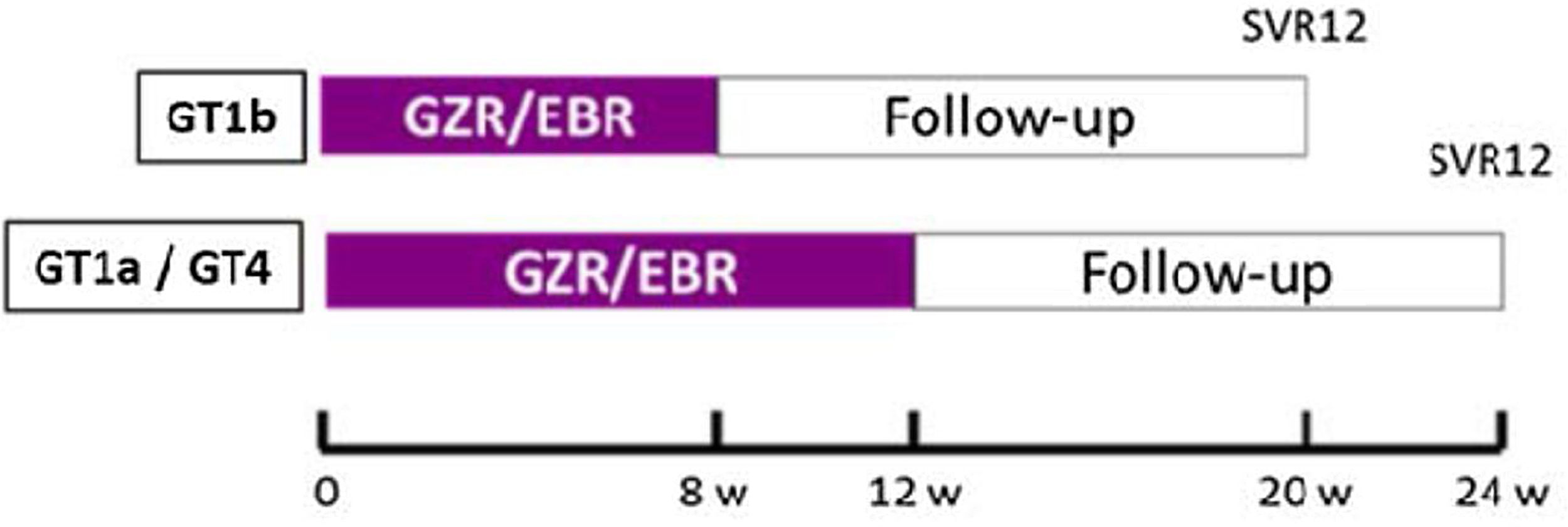
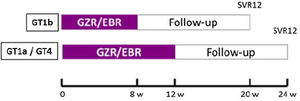
The study design is illustrated in Fig. 1.
PatientsThe study enrolled chronic HIV infected patients with a diagnosis of Early Chronic Hepatitis C (ECHC) infection genotype (GT) 1 or 4, from May 2017 to March 2018. ECHC was defined as an HCV infection lasting less than 4 years and mild liver fibrosis measured by transient elastography (liver stiffness of <8kPa). All the included patients had been diagnosed of acute hepatitis C by clinical or antibody seroconversion in the 4 years prior to start the study. HCV-RNA quantification was determined by the Roche Cobas® 6800 test with a lower quantification limit of 15IU/mL.
They could be naïve for HCV therapy or failure to prior P/R treatment.
HIV infection must be controlled with undetectable plasma HIV viral load (<50copies/ml) under stable Antiretroviral Therapy (ART) for at least 8 weeks prior to study entry using a dual nucleoside reverse transcriptase inhibitor (NRTI) backbone plus raltegravir, or dolutegravir.
Clinical and laboratory protocolsAll patients received the study drug (one co-formulated tablet of Elbasvir 50mg plus Grazoprevir 100mg taken every morning) following the scheme: 8 weeks in patients with GT1b or 12 weeks those patients with GT1a or 4 (Fig. 1).
Plasma hepatitis C virus RNA was quantified at baseline, every 4 weeks during therapy, end of treatment (EOT), 12 weeks post DAA therapy to know the SVR, and 24, 48 and 60 weeks of follow-up to identify re-infection. Additional laboratory analysis obtained at baseline and throughout therapy included quantitative HIV RNA, CD4 T-cell counts, liver chemistries and complete blood count.
