The SARS-CoV-2 pandemic has proven to be a serious challenge for the Spanish healthcare system. The impact of the virus on the liver is not well known, but in patients with chronic liver disease, mostly in advanced stages, it can critically compromise survival and trigger decompensation. Treatment in this subpopulation is complex due to the potential hepatotoxicity of some of the medicinal products used. Moreover, the pandemic has also negatively impacted patients with liver disease who have not contracted COVID-19, since the reallocation of human and material resources to the care of patients with the virus has resulted in a decrease in the treatment, diagnosis and follow-up of patients with liver disease, which will surely have negative consequences in the near future. Efficient reorganisation of hepatology units is a priority to minimise the impact of the pandemic on a population as vulnerable as liver disease patients.
La pandemia producida por SARS-CoV-2 ha supuesto uno de los mayores desafíos del sistema sanitario español. El impacto del virus sobre el hígado no es bien conocido, pero en pacientes con enfermedades hepáticas crónicas, especialmente en estadios avanzados, puede comprometer de forma crítica la supervivencia y desencadenar descompensaciones. El tratamiento en esta subpoblación es complejo por la potencial hepatotoxicidad de algunos fármacos empleados. Más allá, la pandemia también ha impactado negativamente sobre aquellos con enfermedades hepáticas que no han adquirido la enfermedad, ya que la redistribución de los recursos humanos y materiales hacia la atención de pacientes con COVID-19, ha provocado una merma en su tratamiento, diagnóstico y seguimiento que a buen seguro tendrá consecuencias negativas en el futuro. La reorganización eficiente de las unidades de Hepatología es una necesidad de primer orden para aminorar el impacto de la pandemia sobre una población tan vulnerable como los pacientes con hepatopatía.
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the agent that causes the disease called COVID-19, represents one of the greatest challenges to the infrastructure of health systems and public health in recent times.1,2 As of 1 June 2020, this pandemic has caused more than 300,000 deaths worldwide.3 In Spain, the impact of COVID-19 is particularly serious, as it is, in Europe, the country with the third largest confirmed caseload, and, globally, the country with the fifth largest confirmed caseload, after the United States, Brazil, Russia and the United Kingdom.3 In most cases, the disease follows a benign course; however, in others, the outcome may be fatal; its mortality rate in Spain is 11.1%.4 The cause of death is usually acute respiratory failure secondary to diffuse alveolar damage; however, the liver may play a key role in two respects: 1) as it may be affected by the infection itself or its treatment (or both); and 2) owing to the possible implications of underlying chronic liver disease for the patient’s prognosis.
What are the characteristics of liver damage in patients with COVID-19?Today it is known that SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE-2) receptors as a gateway for infection.5 These receptors are present on the cell surface of practically all human organs, rendering COVID-19 a systemic disease which, though it essentially manifests with respiratory symptoms, may similarly affect other vital organs, such as the liver, heart, pancreas, kidneys and bowel.
Liver impairment associated with COVID-19, understood to refer to any clinical or laboratory abnormality tied to liver function, may develop in previously healthy individuals and in individuals with pre-existing liver disease. The initial studies from China offered disparate data on its prevalence, with percentages ranging from 14% to more than 50%.6–8 In the study that enrolled a higher number of patients the main abnormality detected was elevated aminotransferase levels, present in up to 20% of cases, followed by slightly increased bilirubin levels, in 10% of cases.9 By contrast, elevation of other cholestasis parameters, such as gamma-glutamyl transferase (GGT) and alkaline phosphatase (AP), was relatively uncommon. Other more recent accounts have indeed reported elevated GGT levels in up to 50% of cases, but with normal AP levels.10 Hypoalbuminaemia, for its part, is usually a relatively common laboratory finding in patients with serious COVID-19; however, it is not accompanied by other findings suggestive of liver failure, such as hyperammonaemia, hyperbilirubinaemia, hypoglycaemia or prolonged clotting times.9
To date, no cases of serious acute liver failure secondary to SARS-CoV-2 infection have been reported and abnormalities in transaminase levels are usually transient.8,9 Studies that have analysed the relationship between liver impairment and prognosis have yielded contradictory results. Whereas two cohorts of Asian patients identified a relationship between seriousness of COVID-19, need for admission to an intensive care unit (ICU), male sex and high transaminase levels,6,9 others have not detected a higher risk of death in this subgroup.11,12 Although the fact that approximately one-third of patients already have liver impairment on admission could indicate a direct cytopathic effect of the virus on the liver, other pathophysiological mechanisms also present in critical haemodynamic and respiratory situations, or in situations of serious sepsis, are certainly more likely to contribute more to liver damage. This probably makes it difficult to interpret the role played by liver dysfunction in the prognosis of patients with COVID-19.
