The prevalence of ulcerative colitis (UC) and its associated economic burden is increasing in Spain. Oral mesalazines, which are the recommended first-line treatment for mild-moderate UC, show considerable variability in their formulations and prices.
ObjectiveTo carry out a cost-effectiveness assessment of the use of the two formulations of oral gastro-resistant modified-release mesalazine formulations marketed in Spain (Salofalk® and Mezavant®) for the phases of induction of remission and its maintenance.
MethodsWe adapted internationally validated economic models for the management of UC to the Spanish setting. The adaptation focused on the use of oral gastro-resistant modified-release mesalazines. We conducted cost minimization analyses of remission induction (decision tree) and remission maintenance (Markov model).
ResultsFor the remission induction, Salofalk® 3g/day was superior to (same effectiveness at lower costs) Mezavant® 3.6g/day and 4.8g/day in any treatment strategy that included oral gastro-resistant modified-release mesalazines. When compared with Mezavant® 2.4g/day, Salofalk® was the most cost-effective option. For remission maintenance, all treatment strategies using Salofalk® were the most cost-effective option in all the scenarios considered.
ConclusionBecause of the lower cost per gram of Salofalk®, any treatment strategy based on this drug is more cost-effective than Mezavant® for the treatment of mild-moderate UC, whether for the induction of remission or for its maintenance.
La prevalencia de la colitis ulcerosa (CU) en España y su carga económica asociada están aumentando. La mesalazina oral, tratamiento recomendado como primera línea para la CU leve-moderada, presenta sensibles diferencias en cuanto a formulaciones y precios.
ObjetivosEstudio de coste-efectividad entre las 2 formulaciones orales de mesalazinas gastrorresistentes de liberación prolongada que existen en España (Salofalk® y Mezavant®) en las fases de brote y de mantenimiento de la remisión.
MétodosAdaptación de modelos económicos de manejo de la CU validados internacionalmente, al uso de mesalazinas gastrorresistentes de liberación prolongada en España: análisis de minimización de costes para el tratamiento del brote (árbol de decisión) y mantenimiento de la remisión (modelo de Markov).
ResultadosEn el tratamiento del brote, Salofalk® 3g/día domina (ofrece igual resultado en salud a menor coste) a Mezavant® en dosis de 3,6 y 4,8g/día en cualquier estrategia de tratamiento que incluya mesalazinas gastrorresistentes de liberación prolongada. Comparado con Mezavant® a dosis de 2,4g/día, Salofalk® resulta la opción más coste-efectiva. En el modelo de mantenimiento, las estrategias basadas en Salofalk® 3g/día son también dominantes para todos los escenarios considerados.
ConclusionesEl menor precio por gramo de Salofalk® hace que cualquier estrategia basada en Salofalk® sea más coste-efectiva que Mezavant® para el manejo de la CU leve-moderada, tanto para tratar el brote como para mantener la remisión.
Ulcerative colitis (UC) is a chronic inflammatory disease of the gastrointestinal tract, of partly known aetiology.1 It mainly affects the colon and is characterized by flare-ups or relapses, followed by unpredictable periods of remission.2,3 Severity and extension vary among patients4 and in the same patient over time, thus requiring different treatment strategies adapted to each clinical situation.5
Like other European countries, Spain has seen a gradual rise in the incidence of UC,6 with an estimated total of 6–10 new cases per 100,000 population/year7–9 and an increasing impact on paediatric groups.10 Given the chronic nature of the disease and its low mortality rate, prevalence rates have increased in parallel with the economic burden of this disorder on the Spanish National Health System.
The main objective in the treatment of UC is to achieve and maintain disease remission. Treatment of patients with mild to moderate UC is based essentially on the use of 5-aminosalicylic acid (5-ASA or mesalazine) derivatives.4,11 The efficacy, effectiveness and safety of mesalazine as a first-line induction and remission maintenance therapy in mild to moderate UC is backed by ample scientific evidence.12–16 The total cost of this disease, however, is increasing,17 although estimates vary greatly between different European countries.18 The impact of different mesalazine treatment strategies in each of these countries has proven to be a determining factor.19
Mesalazine is currently available in a wide variety of presentations, dosages, routes of administration, formulations and brands, differing mainly in the form of delivery of active mesalazine in the colon and the dosage.20 In Spain, despite this wide range, the only once-daily oral gastro-resistant prolonged-release formulations are Mezavant (1.2g tablets) and Salofalk (granules available in various doses). According to the latest edition of the National Institute for Health and Care Excellence (NICE) guidelines for the treatment of UC, the British National Formulary21 states that the delivery characteristics of the different oral preparations of mesalazine may vary, and that these preparations should not be considered interchangeable; as such, they should be compared with each other independently. Studies and meta-analyses comparing these gastro-resistant prolonged-release preparations12–16 show that they do not differ in effectiveness or safety, so determining their cost-effectiveness is particularly important to help clinicians choose the correct treatment.
To that end, the aim of this study was to assess the cost-effectiveness of mesalazine therapy by comparing two gastro-resistant prolonged-release formulations, Salofalk and Mezavant, in terms of minimizing the cost of inducing and maintaining remission in mild to moderate UC.
MethodsThis study includes an economic evaluation using models adapted for Spain, previously validated internationally by clinical experts, representatives of the pharmaceutical industry and pricing and reimbursement regulatory authorities.13 These cost-effectiveness simulation models weigh up the health gain (measured as utility) obtained by alternative interventions against the cost of obtaining these outcomes in each intervention. The alternative interventions compared were the different mesalazine gastro-resistant prolonged release once-daily oral preparations for the induction and maintenance of remission in patients with mild to moderate UC. The study began with a literature search, review and synthesis of all clinical and epidemiological information relative to UC and its management. This assessment identified and selected 2 up-to-date meta-analyses (Spanish Working Group in Crohn's Disease and Ulcerative Colitis [GETECCU]12 and NICE13 clinical practice guidelines [CPG]) and found that no subsequent clinical trials have reported findings that would alter the evidence reported in these publications and the articles included in their reviews. The synthesized evidence showed that the therapeutic options of interest did not differ as regards efficacy, effectiveness or safety in the management of mild or moderate UC. The results of the review were assessed and corroborated by 2 gastroenterologists specializing in the treatment of this disease (J.P.G., F.G.). On the basis of this evidence, a cost-minimization analysis was proposed to evaluate whether the therapeutic options might be associated with differences in the use of resources. To that end, following the expert review and assessment, we based our study on 2 economic models proposed in the NICE CPG13: one for induction of remission and the other for maintenance of remission. These validated models were adapted to Spain, and were restricted to the use of the treatments compared in this study.
The study population consisted of patients with left-sided or extensive mild to moderate UC, aged over 18 years, treated with gastro-resistant prolonged-release once-daily mesalazines. The scope is Spain in 2014, and the perspective that of the Spanish National Health System, considering only the direct medical costs.
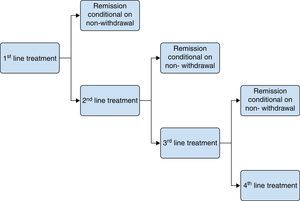
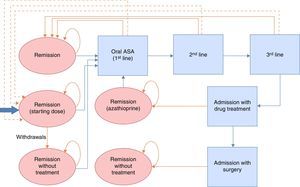
Cost-effectiveness modelsTwo independent models were constructed to analyze the cost-effectiveness of these preparations in both induction and maintenance of remission. Both models differ in their design, as the impact of time on treatment varies in each model and determines different approaches. Induction of remission must necessarily be assessed over short periods, expressed in terms of weeks, until remission has been achieved or, alternatively, the patient is admitted to hospital following therapeutic failure of successive treatment lines. Switching treatment or transition from one health state to another do not depend on time but on patient response. For this reason, the induction of remission model is not based on time cycles but using decision trees, which will last as long as the longest sequence of treatments needed to achieve remission. In the maintenance phase, however, changes in health state can be observed over regular follow-up periods that can be incorporated into proportional risk models; in this case, therefore, Markov models based on time cycles were used.