Data collectionClinical and laboratory data; including patient demographics, transient elastography results, prior HCV treatment, HIV control and antiretroviral therapy were collected and managed using REDCap electronic data capture tools hosted by Eurecat.13
Outcomes and statistical analysisThe primary objective was to assess the efficacy and safety of Elbasvir/Grazoprevir therapy in HIV-HCV co-infected patients with an ECHC, genotype 1 or 4. Moreover we evaluate the re-infection rate during the first year of follow-up.
The efficacy of therapy was defined as the SVR rate: undetectable RNA-HCV in plasma 12 weeks post-treatment.
Qualitative characteristics were summarized using absolute frequency and percentage, quantitative variables using mean and standard deviation (SD) or median and interquartile range (IQR). Analyses of the primary outcome (SVR) included data from all patients who received at least one dose of the study medication. The proportion of patients with SVR was expressed as a percentage with the 95% confidence interval (95%CI). Incidence of adverse events and reinfection were estimated using Poisson regression and reported as number of events per 100 persons-year. The statistical software used for the analyses was Stata (StataCorp. 2017. Stata: Release 15. Statistical Software. College Station, TX: StataCorp LLC).
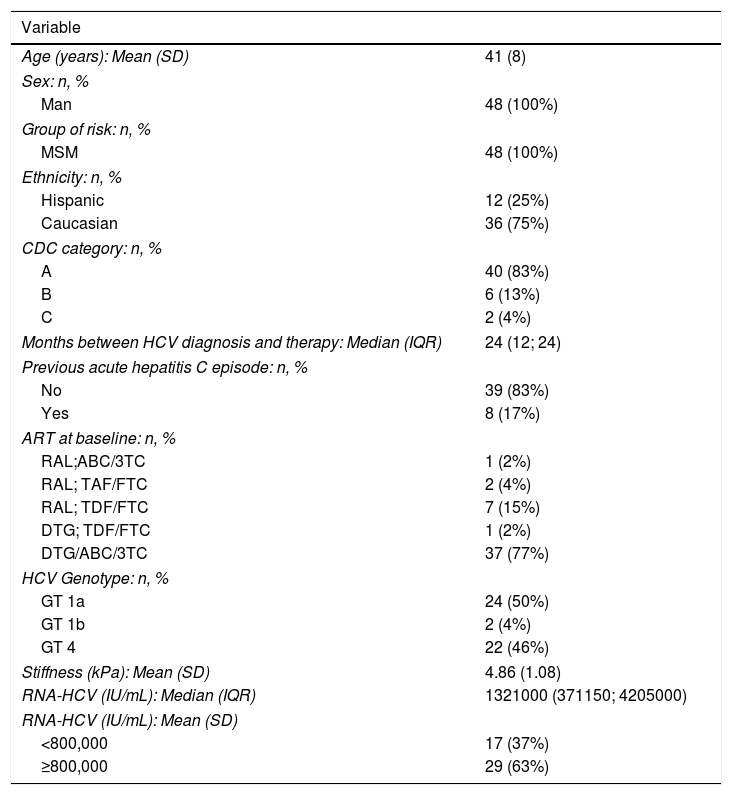
RESULTSClinical characteristics of HIV/HCV coinfected patientsForty-eight patients were included in the study: 26 from Madrid and 22 from Barcelona. All of them were MSM with an average age of 41 years (SD 8); 75% were Caucasian.
Median time since diagnosis of HIV was 6 years (IQR 4-12), all of them had good HIV viral control with ART: 79% with Dolutegravir and 21% with Raltegravir based regimen (see Table 1).
Baseline characteristics REVISADO (v2).
| Variable | |
|---|---|
| Age (years): Mean (SD) | 41 (8) |
| Sex: n, % | |
| Man | 48 (100%) |
| Group of risk: n, % | |
| MSM | 48 (100%) |
| Ethnicity: n, % | |
| Hispanic | 12 (25%) |
| Caucasian | 36 (75%) |
| CDC category: n, % | |
| A | 40 (83%) |
| B | 6 (13%) |
| C | 2 (4%) |
| Months between HCV diagnosis and therapy: Median (IQR) | 24 (12; 24) |
| Previous acute hepatitis C episode: n, % | |
| No | 39 (83%) |
| Yes | 8 (17%) |
| ART at baseline: n, % | |
| RAL;ABC/3TC | 1 (2%) |
| RAL; TAF/FTC | 2 (4%) |
| RAL; TDF/FTC | 7 (15%) |
| DTG; TDF/FTC | 1 (2%) |
| DTG/ABC/3TC | 37 (77%) |
| HCV Genotype: n, % | |
| GT 1a | 24 (50%) |
| GT 1b | 2 (4%) |
| GT 4 | 22 (46%) |
| Stiffness (kPa): Mean (SD) | 4.86 (1.08) |
| RNA-HCV (IU/mL): Median (IQR) | 1321000 (371150; 4205000) |
| RNA-HCV (IU/mL): Mean (SD) | |
| <800,000 | 17 (37%) |
| ≥800,000 | 29 (63%) |
MSM: men who have sex with men; HCV: hepatitis C virus; ART: antiretroviral therapy; RAL: Raltegravir; ABC/3TC: lamivudine+abacavir; TAF/FTC: tenofovir alafenamida+emtricitavine; TDF/FTC: Tenofovir diproxil fumarate+emtricitavine; DTG: dolutegravir; DTG/ABC/3TC: dolutegravir+lamivudine+abacavir.
