Helicobacter pylori (H. pylori) is closely related to pre-neoplastic lesions such as gastric atrophy (GA), gastric intestinal metaplasia (GIM) and eventually gastric cancer (GC). The diagnosis of GIM and GA is usually based on endoscopic and histopathological features. Nowadays, there are no recognized good serological markers of GIM and GA. Neopterin is an important marker of cellular inflammation. In this study, we aimed to comparatively evaluate C-reactive protein (CRP) and neopterin levels in patients with GIM, GA and chronic gastritis, and to show the increased serum neopterin levels in GIM and GA according to non-atrophic and non-metaplastic chronic gastritis.
Patients and methods98 patients with GIM and 68 patients with GA and 70 patients with non-atrophic non-metaplastic gastritis were included in the study. CRP and neopterin levels were assessed in patients and controls.
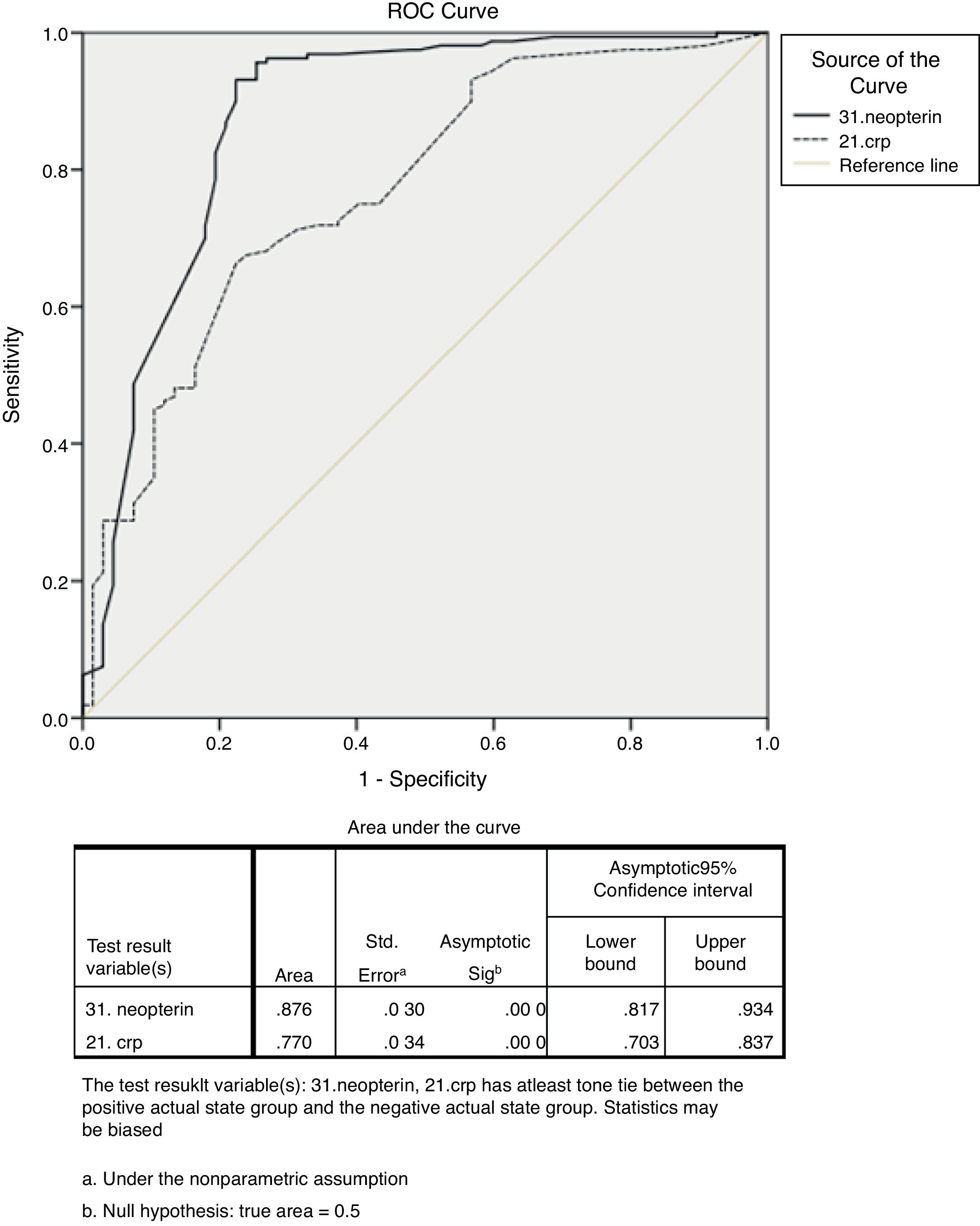
ResultsCRP and neopterin levels were significantly higher in patients with GIM and GA than in controls (p<0.05 and p<0.001, respectively). A multiple logistic regression analysis showed that high levels of serum neopterin were positively correlated with GIM and GA. According to the ROC curve analysis, the best cut-off value to differentiate between patients with GIM and/or GA from controls was ≥10.15nmol/l (p<0.001) for serum neopterin levels and ≥1.95mg/l (p<0.001) for serum CRP levels.
DiscussionCRP and neopterin levels are significantly increased in GIM and GA. Neopterin may be a useful biomarker and diagnostic test for detecting GIM and GA in clinical practice. CRP levels may be helpful for this observation.