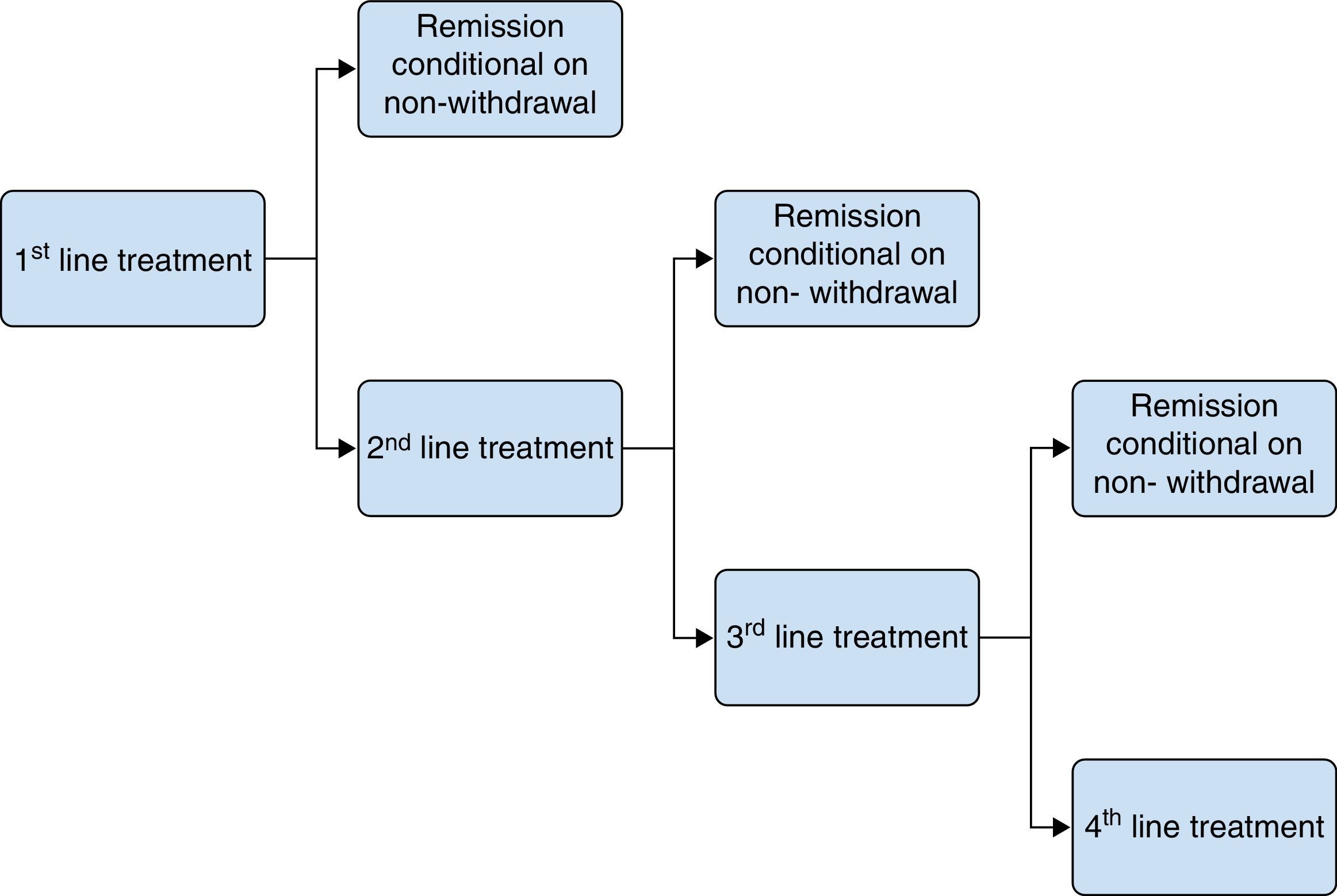
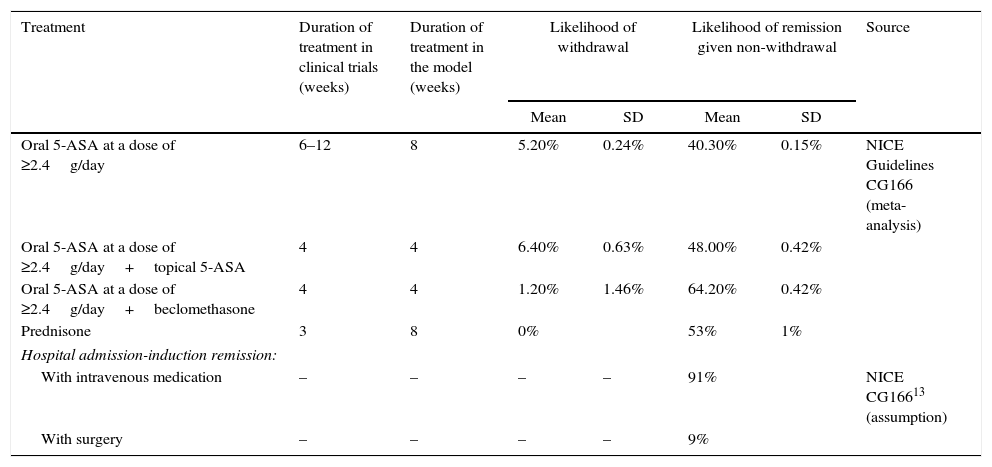
Induction of remission modelThe therapeutic objective in treating flare-ups is to achieve remission and avoid hospital admission. In order to do so, the patient begins a fixed duration regimen (4–8 weeks, depending on the therapeutic alternative), after which they are re-evaluated. The outcomes can be remission due to continuation of treatment, treatment withdrawal with continuation of the flare-up, or continuation of the flare-up whilst on treatment. If the flare-up continued, the patient switched treatment to a new fixed duration regimen. None of the patients repeated the treatment they were previously administered. A timeframe of 20–24 weeks was considered. A probabilistic decision tree model was used to study flare-up treatment (Fig. 1), in which induction of remission was assessed over short periods (weeks) until a state of remission was reached or, alternatively, the patient was admitted to hospital after the therapeutic failure of successive treatment regimens. Switching treatment or transition from one health state to another do not depend on time but on patient response. The treatment withdrawal and remission rates were taken from the online systematic review and meta-analysis included in the NICE Guidelines Development Group,13 detailed in Table 1.
Rates of treatment withdrawal and remission in the induction of remission model.
| Treatment | Duration of treatment in clinical trials (weeks) | Duration of treatment in the model (weeks) | Likelihood of withdrawal | Likelihood of remission given non-withdrawal | Source | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Oral 5-ASA at a dose of ≥2.4g/day | 6–12 | 8 | 5.20% | 0.24% | 40.30% | 0.15% | NICE Guidelines CG166 (meta-analysis) |
| Oral 5-ASA at a dose of ≥2.4g/day+topical 5-ASA | 4 | 4 | 6.40% | 0.63% | 48.00% | 0.42% | |
| Oral 5-ASA at a dose of ≥2.4g/day+beclomethasone | 4 | 4 | 1.20% | 1.46% | 64.20% | 0.42% | |
| Prednisone | 3 | 8 | 0% | 53% | 1% | ||
| Hospital admission-induction remission: | |||||||
| With intravenous medication | – | – | – | – | 91% | NICE CG16613 (assumption) | |
| With surgery | – | – | – | – | 9% | ||
In this study, Salofalk 3g/day was compared with each of the treatment alternatives with Mezavant at doses of ≥2g/day, according to current recommendations12: Mezavant at doses of 4.8, 3.6 or 2.4g/day. These comparisons were made for 3 scenarios based on different treatment strategies with oral mesalazine, taken from among those evaluated in the NICE guidelines13, which include the use of mesalazines at a dose of ≥2g/day. Strategy 1: oral 5-ASA (1st line)→prednisone (2nd line)→hospitalization. Strategy 2: oral 5-ASA (1st line)→oral 5-ASA+topical 5-ASA (2nd line)→prednisone (3rd line)→hospitalization. Strategy 3: oral 5-ASA (1st line)→oral 5-ASA+beclomethasone (2nd line)→prednisone (3rd line)→hospitalization.