The median time between the AHC diagnosis and the study inclusion was 2 years (IQR 1–2). Two patients had HCV GT1b, 24 patients GT1a and 22 GT4. Transient elastography medium rate was 4.9kPa (SD 1.08). The RNA-HCV viral load was >800000IU in 63% overall patients included and 67% in the group of GT1a infected patients.
We included 8 HCV re-infections; four of them had history of a spontaneous clearance and four were cured with P/R treatment.
Forty-two patients (88%) had previous history of sexually transmitted diseases (STD) and 25 of them presented a STD concomitantly with the diagnosis of AHC.
Direct-acting antiviral treatment responseThe rate of viral response achieved at the end of the treatment was 98% (95% CI: 98–100); only one treatment failure was reported in a poorly adherent patient who did not complete the therapy (GT4, transient elastography 4.7kPa and baseline RNA 5.8×106IU/mL). All the patients remained with SVR.
Adverse effects of anti-HCV therapySixty-seven percent of the patients presented adverse effects along the study, of those 46% during therapy. The overall incidence rate of adverse events was 80 events per 100 persons-year (95% CI: 66–94). Most of these side effects were mild (87%) and only 16% were possibly related to the treatment; none grade 4 and none had to stop therapy for this reason.
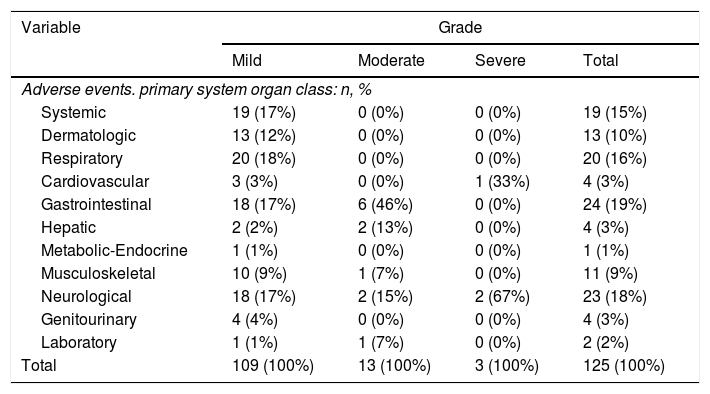
The most frequent side effects were related with gastrointestinal (19%), the central nervous system (18%), respiratory (16%) and systemic symptoms (15%) (Table 2).
Distribution of adverse events for each primary organ system affected and stratifying for grade.
| Variable | Grade | |||
|---|---|---|---|---|
| Mild | Moderate | Severe | Total | |
| Adverse events. primary system organ class: n, % | ||||
| Systemic | 19 (17%) | 0 (0%) | 0 (0%) | 19 (15%) |
| Dermatologic | 13 (12%) | 0 (0%) | 0 (0%) | 13 (10%) |
| Respiratory | 20 (18%) | 0 (0%) | 0 (0%) | 20 (16%) |
| Cardiovascular | 3 (3%) | 0 (0%) | 1 (33%) | 4 (3%) |
| Gastrointestinal | 18 (17%) | 6 (46%) | 0 (0%) | 24 (19%) |
| Hepatic | 2 (2%) | 2 (13%) | 0 (0%) | 4 (3%) |
| Metabolic-Endocrine | 1 (1%) | 0 (0%) | 0 (0%) | 1 (1%) |
| Musculoskeletal | 10 (9%) | 1 (7%) | 0 (0%) | 11 (9%) |
| Neurological | 18 (17%) | 2 (15%) | 2 (67%) | 23 (18%) |
| Genitourinary | 4 (4%) | 0 (0%) | 0 (0%) | 4 (3%) |
| Laboratory | 1 (1%) | 1 (7%) | 0 (0%) | 2 (2%) |
| Total | 109 (100%) | 13 (100%) | 3 (100%) | 125 (100%) |
Three serious adverse events were recorded in two patients during the study and totally recovered after hospitalization; none of them were related with the study medication: one patient developed a heart attack and the other needed two hospitalizations due to a cerebral stroke and a psychotic crisis probably related with the use of illicit drugs.