Helicobacter pylori (H. pylori) está estrechamente relacionado con lesiones preneoplásicas, como la atrofia gástrica (AG), metaplasia intestinal gástrica (MIG) y finalmente cáncer gástrico (CG). El diagnóstico de MIG y AG generalmente se basa en características endoscópicas e histopatológicas. Hoy día, no hay buenos marcadores serológicos reconocidos de MIG y AG. La neopterina es un marcador importante de inflamación celular. En este estudio, nuestro objetivo fue evaluar comparativamente la proteína C-reactiva (PCR) y los niveles de neopterina en pacientes con MIG, AG y gastritis crónica, y mostrar el aumento del nivel sérico de neopterina en MIG y AG sobre la base de gastritis crónica no atrófica y no metaplásica.
Pacientes y métodosSe incluyó en el estudio a 98 pacientes con MIG, 68 pacientes con AG y 70 pacientes con gastritis no atrófica y no metaplásica. Se evaluaron los niveles de PCR y neopterina en pacientes y controles.
ResultadosLos niveles de PCR y neopterina fueron considerablemente más altos en los pacientes con MIG y AG que en los controles (p<0,05 y p<0,001, respectivamente). Un análisis de regresión logística múltiple mostró que el elevado nivel de neopterina sérica se correlacionó positivamente con MIG y AG. Según el análisis de la curva ROC, el mejor valor de corte para diferenciar entre pacientes con MIG y/o AG y controles fue ≥10,15nmol/l (p<0,001) para el nivel de neopterina sérica y ≥1,95mg/l (p<0,001) para el nivel de PCR en suero.
DiscusiónLos niveles de PCR y neopterina aumentan considerablemente en MIG y AG. La neopterina puede ser un biomarcador útil y una prueba de diagnóstico para detectar MIG y AG en el entorno clínico. Los niveles de PCR pueden ser útiles para esta observación.