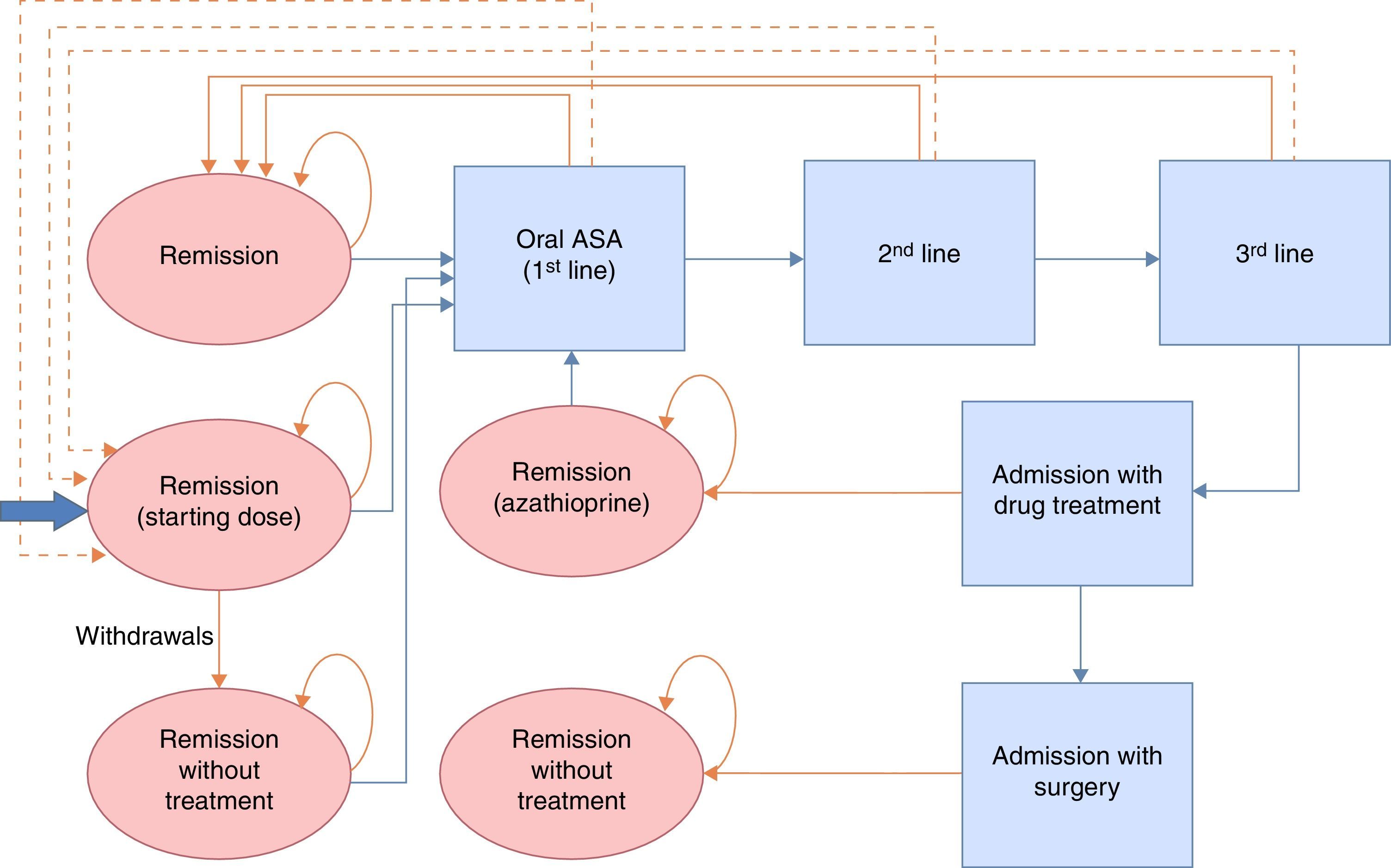
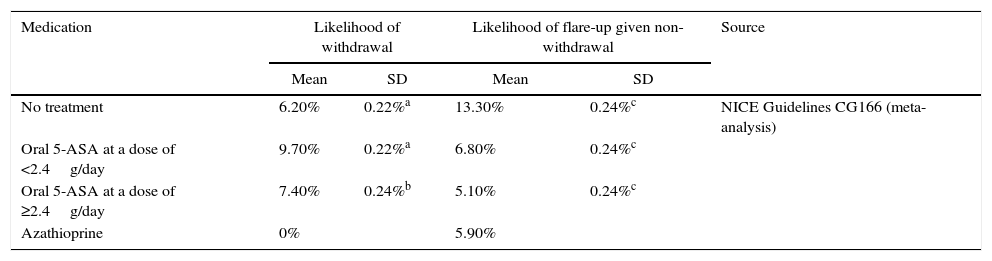
Maintenance of remission modelThe therapeutic objective in remission is to ensure continuity of treatment and to prevent or delay flare-ups. Continuity of treatment is contingent on adherence and onset of flare-ups, and is evaluated at regular 2-monthly intervals over a 2-year follow-up period (corresponding to the duration of the longest clinical trial published, and in accordance with the recommendations of the NICE CPG13). Treatment withdrawal or flare-ups are assessed in each cycle, and the remaining patients are considered to continue on treatment. Patients who withdraw from treatment in 1 cycle increase their likelihood of having a flare-up at the following evaluation. If the patient presented a flare-up, he or she was included in the most cost-effective remission induction treatment strategy in the model validated by NICE (described as number 3 in the induction of remission model) until remission was achieved. Following remission, patients started a new maintenance treatment regimen. Only when remission occurred after hospital admission was azathioprine/mercaptopurine administration considered as maintenance treatment, providing surgery was not required. Patients who required surgery were considered to be cured and did not receive maintenance treatment. A Markov probabilistic model was used to study treatment for maintenance of remission (Fig. 2). The remission rates were taken from the online systematic review and meta-analysis included in the NICE Guidelines Development Group13, detailed in Table 2. Given that the timeframe was greater than 1 year, an annual discount rate of 3.5% was applied to costs and outcomes.
Rates of treatment withdrawal and remission in the maintenance of remission model.
| Medication | Likelihood of withdrawal | Likelihood of flare-up given non-withdrawal | Source | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| No treatment | 6.20% | 0.22%a | 13.30% | 0.24%c | NICE Guidelines CG166 (meta-analysis) |
| Oral 5-ASA at a dose of <2.4g/day | 9.70% | 0.22%a | 6.80% | 0.24%c | |
| Oral 5-ASA at a dose of ≥2.4g/day | 7.40% | 0.24%b | 5.10% | 0.24%c | |
| Azathioprine | 0% | 5.90% | |||
Assumption based on the standard deviation of the likelihood of withdrawal whilst receiving oral 5-ASA at a dose of <2.4g/day provided by NICE Guidelines CG166 for the induction of remission model.