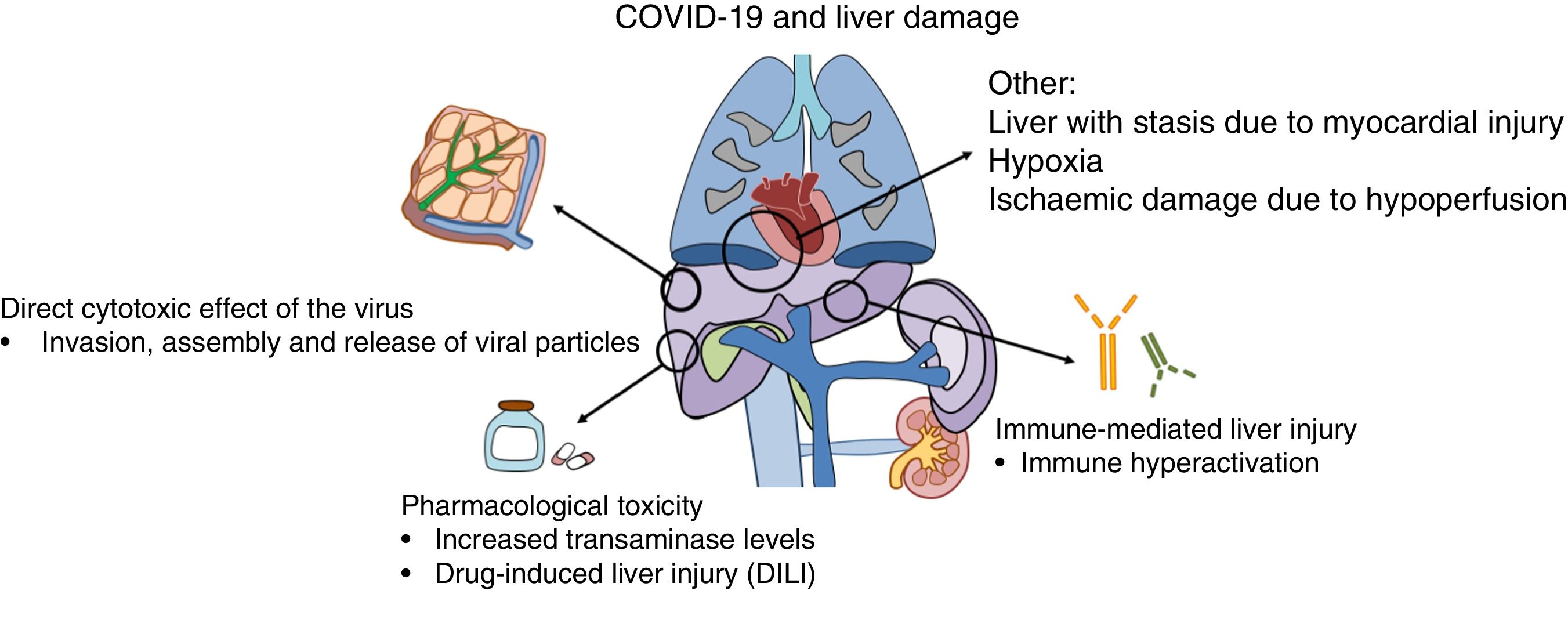
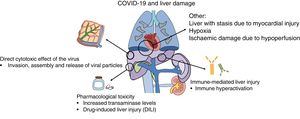
Why does liver damage occur in COVID-19?Liver damage in patients with COVID-19 may be due to several mechanisms, including the action of the virus itself or the immune system on liver cells and the toxicity of the drugs used in its treatment (Fig. 1).