Helicobacter pylori (H. pylori) infection is considered a major cause of acute and chronic gastritis, and of gastric and duodenal ulcer. H. pylori infection is frequent worldwide, with a global prevalence of more than 50% and distinct geographical alterations.1H. pylori infection is closely associated with pre-neoplastic lesions such as gastric atrophy (GA), gastric intestinal metaplasia (GIM) and eventually gastric cancer (GC).2 GC remains the second most frequent cause of cancer-related deaths and 2 ranks 4th in cancer incidence worldwide. The stage is crucial concerning with prognosis of GC. Especially, early GC has so good prognosis and survival rates are over 90%.3 Recent population-based studies reported that the incidence rate of GC in patients with GIM was about 0.38–1.29 per 1000 person per year.4 Thus, it is important to determine at early stages for high-risk group of GC. Therefore, effective surveillance, diagnosis and management of patients with GIM and GA is required to detect GC at an early stage.5 GA is a characterized by the death of the parietal cells and the loss of gastric glandular epithelium. GIM is histologically defined as the replacement of glandular and/or foveolar epithelium by intestinal epithelium and is characterized by its morphological similarity to enterocytes.2 GA and GIM are the histopathologic entities that reflect various phases during conversion of chronic gastric inflammation to carcinoma for whatever reason. The diagnosis of GIM and GA usually based on endoscopic features and histopathological findings. Sometimes it is difficult to make the diagnoses of GA and GIM.6 Noninvasive tests such as serum level of pepsinogen (PG) and gastrin-17 antibodies are not easily accessible tests everywhere. Briefly nowadays, there are no recognized good markers of GIM and GA.
Neopterin is produced by human monocytes/macrophages upon stimulation by interferon-gamma (IFN-γ) and is a sensitive marker for monitoring Th-1 cell immune response in humans. Neopterin is an early inflammation marker, like interleukin-6 (IL-6) and C-reactive protein (CRP), which are related to the increased malignant potential of the neoplasm.7 The accuracy of neopterin measured in blood has been previously investigated for monitoring disease activity in various immune-mediated and chronic inflammatory disorders, such as transplant rejection, graft versus host disease, rheumatoid arthritis, psoriasis, systemic lupus erythematosus, cardiovascular disease and inflammatory bowel disease. It has been shown that serum neopterin levels were elevated in patients with GC in previously report.8 T helper 1(Th 1) predominant inflammatory response to H. pylori has been shown as a key factor of the transdifferentiation to GA and GIM in the previous report.9 The level of neopterin concentrations in serum or urine correlated with the degree of Th1-type immune response in humans.10
To date no studies have investigated whether serum neopterin plays a role in the progression of GA and GIM. Thus, the purpose of this study was the first to comparatively evaluate the changes and relationship between CRP and neopterin levels in patients with GA and GIM and non-atrophic and non-metaplastic chronic gastritis.
Patients and methodsA total of 236 prospective and consecutive patients, consistent with the study criteria, who applied to the Usak University, Gastroenterology polyclinic, during the period between December 2017 and July 2018 and have been included in the study after having obtained their consents. Patients were split into three groups as gastric intestinal metaplasia group (GIMG), gastric atrophy group (GAG) and control group (CG). Ninety-eight patients with GIM and sixty-eight patients with GA and CG consisted of seventy patients with nonatrophic gastritis who had no intestinal metaplasia were enrolled to the study. In this study, population characteristics were consist of sex, age, menopause, current/past smoking status, H. pylori infection and family history of GC. The exclusion criteria were as follows: 1, previous H. pylori eradication; 2, intake of antibiotics, nonsteroidal antiinflammatory drugs, statins, proton pump inhibitors, or H2-receptor blockers in the previous 2 months; 3, impaired renal function (serum creatinine=1.2mg/dl); 4, history or presence of cardiovascular disease; 5,diabetes mellitus; 6, immune-mediated and chronic inflammatory disorders; 7, active infection; 8, evidence of liver and renal disease; 9, chronic obstructive lung disease; 10, pulmonary hypertension; 11, malignancy and cytotoxic medicines; 12, gastric and duodenal ulcer; 13, gastrointestinal hemorrhage; 14, pregnancy and patients who refused the protocol's agreement.
Histopathologic evaluationMucosal samples of all patients were stained with hematoxylin and eosin. Histopathologic parameters were determined according to updated Sydney system.11 Hematoxylin–eosin-stained sections were evaluated for the presence of intestinal metaplasia, gastric atrophy, nonatrophic gastritis (with no intestinal metaplasia) and H. pylori by updated Sydney system.