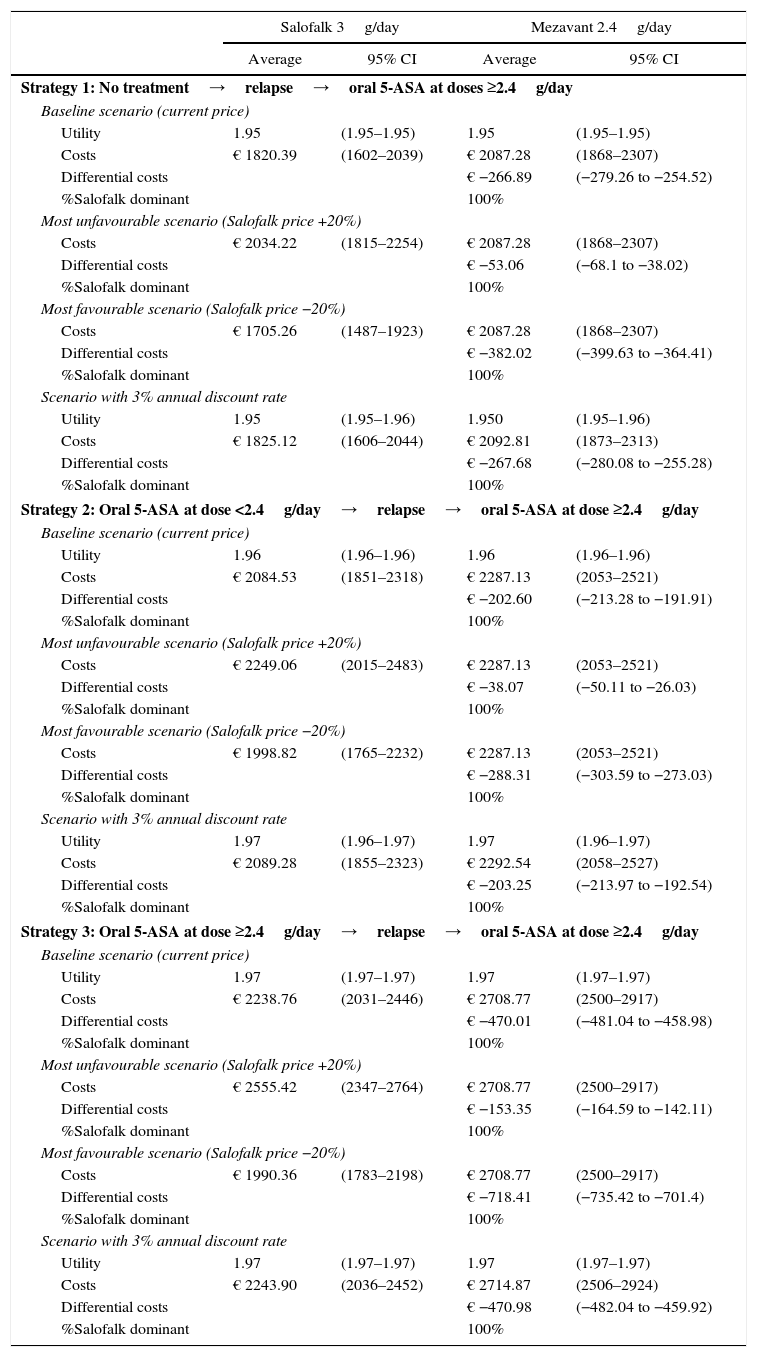
This model compared treatment with Salofalk 3g/day against Mezavant at doses of 2.4g/day according to the 3 oral 5-ASA treatment strategies detailed in the NICE guidelines. Strategy 1: no treatment→relapse→oral 5-ASA at dose ≥2.4g/day. Strategy 2: oral 5-ASA at dose <2.4g/day→relapse→oral 5-ASA at dose ≥2.4g/day. Strategy 3: oral 5-ASA at dose ≥2.4g/day→relapse→oral 5-ASA at dose ≥2.4g/day.
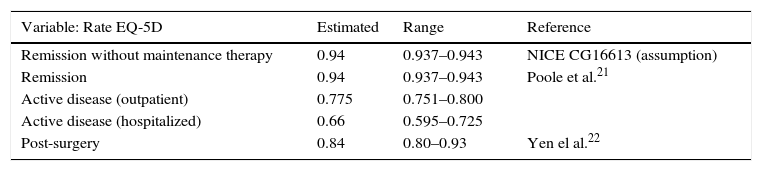
Effectiveness: health gainEffectiveness, measured as a change in a variable that measures health outcomes, can be expressed as health gain (or loss). Health gain in both models was measured in terms of utility, which is a rough estimate of the value given to a particular state of health by individuals in a population who express their preferences by assigning a quality of life score to certain states of health. The most commonly used measure of utility in health is the “quality adjusted life-year” (QALY). This measure was calculated for each treatment and strategy, based on differences between the start and end health state score, adjusted for the time spent in each state, and measured in years. The health states associated with different utility values are remission, active disease treated on an outpatient basis (mild or moderate), active disease requiring hospitalization (exacerbation), and post-surgical recovery. The values used to simulate the distributions were taken from the online systematic review and meta-analyses included in the NICE Guidelines Development Group,13,22,23 detailed in Table 3.
Estimated values for the simulation of the distribution of health states associated with different utility values.
| Variable: Rate EQ-5D | Estimated | Range | Reference |
|---|---|---|---|
| Remission without maintenance therapy | 0.94 | 0.937–0.943 | NICE CG16613 (assumption) |
| Remission | 0.94 | 0.937–0.943 | Poole et al.21 |
| Active disease (outpatient) | 0.775 | 0.751–0.800 | |
| Active disease (hospitalized) | 0.66 | 0.595–0.725 | |
| Post-surgery | 0.84 | 0.80–0.93 | Yen el al.22 |
Calculation of the cost associated with the different therapeutic alternatives included the direct medical costs derived from the costs of the pharmacological intervention, and the costs of clinical management of the UC. The cost of the treatments was obtained using different estimates. The cost of Salofalk and Mezavant were obtained by calculating the number of boxes needed to supply the total number of daily doses required in each case. For medicinal products with a single marketing presentation (beclomethasone, azathioprine, mercaptopurine), the cost was calculated on the basis of the cost/day over the total period considered. For medicinal products with different presentations (topical mesalazines, oral prednisone), the cost was obtained by calculating the average cost of the daily dose of all the presentations according to their summary of product characteristics sheet, and multiplying it by the total number of days of the period considered. In all cases, the price was obtained from secondary databases.24 The unit costs of the medicinal products used for this model are shown in Table 1 of the supplementary material.
The frequency of visits to the doctor and laboratory tests per time period for each model was adapted for Spain from the NICE models by clinical experts (Table 2 of the supplementary material). The cost of clinical management included the cost of consultations (consultant gastroenterologist, specialized nurse consulted in person and by telephone, primary care physician and primary care nursing staff); the cost of laboratory tests (complete blood count, renal and liver function tests and purine methyltransferase test for patients on azathioprine/mercaptopurine treatment); and the cost of hospitalization (Table 3 of the supplementary material).
The cost of follow-up and tests was obtained by estimating the national average from the prices given in healthcare cost bulletins for different regions of Spain, updated for 2014 and adjusted for the year-on-year inflation rate.25–31 Hospital costs were estimated on the basis of diagnosis-related groups (DRG); information for these was obtained from hospital healthcare information included in the minimum basic data set (MBDS) for 2012,32 updated to 2014 prices. DRG 179 (inflammatory bowel disease) was used for hospitalization without surgery. For hospitalization with surgery, we used the average of the prices for DRGs 148, 149, 152 and 153 (major and minor small and large bowel procedures, with and without complications), weighted by the percentage used in 2012.
The costs associated with each intervention and the differential costs obtained by comparing the interventions were calculated in each model. To facilitate comparison, the cost-effectiveness ratio of each intervention in each strategy was considered in terms of net monetary benefit (NMB). The NMB converts the health gain (QALY) into monetary units (€) after assigning a value to 1 health gain unit (€/AVAC), and calculates the balance between this benefit and the cost of obtaining it (NMB [€]=(Utility obtained [QALY]×Cost-effectiveness threshold [€/QALY]−Costs [€]). The value assigned to the health gain is known as the cost-effectiveness threshold, which is the limit amount or series of arbitrary amounts that society is willing to pay to obtain those results. The threshold used in this case was € 30,000/QALY.
Assumptions in both modelsA probabilistic method was used to incorporate the variability arising from uncertainties surrounding the values used. The distributions considered in the model were: lognormal distribution for the effects of treatment (transition probabilities), utility weightings, and reference costs. For the probabilities (dichotomous) relating to transition/use of a resource with no information on the standard deviation (SD), a beta distribution was adopted, using the probability value as average and its corresponding SD, using the formula: SD=ROOT[(p*(1−p))/n]. Finally, a gamma distribution was used to obtain hospitalization costs (Table 3 of the supplementary material).
For the induction of remission model, the variation in the distribution of the number of follow-up blood and renal function tests after the initiation of mesalazine treatment was modelled using a triangular distribution with minimum 0, mean 1 and maximum 3 values over an 8-week period.
For the maintenance of remission model, the SD for the mean was not described in the CPG. Experimentally, therefore, we used the same SD as the induction model (SD=0.22) for the probability of treatment withdrawal in non-treated patients and patients treated with mesalazine at a dose of <2.4g/day. We used SD=0.24 for patients treated with any other medication, and SD=0.24 for the probability of a flare-up whilst on treatment was used in all cases.
For the induction of remission model, it was assumed that remission occurred in the period between each treatment regimen. To calculate the use of resources, it was assumed that treatment lasted for the whole period, but that the remaining medical resources were used only during the first half of the period.
The cost of the medicinal products compared (Salofalk and Mezavant) was taken to be a fixed value determined by the number of days on treatment, and was not part of the probabilistic simulation. For the remaining treatments, the cost was obtained from lognormal distributions based on the mean and SD of the prices of the medicinal products with this denomination in the Spanish market. Treatment adherence was considered 100% in all cases.