Recent publications have suggested that the virus may bind to ACE-2 receptors located in hepatocytes, especially in cholangiocytes where their expression is more abundant.13 After they bind to the receptor and enter the cell, replication mechanisms are initiated that are intended to generate new viral RNA and synthesise structural proteins needed for the assembly and release of new viral particles.14 By contrast, expression of ACE-2 receptors in hepatocytes is limited; this could explain the absence of laboratory and histology data typical of viral forms of hepatitis.15
In some serious cases, proinflammatory immune hyperactivation has occurred; the consequences of this could be more deadly than the cytopathic effect of the virus itself.16 The potential benefit of certain currently investigational drug treatments is predicated on this hypothesis of immune-mediated damage.17,18 However, the consequences for the liver of this immune dysfunction in the context of COVID-19 remain unknown.
Secondly, the toxicity induced by the drugs used to treat COVID-19 also may contribute to liver damage.19 This is particularly important, on the one hand, in patients with pre-existing chronic liver diseases in whom the risk of toxicity is higher and, on the other hand, in liver transplant patients, due to potential interactions with routinely used immunosuppressant drugs. Among the different drug treatment options used to date, remdesivir, a nucleotide analogue, has yielded promising results in decreasing recovery time and duration of hospital stay.20 Initially elevated transaminase levels during treatment with remdesivir were reported in up to 23% of patients, with two patients requiring suspension of the treatment for this reason.20 Nevertheless, the preliminary results of two clinical trials have found this adverse effect in just 4.1%- 5.0% of patients, with no differences versus the placebo group.21,22 Other drugs used in the treatment of COVID-19, such as protease inhibitors (lopinavir) and tocilizumab, could also carry a potential risk of hepatotoxicity.23,24
What is the impact of COVID-19 on patients with chronic liver disease?Several studies have analysed the prevalence of underlying chronic liver disease in long series of patients hospitalised for COVID-19, with figures ranging from 0.6% to 1.4%.9,25,26 A recent meta-analysis found that in approximately 60% of patients with chronic liver disease, the infection followed a serious course, with a mortality rate of 18%.27 The main drawback of the studies included is that no stratified analysis by cause or stage of liver disease was performed.
The liver disease most closely linked to COVID-19 is metabolic-associated fatty liver disease (MAFLD).28 An initial Asian study, which enrolled 202 patients, found indirect MAFLD findings, along with age, male sex and presence of other comorbidities, to be a factor for a poor prognosis. However, coexisting cardiovascular diseases might have acted as confounding factors, complicating the interpretation of the results. In an attempt to control for these factors, another Chinese group reported that the presence of MAFLD and obesity is associated with a worse clinical course only in the population group <60 years of age, regardless of sex, smoking or other metabolic diseases (hypertension, dyslipidaemia or diabetes mellitus).29,30 These findings invite speculation on a possible relationship between baseline systemic inflammation, which is more present in young and obese individuals with MAFLD compared to older individuals, and a stronger systemic inflammatory response associated with SARS-CoV-2 infection. This relationship could in part account for a worse course of the disease in patients fitting this profile. However, in both studies MAFLD was diagnosed using non-invasive methods, which could have represented a significant confounding factor given that the parameters used might have be altered by systemic inflammation itself and other aetiological agents which were not considered, such as alcohol use. Therefore, the possible overestimation of the effect of MAFLD due to the coexistence of other factors with a poor prognosis (hypertension, obesity, cardiovascular disease or chronic obstructive pulmonary disease) means that these results should be interpreted with caution. However, regardless of the cause-effect relationship, these findings should alert the clinician to the importance of close monitoring and optimisation of infection prevention measures in patients with a prior diagnosis of steatohepatitis of metabolic origin.
For its part, liver disease of autoimmune origin, especially when treated with immune-modulating or immunosuppressant drugs, at least theoretically represents a higher risk of complications associated with COVID-19. With this premise, a study based on 148 telephone clinical interviews was conducted in northern Italy, one of the epicentres of the pandemic in Europe. This study found that the incidence of SARS-CoV-2 infection in patients with autoimmune hepatitis is no higher than that in the general population, and that, should it occur, the risk of developing severe complications is low.31 These data suggest a possible modulating effect on the immune system on the part of these drugs in situations of infection-induced immune system hyperstimulation. Together with the possible fatal consequences of abrupt treatment suspension, these enable the conclusion to be drawn that it is advisable to maintain immunosuppressant therapy in all cases, including in areas at high risk of community transmission. Finally, the impact of SARS-CoV-2 on patients with primary biliary diseases, such as primary biliary cholangitis or primary sclerosing cholangitis, has not been evaluated to date. Nevertheless, bearing in mind the virus’s special affinity for cholangiocytes and the presence in this group of a pre-established biliary lesion, the risk of abnormal cholestasis parameters in these patients could be higher.