Assessment of H. pylori infectionH. pylori infection was assessed by both histopathologic examination and local rapid urease test. Subjects were considered H. pylori positive to be positive for H. pylori infection if the bacteria were histopathologically detected and/or the local rapid urease test was positive.
Blood samples and biochemical analysesVenous blood samples were obtained from the patients within 12h of fasting. The blood samples were collected in tubes that contain clot activator. The blood samples were separated into serum or plasma within the hour by centrifugation at 4000rpm for 15min for analyzing CRP and neopterin. The serum CRP levels were analyzed by Abbott Diagnostics on the Abbott Architect c16000 analyzer (Abbott Park, Illinois, U.S.A.) with its original reagents (Archem Diagnostics CRP WR). Plasma neopterin levels were analyzed by enzyme-linked immunosorbent assay (ELISA) method as previously described by Werner et al.12 in an Chemwell Awareness Technology analyzer (Awareness Technology Inc, Palm City, USA) and SunRed Human Neop ELISA Kit (Shanghai Sunred Biological Technology Co., Ltd, China). N values were expressed as nmol/liter (nmol/l). The neopterin levels above 8.7nmol/l were considered high (adults, 95th percentile) according to previous report.12 The serum CRP level was defined as high when serum CRP levels were >5mg/l according to our laboratory reference values.
The present study was carried out according to the ethics guidelines of the Declaration of Helsinki on biomedical research involving human participants. All patients provided their written informed consent before being involved in the study. This study was performed in accordance with the approval of the ethics board of Usak University Faculty of Medicine dated February 12, 2018 Nr.002.