Sensitivity analysisTo evaluate the robustness of the results and their sensitivity to variations in certain parameters, one-way sensitivity analyses were performed. Scenarios for a Salofalk cost of ±20% with respect to the current price were analyzed in both models. For the maintenance model, a sensitivity analysis was also carried out considering an alternative annual discount rate of 3.0%.33
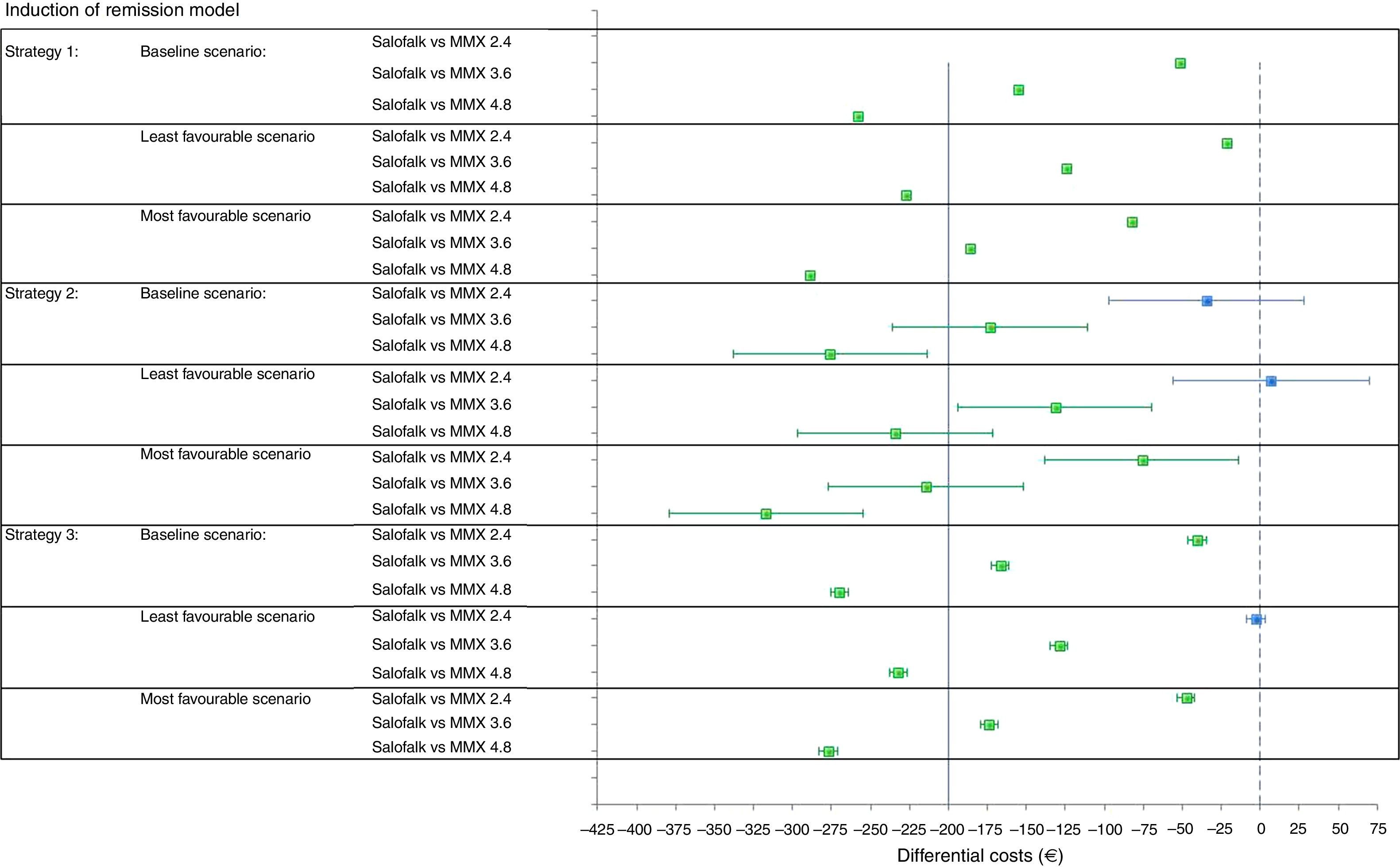
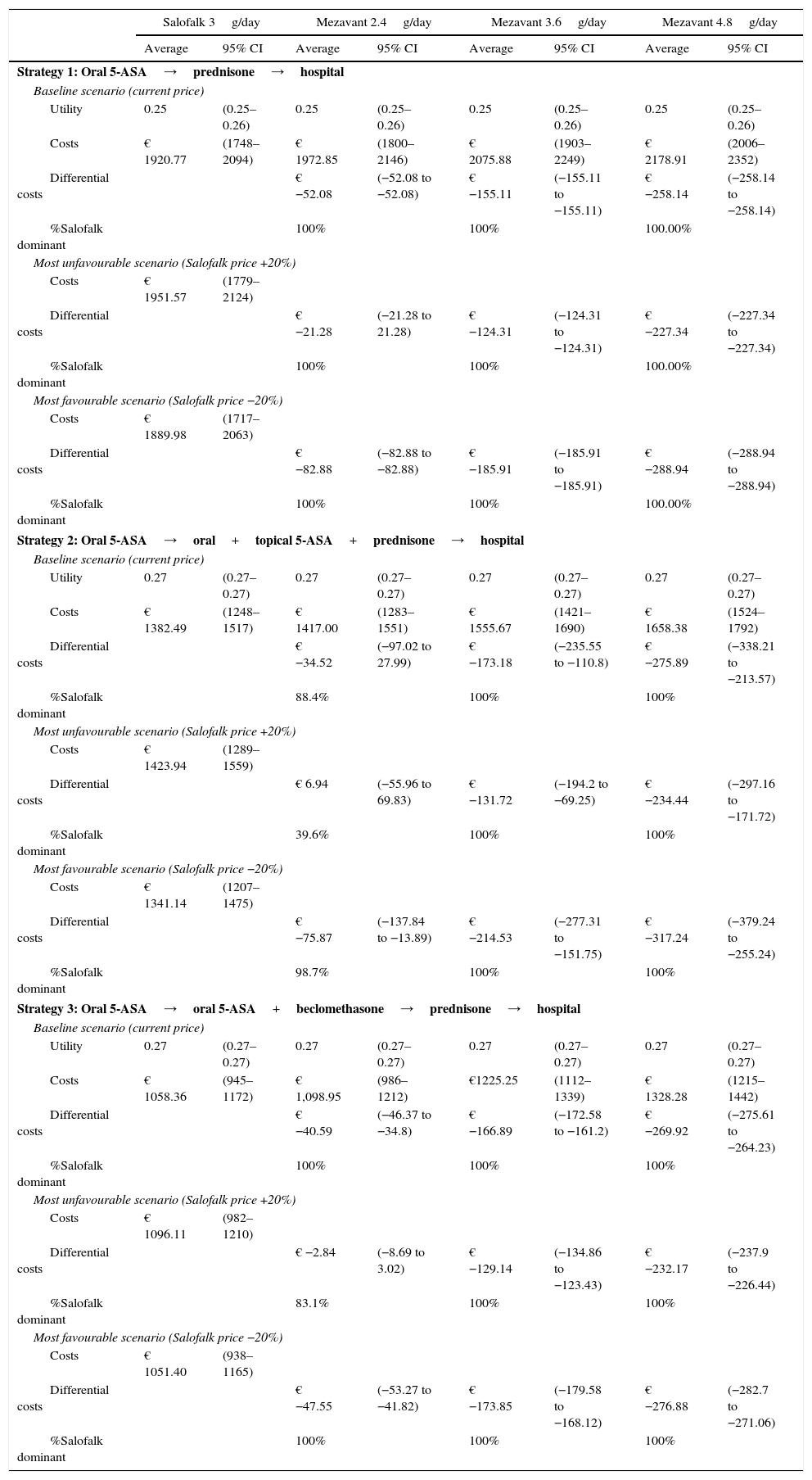
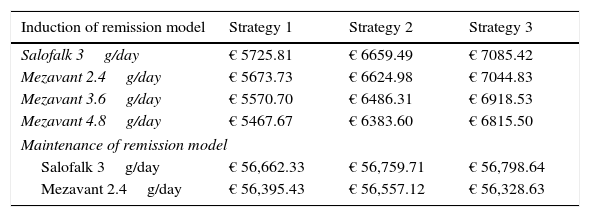
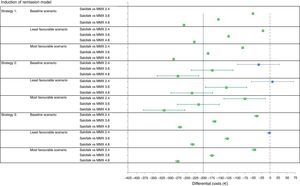
ResultsInduction of remission modelConsidering the baseline scenario, in which the current price of the medicinal products of interest were used, the differential cost per patient treated with Salofalk 3g/day with respect to Mezavant 4.8g/day gave a saving of € 258 over a 20–24 week period with treatment strategy 1, € 276 (95% CI: 214–338) with strategy 2, and € 270 (95% CI: 264–276) with strategy 3. Salofalk 3g/day always provided similar or better health gain outcomes at a lower cost than Mezavant 4.8g/day; i.e. Salofalk 3g/day was dominant in 100% of simulations in all 3 strategies. When Salofalk 3g/day was compared with Mezavant 3.6g/day, the saving was € 152 with strategy 1, € 173 (95% CI: 111–236) with strategy 2 and € 167 (95% CI: 161–173) with strategy 3. In all the strategies, Salofalk 3g/day was dominant over Mezavant 3.6g/day in 100% of simulations. When Salofalk 3g/day was compared with Mezavant 2.4g/day, a saving of € 52 was obtained with strategy 1, € 36 with strategy 2 (95% CI: 28–97) and € 41with strategy 3 (95% CI: 35–46). Salofalk 3g/day was dominant over Mezavant 2.4g/day in 100% of simulations in strategies 1 and 3, and was more cost-effective in 88% of simulations for strategy 2. The most cost-effective strategy with the highest NMB was strategy 3, in which Salofalk 3g/day was more cost-effective than any of the Mezavant dosages and, within each strategy, Salofalk 3g/day was the most cost-effective of all options compared (Table 4 and Fig. 3).