The adherence to the therapy, according to information collected by the Morisky-Green Adherence test, was very good; most of the patients (86%) did not forget to take their therapy throughout the study. Three patients did not complete the assigned 12 weeks of therapy due to personal issues, two stopped after 8 weeks and the third after 10 weeks. Two of them had HCV GT4 and the third GT1a, but all three patients achieved the SVR.
One year of follow-up after anti-HCV therapyDuring the year of follow-up after anti-HCV treatment, eighteen patients were diagnosed with an STD; six of them presented more than one episode. The most frequent diagnoses were: Syphilis and Lymphogranuloma Venereum. After being directly interrogated, 11 of these 18 patients confirmed to be drug users in the sexual context and half of them used these substances intravenously. There was a high number of HCV reinfection: eight patients had presented a previous cured episode of AHC at the beginning of the study and four more patients were re-infected during the 48 weeks of study follow-up (one in week 12, two in week 24 and one in week 48), with a reinfection incidence rate of 9 patients/100py of follow-up (95% CI: 2–19).
DiscussionAcute hepatitis C virus infection has been increasingly recognized among HIV-positive MSM. Treat and cure these patients, as soon as they are diagnosed with the best treatment options is mandatory to eradicate HCV in this population as recently different publications describe.14,15 The therapeutic indication of DAA treatment was not available in Spain in those patients when the present study was planned five years ago and DAA remain still restricted in different countries to patients with advanced liver fibrosis.
Herein we report excellent treatment outcomes and tolerability in this special group of HIV/HCV coinfected patients. The efficacy of Elbasvir/Grazoprevir combination was very high, nearly 100% of SVR although some patients did not complete the assigned treatment scheme.
Our results are comparable to those observed in the pivotal studies of Elbasvir/Grazoprevir, although more than 65% of our patients were GT1a with a baseline HCV viral load>800,000IU/mL who received twelve weeks of therapy following the design of the study, but according to the drug data sheet that should have been treated for 16 weeks. Currently, we have excellent results from two trials that support this option,16,17 even shortening to 8 weeks of therapy in AHC17; but at the beginning of this study these data were unknown.
The therapy was well tolerated without interruptions due to side effects like previously studies report.10–12
In accordance with the published data,18,19 the presence of other sexually transmitted diseases concomitantly to AHC was very common in our patients and, during the follow up period, we diagnose a high number of new STDs. Moreover, we want to highlight the high rate of HCV reinfection in this short period of follow-up that is similar to recently published data in HIV/HCV MSM patients treated in the DDA era.19,20
In one hand, we can consider as a study weakness the low number of patients included, especially those infected with GT1a and GT4, to do hard conclusions in this pilot study. But in the other hand, taking into account that pangenotypic treatment options are not available worldwide, our results on elbasvir/grazoprevir efficacy and tolerability may give information to health care providers on available treatment options for those patients.
Nevertheless, the really special conditions of the population studied (HIV+, MSM, high risk STD) is a remarkable strength, in order to assess the efficacy of early health interventions in those patients.
As other authors have described,19,21 we face a group of patients with risky sexual practices, often related to drug use, who have a high risk of contracting sexually transmitted diseases. In these patients, in addition to providing treatment for HCV and other STDs, we must keep them linked to the medical care system to preserve the effect of the cure and detect possible reinfections, implement behavioral interventions aimed at reducing damage and offer help in the addiction management. This strategy is aligned with the objective proposed by WHO22 to end viral hepatitis in 2030 and is included in a HCV micro elimination program in specific high-risk populations in our environment.23
SummaryTreatment with Elbasvir/Grazoprevir for 8 or 12 weeks in recent chronic hepatitis C in HIV+ coinfected patients is highly effective and safe.
FundingThis study has been founded by MSD through the MSD Research Program (MISP) with the code IIS53625.
Conflicts of interestEM, JLB and JM receive compensation to serve as a consultant for and on the speakers’ bureaus of Gilead Sciences, Janssen Pharmaceuticals, VIIV, and MSD.
For the remaining authors no conflicts of interest were declared.
ML designed the study, collected and analyzed data and wrote the report. MM and BA collected and analyzed data and wrote the report. JM designed the study and revised the report. ELD designed the study, managed and analyzed the data and wrote the report.
The remaining authors: collected data and revised the report.