Chronic infection with hepatotropic viruses, especially hepatitis B virus (HBV), does not seem to lead to a worse prognosis in patients with SARS-CoV-2 infection.9 However, this has taken on particular importance in the management of COVID-19 as the use of drugs with a powerful immunosuppressant effect (glucocorticoids at high doses, tocilizumab, etc.) could increase the risk of serious cases of viral reactivation. Therefore, it is advisable to suitably evaluate the serology status of all patients with a diagnosis of COVID-19 against hepatitis B and C viruses and to evaluate, based on that status, the need for preventive antiviral treatment if very powerful immunosuppressant drugs are needed.
Regarding patients with advanced liver disease, the information available is also limited. The first multi-centre study based on two international registries shed light on the impact of SARS-CoV-2 in a cohort of 152 patients. In this series, 103 of the cases included presented cirrhosis and the most common liver disease aetiology was steatohepatitis of metabolic origin.32 Mortality during admission was as high as 40%, being of respiratory origin in close to 80% of cases. As could be anticipated, this was higher in patients with more severe liver failure (24%, 43% and 63% in Child-Pugh A, B and C, respectively). In addition, it could be confirmed how in 40% of them, SARS-CoV-2 infection involved a triggering of hepatic decompensation (encephalopathy, worsening of ascites and/or varicose bleeding), with this, and not respiratory symptoms, being the dominant sign in up to 24%. Another study, with a smaller number of patients, showed that SARS-CoV-2 infection triggers acute over chronic failure in up to 28% of cases and that the mortality rate is comparatively higher than in patients with cirrhosis admitted for bacterial infection, especially in those with a Model for End-Stage Liver Disease (MELD) score ≥15 and/or respiratory failure.33 The updated data from the combined SECURE Cirrhosis and COVID-Hep registries, which already include 352 patients with cirrhosis, confirm a high mortality rate — up to 34%.34
To date, no data are available regarding the impact of COVID-19 on patients with hepatocellular carcinoma. Nonetheless, in them it is highly likely that the effect is negative, as in most cases there are two clinical conditions associated with a worse prognosis: oncologic disease per se and advanced liver disease.35 Moreover, logistical limitations on healthcare caused by the pandemic have had a negative impact on both the diagnostic process and the treatment of liver cancer in some cases. This will probably also have an unfavourable indirect impact on the prognosis for these patients.
Finally, liver transplant candidates merit special consideration as they comprise a particularly vulnerable population with a narrower therapeutic window of opportunity. The high morbidity and mortality rates of surgical procedures in COVID-19 patients,36 together with the scarcity of ICU beds, the drastic decrease in the number of donors, and the risk of nosocomial transmission of SARS-CoV-2 infection, have led to an unprecedented drop in transplant numbers in Spain. In fact, we were able to confirm how Spain went from 20 to 30 liver transplants per week, in the first two months of 2020, to less than a third of that in March and April, then increased to just half its prior level of activity in May.37 The reality is that, in recent months, in most units, transplants have only been performed in selected cases of an extremely serious nature (fulminant liver failure) and/or with a high likelihood of departure from the waiting list. Although for the moment definitive data are pending, the pandemic can be predicted to have a significant impact on the survival of candidates on a waiting list, not only due to loss of opportunity in some cases, but also because delaying surgery may mean performing it later in a potentially more complex clinical scenario. However, it should be noted that Spain has one of the most cutting-edge, efficient transplant systems in the world, which has meant that, even in situations of extreme difficulty, it has been possible to perform procedures with maximum safety assurances.
What should be borne in mind when treating COVID-19 in patients with chronic liver disease?As of today, it is not known which drug treatment is best for COVID-19 and in which specific clinical situations it should be used. Therefore, currently a large number of clinical studies are being actively conducted in order to obtain more solid scientific evidence in this regard. Uncertainty around therapeutic management of SARS-CoV-2 infection also affects patients with chronic liver diseases, in whom the risk of liver toxicity and drug interactions take on greater importance.