Results of the induction of remission model and sensitivity analysis: utility, total costs, differential costs (Salofalk–Mezavant) and percentage dominance.
| Salofalk 3g/day | Mezavant 2.4g/day | Mezavant 3.6g/day | Mezavant 4.8g/day | |||||
|---|---|---|---|---|---|---|---|---|
| Average | 95% CI | Average | 95% CI | Average | 95% CI | Average | 95% CI | |
| Strategy 1: Oral 5-ASA→prednisone→hospital | ||||||||
| Baseline scenario (current price) | ||||||||
| Utility | 0.25 | (0.25–0.26) | 0.25 | (0.25–0.26) | 0.25 | (0.25–0.26) | 0.25 | (0.25–0.26) |
| Costs | € 1920.77 | (1748–2094) | € 1972.85 | (1800–2146) | € 2075.88 | (1903–2249) | € 2178.91 | (2006–2352) |
| Differential costs | € −52.08 | (−52.08 to −52.08) | € −155.11 | (−155.11 to −155.11) | € −258.14 | (−258.14 to −258.14) | ||
| %Salofalk dominant | 100% | 100% | 100.00% | |||||
| Most unfavourable scenario (Salofalk price +20%) | ||||||||
| Costs | € 1951.57 | (1779–2124) | ||||||
| Differential costs | € −21.28 | (−21.28 to 21.28) | € −124.31 | (−124.31 to −124.31) | € −227.34 | (−227.34 to −227.34) | ||
| %Salofalk dominant | 100% | 100% | 100.00% | |||||
| Most favourable scenario (Salofalk price −20%) | ||||||||
| Costs | € 1889.98 | (1717–2063) | ||||||
| Differential costs | € −82.88 | (−82.88 to −82.88) | € −185.91 | (−185.91 to −185.91) | € −288.94 | (−288.94 to −288.94) | ||
| %Salofalk dominant | 100% | 100% | 100.00% | |||||
| Strategy 2: Oral 5-ASA→oral+topical 5-ASA+prednisone→hospital | ||||||||
| Baseline scenario (current price) | ||||||||
| Utility | 0.27 | (0.27–0.27) | 0.27 | (0.27–0.27) | 0.27 | (0.27–0.27) | 0.27 | (0.27–0.27) |
| Costs | € 1382.49 | (1248–1517) | € 1417.00 | (1283–1551) | € 1555.67 | (1421–1690) | € 1658.38 | (1524–1792) |
| Differential costs | € −34.52 | (−97.02 to 27.99) | € −173.18 | (−235.55 to −110.8) | € −275.89 | (−338.21 to −213.57) | ||
| %Salofalk dominant | 88.4% | 100% | 100% | |||||
| Most unfavourable scenario (Salofalk price +20%) | ||||||||
| Costs | € 1423.94 | (1289–1559) | ||||||
| Differential costs | € 6.94 | (−55.96 to 69.83) | € −131.72 | (−194.2 to −69.25) | € −234.44 | (−297.16 to −171.72) | ||
| %Salofalk dominant | 39.6% | 100% | 100% | |||||
| Most favourable scenario (Salofalk price −20%) | ||||||||
| Costs | € 1341.14 | (1207–1475) | ||||||
| Differential costs | € −75.87 | (−137.84 to −13.89) | € −214.53 | (−277.31 to −151.75) | € −317.24 | (−379.24 to −255.24) | ||
| %Salofalk dominant | 98.7% | 100% | 100% | |||||
| Strategy 3: Oral 5-ASA→oral 5-ASA+beclomethasone→prednisone→hospital | ||||||||
| Baseline scenario (current price) | ||||||||
| Utility | 0.27 | (0.27–0.27) | 0.27 | (0.27–0.27) | 0.27 | (0.27–0.27) | 0.27 | (0.27–0.27) |
| Costs | € 1058.36 | (945–1172) | € 1,098.95 | (986–1212) | €1225.25 | (1112–1339) | € 1328.28 | (1215–1442) |
| Differential costs | € −40.59 | (−46.37 to −34.8) | € −166.89 | (−172.58 to −161.2) | € −269.92 | (−275.61 to −264.23) | ||
| %Salofalk dominant | 100% | 100% | 100% | |||||
| Most unfavourable scenario (Salofalk price +20%) | ||||||||
| Costs | € 1096.11 | (982–1210) | ||||||
| Differential costs | € −2.84 | (−8.69 to 3.02) | € −129.14 | (−134.86 to −123.43) | € −232.17 | (−237.9 to −226.44) | ||
| %Salofalk dominant | 83.1% | 100% | 100% | |||||
| Most favourable scenario (Salofalk price −20%) | ||||||||
| Costs | € 1051.40 | (938–1165) | ||||||
| Differential costs | € −47.55 | (−53.27 to −41.82) | € −173.85 | (−179.58 to −168.12) | € −276.88 | (−282.7 to −271.06) | ||
| %Salofalk dominant | 100% | 100% | 100% | |||||
Considering a scenario in which Salofalk was 20% more expensive than its current price while Mezavant retained its current price, Salofalk 3g/day would continue to be dominant over any strategy that included Mezavant at doses of 3.6 or 4.8g/day in 100% of simulations, with savings per patient ranging from a minimum of € 69 (lower limit of the 95% CI for the comparison with Mezavant 3.6g/day in strategy 2) to a maximum of € 297 (upper limit of the 95% CI for the comparison with Mezavant 4.8g/day in strategy 2) over 20–24 weeks of treatment. However, the difference is less significant when compared with Mezavant 2.4g/day, since combined oral and topical mesalazine treatment in the model lasts 4 weeks, and a single box of Mezavant is required for this period at this dose. In this scenario, Salofalk would continue to be dominant in 100% of simulations in strategy 1, it would be more cost-effective in 83% of simulations with strategy 3, and would obtain similar efficacy outcomes with strategy 2, with 40% of outcomes in favour and a 95% CI that includes results in favour and against, yielding an average higher cost of € 7 per patient.
Considering the scenario in which Salofalk was 20% cheaper than its current price while Mezavant retained its current price, Salofalk 3g/day gives the same health gain at a lower cost than Mezavant 4.8, 3.6 or 2.4g/day in all strategies and simulations.