Statistical analysisData were examined by SPSS 23.0 software. Differences between groups were calculated by analysis of variance (ANOVA) test with post hoc Scheffe method. A correlation between the neopterin levels and variables was assessed by using the Pearson's or Spearman's tests according to the distribution pattern of the variables. Stepwise multiple logistic regression was used, considering a normal/high neopterin and CRP levels as the type of study groups including gastric intestinal metaplasia, gastric atrophy and non-atrophic non-metaplastic chronic gastritis p<0.05 was considered to be statistically significant. The Spearman's test and stepwise multiple logistic regression analysis were used for the relationship between normal/high neopterin and CRP levels and the H. pylori status of the histological study groups. The sensitivity and specificity of serum neopterin and CRP levels in predicting gastric intestinal metaplasia and/or gastric atrophy were calculated using an optimal cut-off point on the receiver operating characteristic (ROC) curve analysis with a 95% confidence interval.
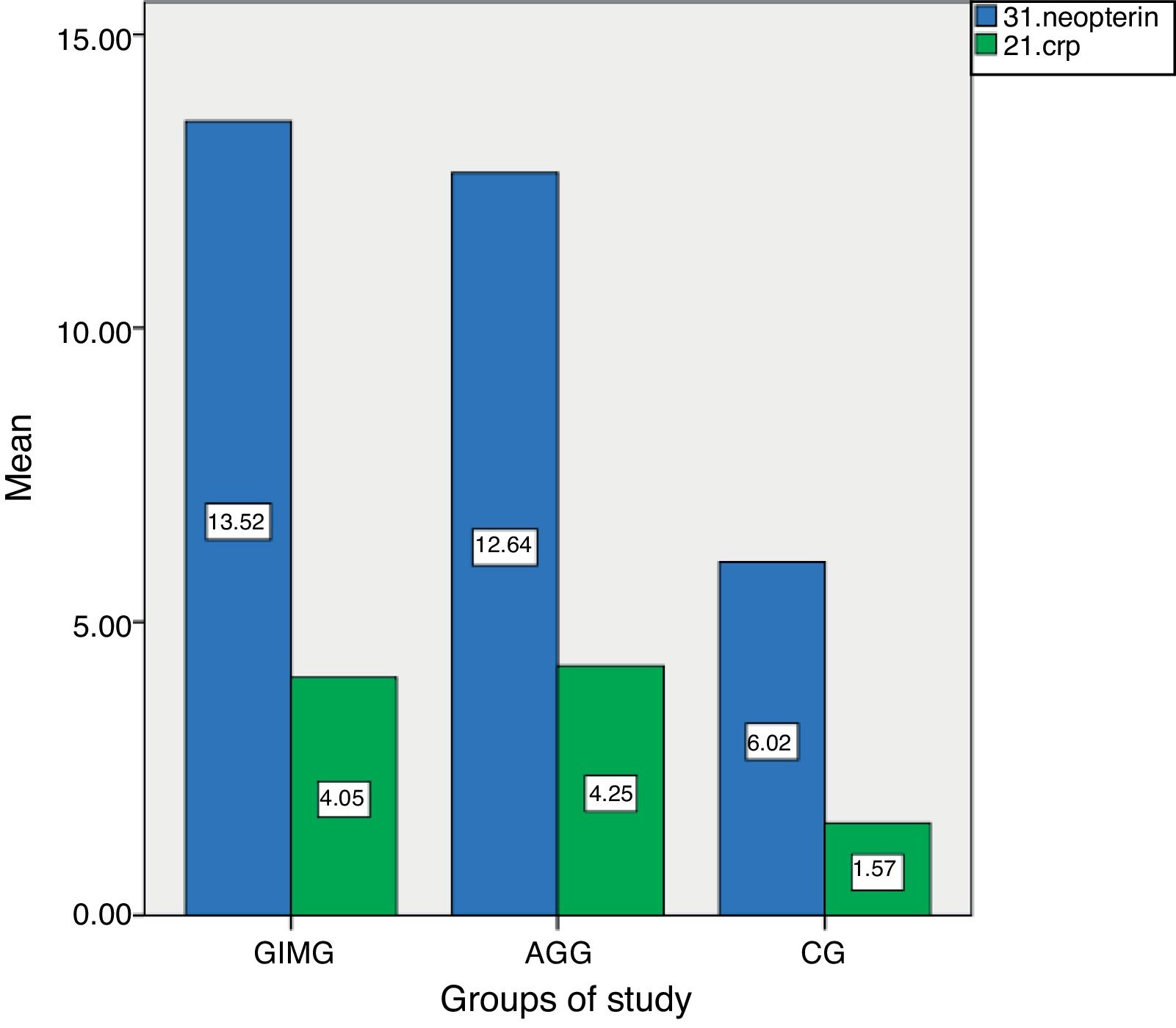
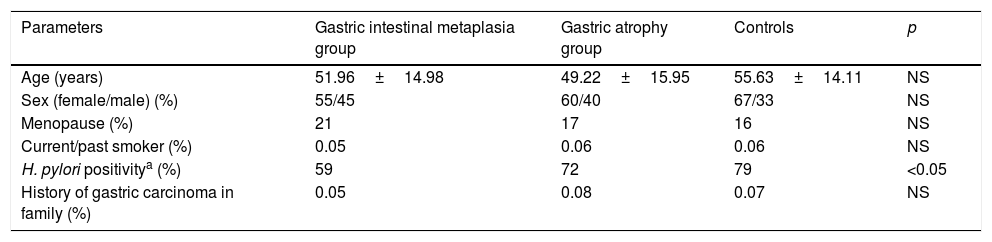
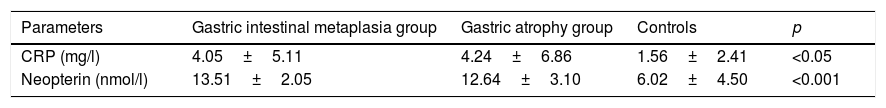
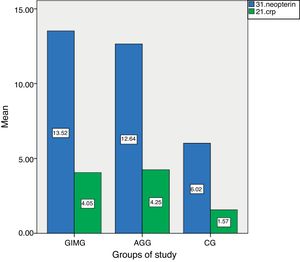
ResultsThe characteristics of the GIMG, GAG and CG are shown in Table 1. There were no significant differences in age, sex, menopause, smoking status, history of gastric carcinoma in family among three groups. But H. pylori positivity was significantly higher in CG (79%) than in GIMG (52%) (p=0.017). There was no significant difference in H. pylori positivity between CG (79%) and GAG (59%) groups (p=0.671). In this study, 62.5% (60/98) of cases with GIM were accompanied by GA. We had no patients with GC in this study. Serum CRP and neopterin levels of the groups are shown in Table 2. Serum CRP levels were significantly higher in GIMG and GAG than in controls (4.05±5.0mg/l, 4.24±6.86mg/l versus 1.56±2.41mg/l, p=0.007 and, p=0.008 respectively) (Table 2). There was no significant difference in serum CRP levels in GIMG and GAG groups (p=0.969). Plasma neopterin levels were significantly higher in GIMG and GAG than in controls (13.51±2.05nmol/l, 12.64±3.10nmol/l versus 6.02±4.50nmol/l, p<0.001 and, p<0.001 respectively) (Table 2). There was no significant difference in plasma neopterin levels in GIMG and GAG groups (p=0.216). Fig. 1 shows that neopterin, CRP the groups of study. In CG, 25.3% of the subjects had plasma neopterin level higher than 8.7nmol/l. This prevalence was 97.8% and 90.7% in the GIMG and GAG respectively. There were significant differences between the groups (p<0.001 and, p<0.001 respectively).