Among the drugs used to manage symptoms, the potential toxicity of paracetamol is worthy of note. A paracetamol overdose can induce hepatocellular injury, especially in patients with liver disease of alcoholic origin.38 Compared to antivirals, remdesivir is associated with a reversible elevation of transaminase levels in 5%-30% of patients. Therefore its use is not advised when AST levels or ALT levels are found to be more than five times the upper limit of normal.39 Chloroquine and hydroxychloroquine, with or without azithromycin, have also been proposed as antiviral agents against COVID-19, as they have managed to block the virus’s entry into target cells in preclinical studies.40 Although no significant hepatotoxic effects have been reported with these drugs, their use is not recommended outside the context of clinical trials owing to the potential (primarily cardiologic) adverse effects and doubts around their efficacy.41 Finally, immune-modulating agents, such as tocilizumab (an IL-6 receptor antagonist), sarilumab and siltuximab (both anti–IL-6 monoclonal antibodies), are being researched for their potential to counteract the immune hyperactivation associated with the most serious cases. With these drugs, reversible elevations of transaminase levels have also been detected;42 hence, their use is discouraged if AST and/or ALT levels are greater than or equal to five times the upper limit of normal.43
The implications of immunosuppressant treatment associated with solid-organ transplant for the course of COVID-19 is only partly known. Today there is inconclusive information collected in small series on the prognosis for the disease in this subgroup, with variable reported mortality rates ranging from 4.8% to 19%.34,44,45 Regarding pharmacological management, the use of hydroxychloroquine, azithromycin, remdesivir, tocilizumab and steroid boluses has been reported in these patients, but to date it has not been possible to draw definitive conclusions with regard to which treatment is best in these cases. In general, there is advocacy for decreasing the dose of immunosuppressants (or suspending them in rare cases), since concomitant serious nosocomial infections are associated with a worse prognosis.46 Administration of lopinavir/ritonavir (Kaletra®) is discouraged due to the high risk of interactions and potential side effects. This is rooted in the fact that ritonavir is a powerful cytochrome CYP3A4 inhibitor, involved both in the metabolism of calcineurin inhibitors and in the metabolism of sirolimus and everolimus. If joint administration of the two drugs is required, it is recommended that the dose of tacrolimus be decreased to 1/20-1/50 relative to the usual regimen.46
What impact has the pandemic had on patients with chronic liver disease, and what impact will it have on them?The SARS-CoV-2 pandemic has had a serious impact on the inpatient and outpatient care system in Spain, which will surely result in changes to the protocols for intervention and clinical practice adopted to date. Although the impact of the epidemic has not been the same throughout Spain, there is a common denominator: the need for immediate reorganisation of all hepatology units. At most centres, these units have seen their human and material resources diminish due to redistribution thereof to priority care areas for patients with COVID-19. The first consequence of this was a reduction in healthcare activity aimed at the treatment, diagnosis and follow-up of chronic liver diseases.
Today it seems that patients and physicians have already experienced the peak of the pandemic in Spain, which implemented mandatory special measures such as social distancing, self-quarantining and using face masks in public places.47 In view of the latest epidemiological data available, these measures have enabled a significant decrease in virus transmission. However, Spain is far from getting back to normal. The impact of this "first wave" on a particularly vulnerable group — patients with chronic liver diseases — remains uncertain. Although the current epidemiological situation is more favourable, the repercussions of the pandemic for the medical care of this group are still serious, and it may be that a "second wave" is approaching in which the consequences of healthcare delays and forced changes in clinical care models may have a greater negative impact on the patients’ clinical courses than the infection itself. Some authors have even suggested the possibility of a future "third wave" in which suspension of preventive efforts, such as screening for hepatocellular carcinoma, will result in patients seeking care for problems that were initially preventable or easier to treat.48
It is well-known that the responsibility of the physician transcends the pure act of patient care. Physicians are also guarantors of judicious distribution of resources, even in situations of resource scarcity and extraordinary healthcare pressure. As a result, the different scientific associations, including the Asociación Española para el Estudio del Hígado (AEEH), American associations (AASLD) and European associations (EASL), have prepared position statements to design the guidelines that the different hepatology units should follow at present and in the next few months (Table 1).46,49,50
Summary of recommendations based on consensus documents from the AEEH, EASL and AASLD.46,49,50.
