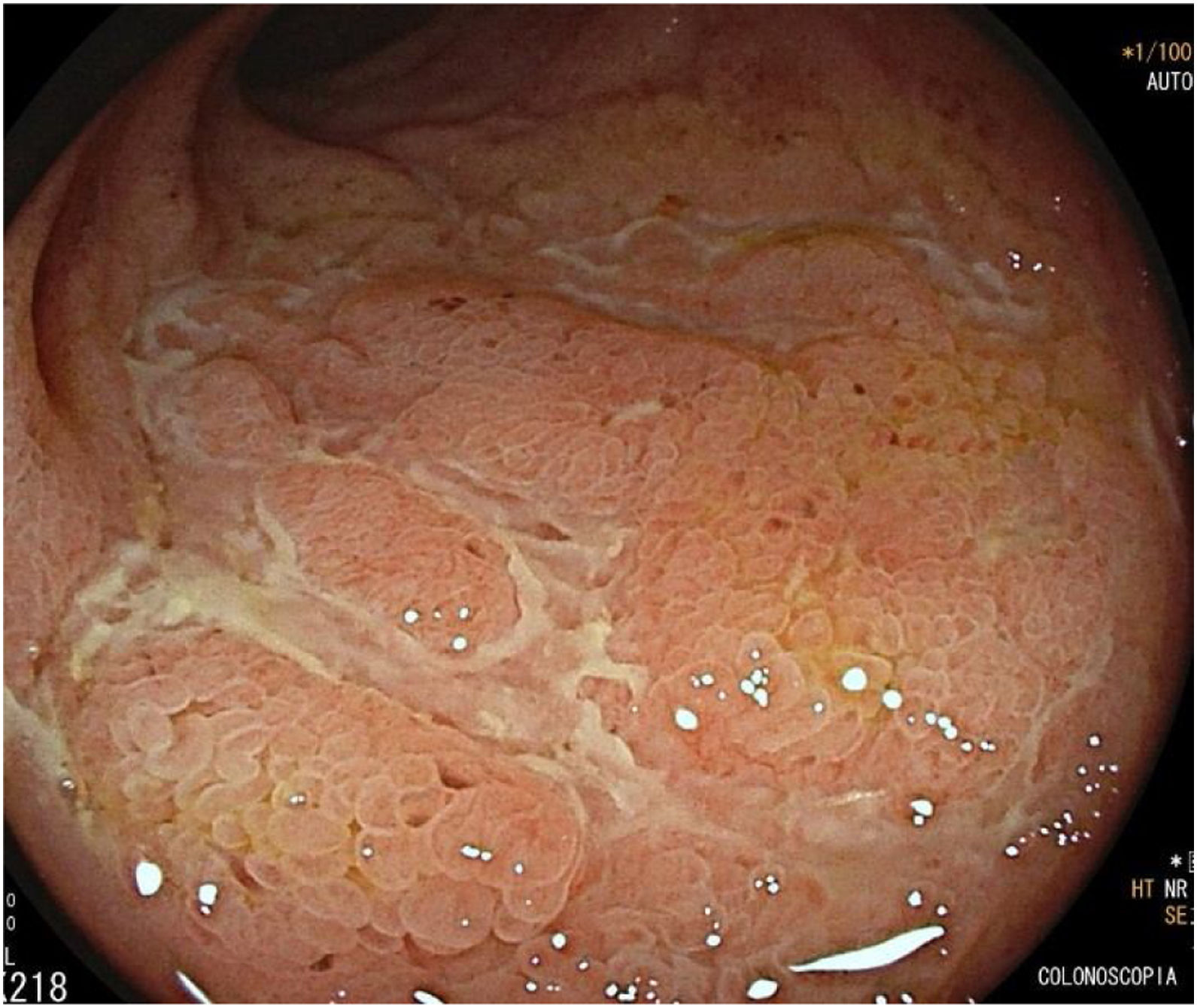
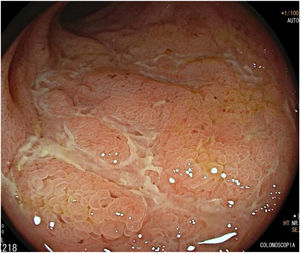
We present the case of a 42-year-old man with a history of smoking, sleep apnoea/hypopnoea syndrome (SAHS) and mainly hard-to-control inverse psoriasis, for which he had been on treatment with multiple biologicals, such as anti-TNF (etanercept, adalimumab) and anti-IL12 and 23 (ustekinumab), and immunomodulators (methotrexate), which had been discontinued due to adverse effects and/or intolerance, and currently on cyclosporine treatment with partial response. Therefore, treatment with ixekizumab (anti-IL17 monoclonal antibody) was initiated. Two weeks after starting the treatment, the patient developed abdominal pain, fever and diarrhoea with up to 10 stools a day, and was admitted to the Gastroenterology ward where complementary tests were carried out: microbiological study of faeces (Clostridium difficile, parasites and stool culture all negative), blood cultures (negative) and colono-ileoscopy (with a finding of aphthoid erosions and deep fibrinous patchy ulcers from rectum to caecum, with involvement of the first 15cm of the ileum, all highly suggestive of ileocolonic Crohn's disease) (Fig. 1). Biopsies were taken that showed fissuring ulcers, neutrophilic lymphoplasmacytic and polynuclear inflammatory infiltrate, cryptitis with cryptic microabscesses and non-necrotising granulomas, findings consistent with active phase Crohn's disease (CD). Treatment with systemic corticosteroids was started, with a good response, and a follow-up MRI showed involvement of the entire colon and terminal ileum. The rest of the small intestine appeared normal. The patient was subsequently admitted for diarrhoea secondary to Clostridium difficile, which was successfully treated with metronidazole. After nine months, he is currently on treatment with vedolizumab (started due to severe endoscopic involvement and corticodependence criteria).
Psoriasis and inflammatory bowel disease (IBD), both CD and ulcerative colitis (UC), are chronic immune-mediated diseases that share a genetic profile and pathogenic pathways, the prevalence of IBD being higher in patients with psoriasis than in the general population.1