Population characteristics in controls and patients with gastric intestinal metaplasia and atrophic gastritis.
| Parameters | Gastric intestinal metaplasia group | Gastric atrophy group | Controls | p |
|---|---|---|---|---|
| Age (years) | 51.96±14.98 | 49.22±15.95 | 55.63±14.11 | NS |
| Sex (female/male) (%) | 55/45 | 60/40 | 67/33 | NS |
| Menopause (%) | 21 | 17 | 16 | NS |
| Current/past smoker (%) | 0.05 | 0.06 | 0.06 | NS |
| H. pylori positivitya (%) | 59 | 72 | 79 | <0.05 |
| History of gastric carcinoma in family (%) | 0.05 | 0.08 | 0.07 | NS |
C-reactive protein and neopterin levels in controls and patients with gastric intestinal metaplasia and atrophic gastritis.
| Parameters | Gastric intestinal metaplasia group | Gastric atrophy group | Controls | p |
|---|---|---|---|---|
| CRP (mg/l) | 4.05±5.11 | 4.24±6.86 | 1.56±2.41 | <0.05 |
| Neopterin (nmol/l) | 13.51±2.05 | 12.64±3.10 | 6.02±4.50 | <0.001 |
Results were presented as mean±SD. CRP; C-reactive protein. ANOVA test was used. p<0.05 was considered to be statistically significant.
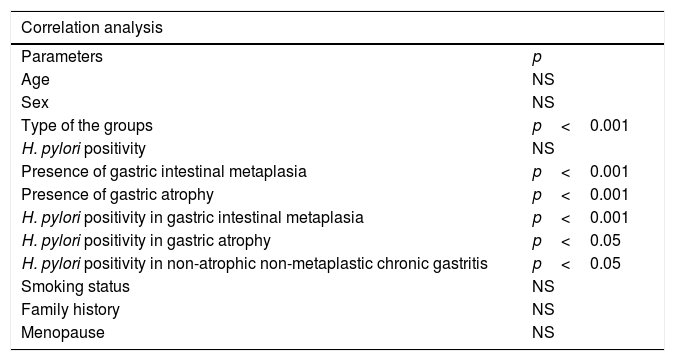
The associated parameters of high serum neopterin level are shown in Table 3. When Pearson's correlation analysis was used, high serum neopterin level was correlated positively with type of study groups (p<0.001), and presence of gastric intestinal metaplasia (p<0.001) and presence of gastric atrophy (p<0.001). According to Spearman's rank correlation analysis, CRP level was only correlated positively with type of study groups (p<0.001). According to multiple logistic regression analysis, the presence of gastric intestinal metaplasia (R2=0.615, β=4.91, p<0.001) and presence of gastric atrophy (R2=0.615, β=3.36, p<0.001) were both predictors of high level of serum neopterin.