| Clinical scenario | |
|---|---|
| Stable outpatient | |
| Outpatient | Limit in-person follow-up visits and facilitate remote visits46,49,50Limit the first visits to patients with jaundice, AST or ALT > 500 and/or hepatic decompensation39Maintain active antiviral (HBV and HCV) treatments46,49,50Defer the start of HCV treatment,46,49,50 except in decompensated patients (AEEH)42Maintain immunosuppressant treatment, without reducing the dose, in patients with autoimmune hepatitis46,49,50In patients with a de novo diagnosis of serious autoimmune hepatitis, do not delay the start of corticosteroids, but do delay the start of azathioprine42 |
| Screening, diagnosis and treatment of hepatocellular carcinoma | Delay screening by two months39 or until ultrasound units allow the procedure to be performed safely42,43Prioritise the indication of imaging tests for high-risk patients (elevated AFP levels, advanced cirrhosis on a waiting list or high-risk chronic hepatitis B)39Maintain multidisciplinary committees, preferably virtual ones46,49,50Start and maintain oncologic treatments indicated according to guidelines46,49,50In cases in which this is not possible, deviations from clinical practice guidelines should be approached in multidisciplinary teams and may be guided by the recommendations published by the International Liver Cancer Association (https://ilca-online.org/management-of-hcc-during-covid-19-ilca-guidance/) |
| Screen for oesophageal varices and engage in primary and secondary prophylaxis | Universally apply the Baveno VI consensus criteria to indicate screening43Indicate non-cardioselective beta blockers in primary and secondary prophylaxisMaintain the endoscopic band ligation programme in high-risk patients in secondary prophylaxis42Perform only emergency endoscopies in case of suspected gastrointestinal bleeding in patients with COVID-1943 |
| Patient with decompensation and transplant candidates | |
| Decompensated cirrhosis | Promote telemedicine46,49,50Limit numbers of visits and review patients who are to be seen in person39,43Limit the time of admission, especially in pre-transplant assessments43Ensure that patients have treatment and facilitate long-term prescription thereof39Screen for SARS-CoV-2 in all patients admitted for acute decompensation42,43Carefully assess treatment with corticosteroids in acute alcoholic hepatitis39Continuously maintain transplant evaluation, adjusting the indication to the epidemiological situation at all times42Create "COVID-19–free" pathways to perform pre-transplant evaluations39 |
| Liver transplant | |
| Time of transplant | Reinforce the use of the telephone interview about COVID-19 symptoms in the potential recipient46,49,50Universally screen for SARS-CoV-2 by PCR of the nasopharyngeal exudate and perform imaging tests in potential donors and recipients46,49,50Rule out people who test positive for active (symptomatic or asymptomatic) SARS-CoV-2 infection as potential donors and as potential recipients42,*Consider accepting organs with low ischaemic times to avoid prolonged postoperative stays39Consider temporarily suspending live-donor programmes, except for paediatric patients39 |
| Immediate post-transplant period | Follow regularly used immunosuppression protocols46,49,50Maximise isolation and measures for preventing nosocomial transmission of infections, thus avoiding external visits46,49,50 |
| Follow-up of the transplant recipient | Maintain immunosuppressant treatment46,49,50Promote non-face-to-face visits46,49,50 |
| General recommendations | |
| Measures to prevent contagion | Observe social distancing and wear a face mask46,49,50Avoid travel to areas with a higher risk of contagion39Instruct patients to avoid attending meetings, even support group meetings (e.g. alcohol addiction recovery)39Collaborate closely with primary care physicians43 |
| Patient with liver disease and COVID-19 | |
| Admit patients with cirrhosis and COVID-19 early46,49,50Consider reducing the dose or, occasionally, suspending immunosuppressant treatment in patients with a liver transplant or autoimmune disease46,49,50Adjust the indication for antiviral drugs to the degree of liver failure46,49,50 | |
| Research and development | |
| Limit to essential activity and minimise face-to-face visits39Do not start new clinical trials, except in the absence of another therapeutic alternative39,42Do not recruit new patients to any non-essential clinical trials39Remote work for research personnel39 | |
AASLD: American Association for the Study of Liver Diseases; ALT: alanine aminotransferase; AEEH: Asociación Española para el Estudio del Hígado [Spanish Association for the Study of the Liver]; AFP: alpha-fetoprotein; AST: aspartate aminotransferase; EASL: European Association for the Study of the Liver; TACE: transarterial chemoembolisation; HBV: hepatitis B virus; HCV: hepatitis C virus.
The possibility of false positive or false negative COVID-19 test results should be considered, taking into account the epidemiological situation at the time of the donation and the presence of symptoms, suggestive radiological signs (or both).39.