Ixekizumab, like secukinumab, is an anti-IL-17 monoclonal antibody that has shown great efficacy in the treatment of moderate-to-severe psoriasis and psoriatic arthritis. However, in murine models, blocking IL-17 has been associated with increased intestinal permeability, an imbalance between regulatory and effector CD4-T lymphocytes and bacterial translocation that results in the appearance and/or exacerbation of colitis.2
There are two post hoc analyses in 4209 patients with moderate-to-severe psoriasis treated with ixekizumab, in which 19 cases of IBD (7 CD and 12 UC) were identified, highlighting that 15 were “denovo” cases.3
In the study by Armstrong et al., 5898 patients with moderate-to-severe psoriasis treated with ixekizumab were analysed, of whom less than half were exposed to the drug for four or more years. As a treatment-related adverse event (AE), 26 patients developed IBD (7 CD, 18 UC, and one unclassified colitis), representing less than 0.5% of the patients, and most of the cases occurred in the first two years of treatment.4
Moreover, in the recent analysis by Langley RG et al., which included 5689 patients from 11 clinical trials, AEs were observed in 84% of patients (severe in 11.8%), the majority being non-opportunistic infections, severe injection site reactions or allergic reactions. Both the frequency of AEs and mortality increased with time of exposure to the drug, both of them greater after two years of treatment. Regarding the known risk of IBD, in this analysis the results were very similar to those reported in other studies, with a risk of developing CD and UC after three years of exposure of around 0.0% and 0.1% patients/year, respectively.5
The reported cases of IBD secondary to treatment with anti-IL17 drugs are mostly of the ulcerative colitis type and are more frequent the longer the exposure time to the drug. However, our patient first developed CD in the first weeks of treatment. Therefore, despite the fact that IBD rates in patients exposed to ixekizumab are relatively low (<1%), this risk must be emphasised, especially in patients with other added risk factors such as smoking (as in our case) or a family history of IBD, and these patients must be monitored closely during treatment.1,5
Please cite this article as: Merino Gallego E, Gómez Torres K, Martínez Amate E. Debut de enfermedad inflamatoria intestinal secundaria a tratamiento con ixekizumab en paciente con psoriasis moderada de difícil manejo. Gastroenterol Hepatol. 2020. https://doi.org/10.1016/j.gastrohep.2020.04.009