The association of high serum neopterin levela to various parameters.
| Correlation analysis | |
|---|---|
| Parameters | p |
| Age | NS |
| Sex | NS |
| Type of the groups | p<0.001 |
| H. pylori positivity | NS |
| Presence of gastric intestinal metaplasia | p<0.001 |
| Presence of gastric atrophy | p<0.001 |
| H. pylori positivity in gastric intestinal metaplasia | p<0.001 |
| H. pylori positivity in gastric atrophy | p<0.05 |
| H. pylori positivity in non-atrophic non-metaplastic chronic gastritis | p<0.05 |
| Smoking status | NS |
| Family history | NS |
| Menopause | NS |
Pearson's or Spearman's tests were used according to the distribution pattern of the variables. p<0.05 was considered to be statistically significant.
When Spearman's rank correlation analysis was used, high serum neopterin and CRP level was correlated positively with H. pylori presence in GIMG (p<0.001 and p=0.045 respectively), H. pylori presence in GAG (p=0.017 and p=0.013 respectively) and H. pylori presence in CG (p<0.001 and p<0.001 respectively) (Table 3). According to multiple logistic regression analysis, the presence of H. pylori in GIMG (R2=0.506, β=18.51, p<0.001) and presence of H. pylori in CG (R2=0.506, β=4.91, p=.043) were both predictors of high level of serum neopterin. Among the groups, only the presence of H. pylori with gastric intestinal metaplasia was predictors of high level of serum CRP (R2=0.506, β=16.39, p=0.008).
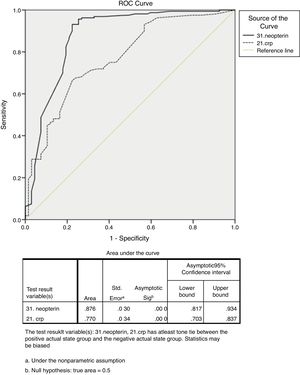
ROC curve analysis revealed that serum neopterin level ≥10.15nmol/l was (Fig. 2) the best cut-off value to differentiate between patients with GIM and/or GA from non-atrophic non-metaplastic chronic gastritis (sensitivity, 93.10%; specificity, 76.10%; +likelihood ratio [LR], 3.89; −LR, 0.09; 95% confidence interval [CI]: 0.81–0.93; standard error [SE], 0.030; p<0.001). The area under the ROC curve (AUC) was 0.876. ROC curve analysis revealed that serum CRP level ≥1.95mg/l was (Fig. 2) the best cut-off value to differentiate between patients with GIM and/or GA from non-atrophic non-metaplastic chronic gastritis (sensitivity, 66.30%; specificity, 77.60%; +LR, 2.95; −LR, 0.33; 95% CI: 0.70–0.83, SE, 0.034; p<0.001). The AUC was 0.770.
DiscussionTo the best of our knowledge, our study is the first one in literature in cases with GIM and GA that investigating the changes concerning with the levels of plasma neopterin and correlation between them. We also analyzed serum levels of CRP in this study. Our main finding was that serum neopterin level, increased significantly in patients with GIM and GA compared with the non-atrophic non-metaplastic chronic gastritis.