In general, the care of patients with more advanced liver disease, such as patients with decompensated cirrhosis, liver transplant candidates and patients with a diagnosis of hepatocellular carcinoma, should be prioritised. The use of remote assistance tools, be they telephone calls, video calls or e-mails, is stressed. The objective of using these tools is to avoid contact with patients in the healthcare setting, especially in situations which a priori contribute no added value, such as visits made to report that the results of a diagnostic test are normal. One goal of remote visits is to bolster treatment adherence in patients with chronic diseases, such as viral hepatitis and hepatitis of autoimmune origin. To this end, it is also essential to coordinate with primary care and hospital pharmacy departments, so as to facilitate access to treatment effectively and prevent unnecessary delays. In-person care should be reserved for patients with serious or decompensated disease while always maximising personal protective measures and shortening waiting times.
In patients with hepatocellular carcinoma, the pandemic may have delayed the start of some treatments such as surgery, local ablation therapy and chemotherapy. Multidisciplinary committees for managing hepatocarcinoma have proven to be effective tools.51 Therefore, these meetings should be promoted by videoconference or with suitable social distancing, as they allow the start of these treatments to be prioritised, regardless of epidemiological circumstances or local availability of resources at any given time.
Possible liver transplant candidates have also seen the probability of their being added to a waiting list decrease in recent months.52 Therefore, restoring multidisciplinary committees to assess candidates is deemed a high-priority measure. It is recommended that, at the time of the transplant, both the donor and the recipient be carefully evaluated with a view to ruling out active SARS-CoV-2 infection by means of virological tests (serology and PCR), a physical examination and imaging tests.
Many complementary tests used in the diagnosis and follow-up of chronic liver disease, such as ultrasound, liver biopsy, elastography, endoscopy and liver haemodynamics, have been delayed or cancelled. In this regard, various scientific associations advocate the gradual restoration of these complementary tests; those patients with the highest risk must be prioritised. It is advisable to perform screening ultrasounds for hepatocellular carcinoma as soon as possible and to delay other complementary tests which are rarely urgent, such as elastography and liver haemodynamics.53 Liver biopsies, both percutaneous and transjugular, should be initially reserved for serious cases in which the biopsy result entails a change in therapeutic strategy. Measurement of the hepatic venous pressure gradient, for its part, should be initially reserved for patients who are candidates for hepatocarcinoma resection surgery. Upper gastrointestinal endoscopy is a procedure that generates aerosols and, as a result, a procedure that carries a risk of SARS-CoV-2 transmission.54 Therefore, it is important to adhere to the criteria proposed at the Baveno VI consensus conference which, through non-invasive methods, allow the risk of developing oesophageal varices to be stratified.55
Finally, it should be noted that the COVID-19 pandemic arrived at a very important time in the history of hepatology, when plans for the macroelimination (in the general population) and microelimination (in risk populations) of the hepatitis C virus (HCV) were starting to be developed. It is clear that the pandemic may have negative repercussions for the achievement of such important and ambitious goals as eradication of the infection before the year 2024. However, healthcare contact due to SARS-CoV-2 infection for many otherwise healthy patients may also facilitate opportunistic diagnoses of chronic HCV infection. Therefore, re-establishment of outpatient hepatology activity must continue to maintain simplified, universal access to antiviral treatments as an objective.
Future needs and conclusionsThe pandemic caused by SARS-CoV-2 represents one of the greatest challenges in recent decades for healthcare professionals and systems worldwide. COVID-19 not only compromises the survival of patients suffering from this disease, but also has major repercussions for medical care for other conditions, including chronic liver diseases. In this regard, it is important to generate more and better evidence with regard to the safety and efficacy of the treatments, both in the general population and in specific subgroups, and, at the same time, adapt routine care protocols to the current situation, in which the availability of complementary tests and treatment options has been compromised.
FundingThis study has received no specific funding from public, private or non-profit organisations.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank Francisco Gea for his invaluable help with the critical review of the article and the Gastroenterology and Hepatology Department at Hospital Ramón y Cajal [Ramón y Cajal Hospital] for its unwavering commitment to constantly updating scientific knowledge, even under special circumstances.
Please cite this article as: Téllez L Martín Mateos RM. Actualización en COVID-19 y enfermedad hepática. Gastroenterol Hepatol. 2020;43:472–480.