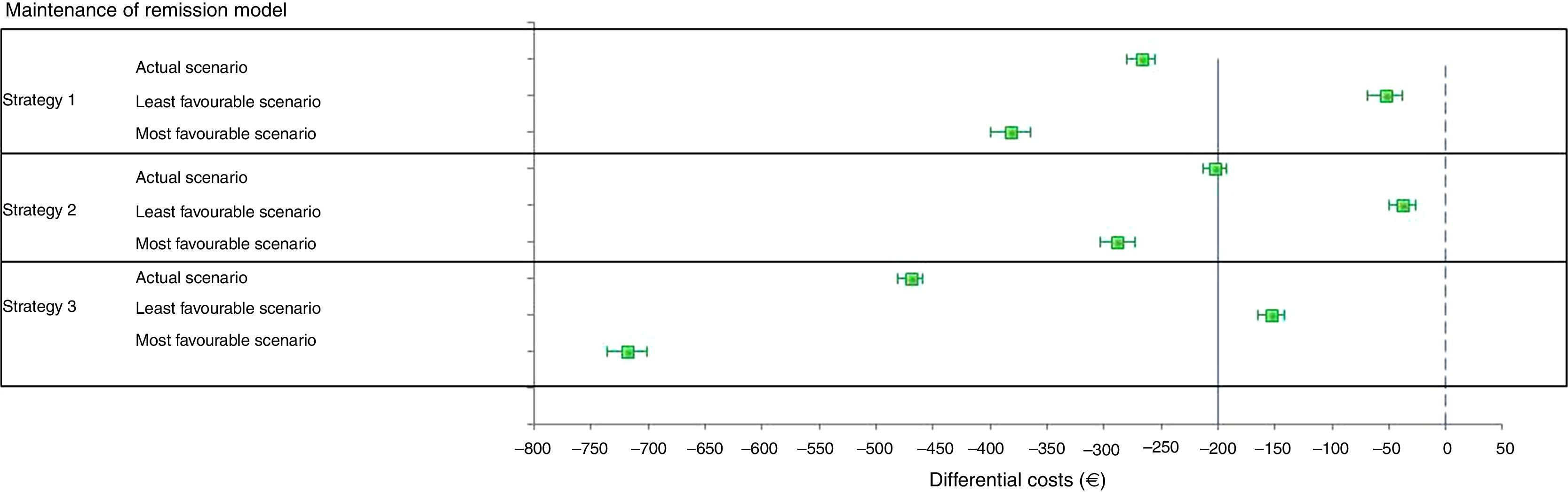
Maintenance of remission modelThe average saving per patient over 2 years of maintenance treatment in the baseline scenario with current treatment prices, using Salofalk 3g/day with respect to Mezavant 2.4g/day, ranged from € 203 (95% CI: 192–213) with strategy 2 to € 470 (95% CI: 459–481) with strategy 3. Salofalk always provides similar health gain outcomes at a lower cost than Mezavant 2.4g/day; in other words, it is 100% dominant in all strategies and simulations (Table 5).
Results of the maintenance of remission treatment model and sensitivity analysis: utility, total costs, differential costs and percentage dominance.
| Salofalk 3g/day | Mezavant 2.4g/day | |||
|---|---|---|---|---|
| Average | 95% CI | Average | 95% CI | |
| Strategy 1: No treatment→relapse→oral 5-ASA at doses ≥2.4g/day | ||||
| Baseline scenario (current price) | ||||
| Utility | 1.95 | (1.95–1.95) | 1.95 | (1.95–1.95) |
| Costs | € 1820.39 | (1602–2039) | € 2087.28 | (1868–2307) |
| Differential costs | € −266.89 | (−279.26 to −254.52) | ||
| %Salofalk dominant | 100% | |||
| Most unfavourable scenario (Salofalk price +20%) | ||||
| Costs | € 2034.22 | (1815–2254) | € 2087.28 | (1868–2307) |
| Differential costs | € −53.06 | (−68.1 to −38.02) | ||
| %Salofalk dominant | 100% | |||
| Most favourable scenario (Salofalk price −20%) | ||||
| Costs | € 1705.26 | (1487–1923) | € 2087.28 | (1868–2307) |
| Differential costs | € −382.02 | (−399.63 to −364.41) | ||
| %Salofalk dominant | 100% | |||
| Scenario with 3% annual discount rate | ||||
| Utility | 1.95 | (1.95–1.96) | 1.950 | (1.95–1.96) |
| Costs | € 1825.12 | (1606–2044) | € 2092.81 | (1873–2313) |
| Differential costs | € −267.68 | (−280.08 to −255.28) | ||
| %Salofalk dominant | 100% | |||
| Strategy 2: Oral 5-ASA at dose <2.4g/day→relapse→oral 5-ASA at dose ≥2.4g/day | ||||
| Baseline scenario (current price) | ||||
| Utility | 1.96 | (1.96–1.96) | 1.96 | (1.96–1.96) |
| Costs | € 2084.53 | (1851–2318) | € 2287.13 | (2053–2521) |
| Differential costs | € −202.60 | (−213.28 to −191.91) | ||
| %Salofalk dominant | 100% | |||
| Most unfavourable scenario (Salofalk price +20%) | ||||
| Costs | € 2249.06 | (2015–2483) | € 2287.13 | (2053–2521) |
| Differential costs | € −38.07 | (−50.11 to −26.03) | ||
| %Salofalk dominant | 100% | |||
| Most favourable scenario (Salofalk price −20%) | ||||
| Costs | € 1998.82 | (1765–2232) | € 2287.13 | (2053–2521) |
| Differential costs | € −288.31 | (−303.59 to −273.03) | ||
| %Salofalk dominant | 100% | |||
| Scenario with 3% annual discount rate | ||||
| Utility | 1.97 | (1.96–1.97) | 1.97 | (1.96–1.97) |
| Costs | € 2089.28 | (1855–2323) | € 2292.54 | (2058–2527) |
| Differential costs | € −203.25 | (−213.97 to −192.54) | ||
| %Salofalk dominant | 100% | |||
| Strategy 3: Oral 5-ASA at dose ≥2.4g/day→relapse→oral 5-ASA at dose ≥2.4g/day | ||||
| Baseline scenario (current price) | ||||
| Utility | 1.97 | (1.97–1.97) | 1.97 | (1.97–1.97) |
| Costs | € 2238.76 | (2031–2446) | € 2708.77 | (2500–2917) |
| Differential costs | € −470.01 | (−481.04 to −458.98) | ||
| %Salofalk dominant | 100% | |||
| Most unfavourable scenario (Salofalk price +20%) | ||||
| Costs | € 2555.42 | (2347–2764) | € 2708.77 | (2500–2917) |
| Differential costs | € −153.35 | (−164.59 to −142.11) | ||
| %Salofalk dominant | 100% | |||
| Most favourable scenario (Salofalk price −20%) | ||||
| Costs | € 1990.36 | (1783–2198) | € 2708.77 | (2500–2917) |
| Differential costs | € −718.41 | (−735.42 to −701.4) | ||
| %Salofalk dominant | 100% | |||
| Scenario with 3% annual discount rate | ||||
| Utility | 1.97 | (1.97–1.97) | 1.97 | (1.97–1.97) |
| Costs | € 2243.90 | (2036–2452) | € 2714.87 | (2506–2924) |
| Differential costs | € −470.98 | (−482.04 to −459.92) | ||
| %Salofalk dominant | 100% | |||
In the scenario in which Salofalk is 20% more expensive and Mezavant retains its current price, Salofalk 3g/day remains dominant (it always offers similar health gain outcomes at a lower cost), although the saving per patient over 2 years falls to between € 38 (95% CI: 26–50) with strategy 2 and € 153 (95% CI: 142–165) with strategy 3.
Strategies based on Salofalk 3g/day were clearly dominant, and Salofalk 3g/day was the most cost-effective choice (higher NMB) in all strategies compared (Table 6 and Figs. 3 and 4). Of all remission maintenance treatment options, strategy 3 was the most cost-effective, with the highest NMB (Fig. 4).
Net monetary benefit per patient for a threshold of €30 000/QALY in the induction of remission model and in the maintenance of remission model.
| Induction of remission model | Strategy 1 | Strategy 2 | Strategy 3 |
|---|---|---|---|
| Salofalk 3g/day | € 5725.81 | € 6659.49 | € 7085.42 |
| Mezavant 2.4g/day | € 5673.73 | € 6624.98 | € 7044.83 |
| Mezavant 3.6g/day | € 5570.70 | € 6486.31 | € 6918.53 |
| Mezavant 4.8g/day | € 5467.67 | € 6383.60 | € 6815.50 |
| Maintenance of remission model | |||
| Salofalk 3g/day | € 56,662.33 | € 56,759.71 | € 56,798.64 |
| Mezavant 2.4g/day | € 56,395.43 | € 56,557.12 | € 56,328.63 |
UC is becoming increasingly common in Spain, with recent data showing that it affected 100/100 000 population in 2014.7 Most patients treated in Spain are diagnosed with mild to moderate UC,9 so determining the most cost-effective treatment could considerably lighten the economic burden associated with this disease.