H. pylori is major cause of chronic gastritis. Duodenal ulcer, gastric ulcer and GC are well known disease associated with H. pylori. Conservative estimates suggest that more than 50% of the world's population is affected.1 GIM and GA are most often secondary to chronic long-standing Helicobacter pylori gastritis. The overall prevalence of GA among individuals with H. pylori infection was 82.9% compared with 9.8% among noninfected individuals; GIM was detected in 43.1% of H. pylori-positive persons compared with 6.2% of non-H. pylori-infected persons.13 The Pelayo Correa “cascade” describes a sequence of histological lesions leading to the possible development of GC, beginning with chronic gastritis and evolving into GA, GIM, and finally, cancer.14 GA and GIM are the histopathologic entities that reflect various phases during conversion of chronic gastric inflammation to carcinoma for whatever reason. It is not clear whether GIM almost always accompanies with GA.15 In our study, we determined presence of GA in 62.5% of the patients with GIM. Some studies have demonstrated the following as risk factors for developing GIM: an age of ≥61, the ingestion of spicy food, a history of smoking, male gender, a family history of gastric cancer, the consumption of dairy products, and dietary factors, such as excessive salt consumption, deficient ascorbic acid, and insufficient ingestion of carotene. However, among the risk factors, H. pylori is a major cause of the transdifferentiation into GIM and GA.16 Nevertheless, the molecular pathogenesis of this transdifferentiation has just begun to be understood. The nature of the host immune or inflammatory responses is also important in determining disease outcome after chronic H. pylori gastritis.17 Th1 immune response and IFN-γ released from Th1-cells might be important in the progression of GA and intestinal metaplastic formation in a concurrent infection model with Helicobacter felis and helminth. Hence, Th1 predominant inflammatory response to H. pylori may be a key factor of the transdifferentiation to GA and GIM.9 The nuclear factor kappa B (NF-κB) pathway, which regulates the expression of a wide variety of inflammatory cytokines, is another critical pathway involved in H. pylori induced gastritis and metaplasia.18,19 The increased neopterin concentration has been demonstrated in diseases, such as infections, autoimmune disorders, malignancy, and post-transplantation complications, in which cellular are known to play a role. Concentrations of neopterin, which is released in large amounts from human monocyte-derived macrophages upon stimulation with Th1-type cytokine IFN-γ, reflect cellular immune activation.10 The accumulation of neopterin secondary to IFN-γ-induced activation of macrophages reflects not only macrophage activation but also the activity of cellular immunity.8 There is a close relationship between increased neopterin production and activation of NF-κB.20 According to the results of our study, distinctive statistical significant highness in serum neopterin levels in cases with GA and GIM might be considered as clinical serological reflection (or indirect serological markers) of Th1, IFN-γ, NF-κB related immunoreaction in development of atrophy and intestinal metaplasia out of chronic gastritis. In our study, H. Pylori was detected to be lower in the GA and GIMG groups, comparing to the CG group. Especially in the GIMG group, this difference is statistically significant. This figure may be related with more long duration of gastric inflammation and “vanishing” phenomenon of the H. pylori infection. However, H. pylori presence in cases with GIM and GA strongly related with high CRP and neopterin values. This might be interpreted as an enhanced continuation of the chronic inflammatory process triggered by H. pylori.
In the recent study serum CRP levels were significantly higher in patients with GIM and GA than in controls. Few studies have shown that there was a correlation between inflammatory mediators and the presence of H. pylori infection. The possible association with high-sensitivity CRP and H. pylori has been observed before.21 There are also studies that any correlation determined between the levels of H. pylori and high-sensitivity CRP.22 Our observation causes to consider that severity of chronic inflammatory process (GIM and GA) which stems from H. pylori is related to the levels of CRP, other than H. pylori positivity.
Finally, not only GA but also GIM plays an important role in vitamin deficiency, inflammatory process and gastric carcinogenesis. Serum neopterin levels in GIM and GA rises significantly. It is promising that the sensitivity and specificity of ≥10.15nmol/l neopterin level as a diagnostic test is high in patients with GIM and or GA. Neopterin may be a useful biomarker for detecting of GIM and GA in the clinical setting. CRP levels may be helpful to this observation.
Conflict of interestWe have no any intervening grant or financial support or conflict of interest in this study.