Mesalazines have been shown to be far more effective in this patient group, as demonstrated in recent articles on the management of UC, including the GETECCU CPG,12 CPG on the general management of UC published by the British National Health Service's NICE in 2013,13 and 2 Cochrane reviews published in 2012.14,15 These documents make up the latest meta-analyses in which both the efficacy and safety of various treatments for different disease presentations (remission and maintenance) are evaluated, and compare gastro-resistant prolonged-release (or other) formulations, dosages, once-daily vs multiple dose regimens, and perform an aggregated analysis of the different treatments.
The review and assessment of studies on the cost-effectiveness of UC treatment together with 2 new models for evaluating the cost-effectiveness of different strategies for managing different forms of the disease included in the NICE CPG was used in this study. It is also a consensus document that has been submitted to various organizations, including the pharmaceutical companies that manufacture the medicinal products of interest, for consultation and review. Based on this evidence, our study has adapted the NICE models to Spain, focusing specifically on management of UC with once-daily mesalazine-based gastro-resistant prolonged-release formulations. More recent studies have shown that a once-daily regimen is at least as effective and safe as multiple daily doses in inducing remission in patients with mild to moderate UC.34,35 Indeed, the once-daily regimen of mesalazine facilitates therapeutic compliance, and could therefore be even more effective.36 Studies on patient preference also indicate a predilection for treatment with a once-daily regimen compared to conventional treatment involving multiple daily doses.34
When the importance of mesalazine dosage for the treatment of a UC flare-up is analyzed, GETECCU guidelines12 show a tendency towards a higher rate of remission with increased doses of oral salicylates, and specify that when the variable analyzed is endoscopic remission, doses of ≥3g/day are more effective. The authors, therefore, recommend a minimum once-daily mesalazine dose of 2.4g/day and probable optimal dose of ≥3g/day to induce remission in patients with mild to moderate flare-ups of UC. With respect to the maintenance dose for these formulations, Kruis et al.37 suggest (with moderate evidence according to the Grading of Recommendations Assessment, Development and Evaluation [GRADE] classification) that 1 Salofalk dose of 3g/day is superior to 1.5g/day, with no significant differences in safety between groups. Studies comparing different doses of Mezavant (2.4, 3.2 and 4.8g) for the induction of UC remission show no significant differences with respect to clinical remission, but report a tendency towards greater endoscopic remission with higher doses, although the differences were not statistically significant.13
Based on the foregoing and the findings of this study, treatment with Salofalk 3g in clinical practice in Spain is associated with lower overall costs than treatment with Mezavant 2.4, 3.2 and 4.8g, in both induction and maintenance of remission. Furthermore, comparing costs against the outcomes achieved with each treatment, our results show that in terms of NMB, Salofalk 3g is superior in all clinical scenarios and strategies considered. Consequently, the use of Salofalk 3g instead of Mezavant would be equally effective overall, but more economically beneficial for the Spanish National Health System.
The interpretation of the results obtained in this study are limited, insofar as it is based on the adaptation of models that include an overall approach to mild to moderate UC with different management and treatment strategies, not specifically mesalazines. As a result, many aspects of interest for our study objectives were pooled in the model, such as the analysis of different treatment sequences (here called strategies), or the selection of an average treatment with topical mesalazine for the combination of oral and topical mesalazines. This aggregation should be taken into account when interpreting our results, as any oral mesalazine treatment at a dose of ≥2.4g/day was replaced by the specific mesalazines compared in this study. Similarly, it should be noted that this study uses the best evidence currently available on the effectiveness, adherence and safety of the treatments compared, from the most up-to-date systematic reviews and meta-analyses, although no data from clinical trials directly comparing the treatments evaluated is available. Finally, this is a model validated by scientific experts, and by the regulatory authorities in the case of the United Kingdom, although it was adapted to Spain by gastroenterologists specializing in the clinical management of UC in Spain.
Another aspect that needs to be considered is that in this evaluation it was assumed that patients had 100% treatment adherence. Poor treatment compliance is known to be associated with an increase in the likelihood of relapses, poorer quality of life, and ultimately, an increase in the total cost associated with the disease.38 The different formulations (sachets vs tablets) could lead to variations in therapeutic adherence, and this could affect the model. This, however, seems unlikely given the results of previous adherence studies.
Another limitation to consider in this study concerns the combined use of oral and rectal mesalazines as first-line therapy for induction of remission, which was not considered among the treatment options. Some studies have suggested that this strategy could induce a more rapid response and be more effective than the administration of each of these agents separately39,40; in fact, some of the most recent guidelines on which we have drawn include it as a first-line strategy. Thus, the current recommendation for clinical practice is to induce remission with combined oral and topical mesalazines for between 4 and 8 weeks, followed by prednisolone in patients who do not respond to this initial regimen. The model used in this study includes combined treatment with oral and topical mesalazines followed by prednisolone in strategy 2, as second- and third-line treatments. Unlike current recommendations, however, it restricts the duration of combined treatment to 4 weeks, as this was the maximum duration evaluated in clinical trials. Due to this shorter duration, fewer patients achieve remission than would be expected from these recommendations. This, in turn, would mean that more patients will need prednisolone treatment, and that the number of patients needing hospital admission would eventually be higher. Accordingly, including a strategy such as the one described in the clinical recommendations (which only considers combined treatment followed by prednisolone) would, paradoxically, shorten the treatment strategy as a whole, and ultimately prove more costly. Therefore, in the interest of consistency with the model originally validated by NICE, we decided to simply reproduce the evaluation of the strategies as set out in the aforementioned guideline.
When we analyzed the cost-effectiveness of different mesalazine formulations reported in the literature, we noted that studies found the formulations evaluated in our study (single dose gastro-resistant prolonged-release preparations) to be more cost-effective than other mesalazine formulations,41,42 but no studies have compared the different formulations within this latter group.
Finally, since there are no high quality cost-effectiveness models in Spain that compare the use of mesalazines in the treatment of UC flare-ups, we were unable to compare the results of our study with other Spanish publications. A recent study (published as a poster presentation at a conference) conducted in 4 Spanish hospitals assessed the annual average cost of maintaining remission of UC with different formulations of mesalazine, showing that Salofalk (€ 834.07) was less expensive than Mezavant (€ 1697.97).43
In conclusion, the results obtained in this study suggest that the lower price per gram of Salofalk compared to Mezavant make Salofalk-based strategies for the management of mild to moderate UC more cost-effective in both the induction and maintenance of remission.
FundingThis study was funded by Dr. Falk Pharma through a contract with Oxon Epidemiology Limited. Oxon Epidemiology Limited is a Contract Research Organization that provides services to different pharmaceutical companies. Dr. Falk did not participate in any way or influence any scientific aspect of the study.
Conflict of interestsDr. Gisbert provides scientific advice, support for research and/or training activities for the following pharmaceutical companies: MSD, Abbvie, Hospira, Kern Pharma, Takeda, Janssen, Pfizer, Ferring, Faes Farma, Shire Pharmaceuticals, Dr, Falk Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical and Vifor Pharma.
Dr. Gomollón provides scientific advice, support for research and/or training activities for the following pharmaceutical companies: MSD, Abbvie, Shire Pharmaceuticals, Dr, Falk Pharma, Vifor Pharma, FAESFARMA and Pharmacosmos.
Ignacio Méndez is employed by Oxon Epidemiology.
The authors would like to thank Antonio González del Castillo from Oxon Epidemiology for his logistic support during the project, and Ana Isabel Ortega for her help in drafting the manuscript.
Both authors contributed equally to this article.
Please cite this article as: Gisbert JP, Gomollón F, Méndez I. Evaluación de coste-efectividad mediante análisis teórico de minimización de costes del uso de dos mesalazinas gastrorresistentes de liberación prolongada en el manejo de la colitis ulcerosa en España. Gastroenterol Hepatol. 2016;39:199–212.