Biological drugs targeting molecules involved in the immune response have become the basis of treatment for many patients with autoinflammatory diseases such as inflammatory bowel disease (IBD).
Drugs which block tumour necrosis factor alpha (TNF-α) are the most widely-used biological agents in the treatment of IBD. The paradoxical manifestation of induction or exacerbation of psoriasis has been widely reported as an adverse effect of anti-TNF-α drugs. However, there are fewer published cases of the opposite situation, the onset of IBD as an adverse effect of the biological therapy used for other diseases.
Secukinumab is an anti-interleukin-17A monoclonal antibody used in the treatment of psoriasis, psoriatic arthritis and ankylosing spondylitis. Previous studies have shown it to be ineffective at treating Crohn’s disease (CD),1 although there are no studies with ulcerative colitis (UC). The risk of underlying IBD being exacerbated in patients receiving this drug is open to debate.
We present the case of a 52-year-old man diagnosed with psoriasis, with no other relevant history, who was on treatment with secukinumab for two weeks.
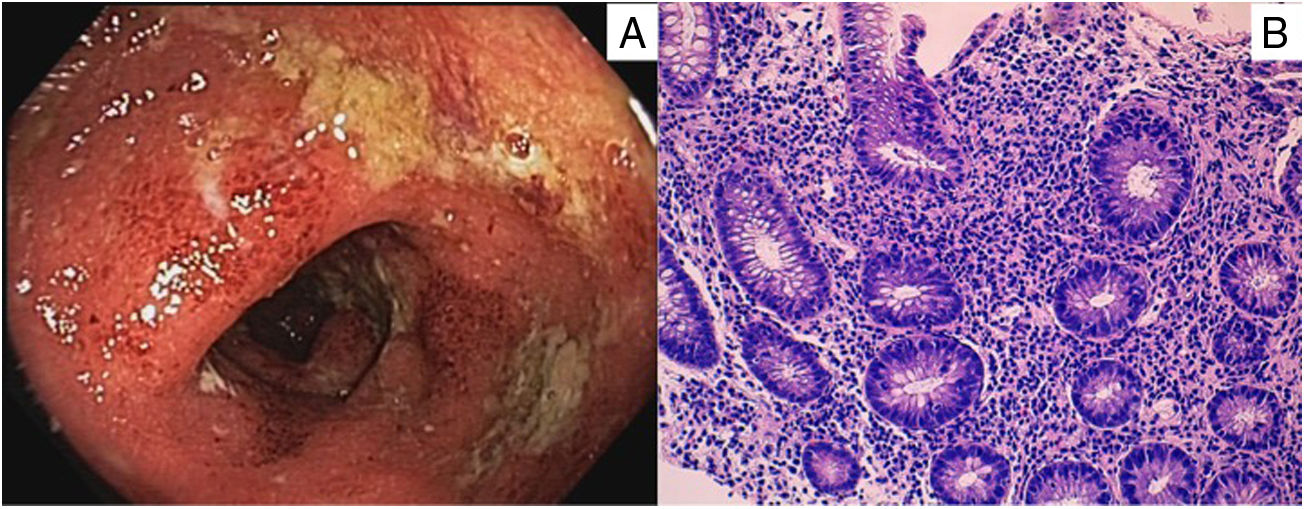
He was admitted to the Gastroenterology Unit with a five-day history of diarrhoea, with approximately 20–30 bowel movements a day, accompanied by colicky abdominal pain. In the last few days, blood was visible in the stools, he had pyrexia of up to 38.5°C and his general condition had worsened. Blood tests showed that the patient had elevated acute phase reactants, with a CRP of 194.6 mg/l (normal <5 mg/l). Stool culture and C. difficile toxin were both negative. Proctoscopy three days after admission showed oedematous and erythematous mucosa, with loss of the vascular pattern and purulent exudate, as well as several superficial, fibrin-bearing sigmoid ulcers (Fig. 1A). Biopsies showed a diffuse increase in mononuclear inflammatory infiltrate in the lamina propria of the rectal mucosa, with cryptitis and microabscesses and crypt architectural distortion, respecting the muscularis mucosae, histopathological findings compatible with UC-type IBD with moderate activity (Fig. 1B).
A) Oedematous and erythematous sigmoid mucosa, with loss of vascular pattern, purulent exudate and fibrin-covered superficial ulcers.
B) Diffuse increase in mononuclear inflammatory infiltrate in the lamina propria of the rectal mucosa, with cryptitis and microabscesses and crypt architectural distortion, respecting the muscularis mucosae.
Treatment was started with systemic corticosteroids, prednisone at a dose of 60 mg in a descending regimen, plus mesalazine, both topical (1 × 500-mg suppository every 12 h) and oral (3200 mg/24 h), with gradual remission of symptoms and disappearance of the lesions in the follow-up colonoscopy three months later. The patient was put on maintenance therapy with 5-ASA, but this was stopped by his dermatologist four months after diagnosis when starting therapy with adalimumab to treat the psoriasis. After 24 months of follow-up, he is in remission of both his skin and gastrointestinal diseases.
IL-17 is a pro-inflammatory cytokine produced by T helper 17 (Th17) cells which has been linked to various chronic inflammatory diseases. The intestines of IBD patients have been shown to have high infiltration of Th17 lymphocytes, which produce large amounts of IL-17A. However, the role of IL-17A in the pathogenesis of IBD is subject to debate; while it has been linked to intestinal inflammation in some studies, in others it has been assigned a protective role, with evidence that its inhibition has led to an exacerbation of IBD.2
A study was published in 2012 which investigated the utility of secukinumab in Crohn’s disease, showing little effect and an increase in adverse reactions compared to placebo.1 However, very few studies have reported the onset or exacerbation of Crohn’s disease in patients taking secukinumab, with even fewer studies reporting UC as a side effect.3 In a review published in 2019, which included 21 clinical trials with patients on secukinumab for ankylosing spondylitis, psoriasis and psoriatic arthritis, new cases of IBD were observed only rarely (<1%), but there was a 22% exacerbation rate in patients with a previous history of IBD.4
A case of secukinumab-induced ulcerative colitis was published recently5, although, unlike our case, in which there were no gastrointestinal symptoms until after starting the drug, in this case the patient had previous intestinal symptoms compatible with IBD. It should be noted that recent publications have reported worsening of IBD and the development of new-onset IBD after treatment with other IL-17A inhibitor drugs, such as ixekizumab6 and brodalumab2.
In summary, we present the onset of UC as a probable adverse effect of an IL-17A inhibitor, which supports the hypothesis of the protective effect of this interleukin in the intestine. We recommend intensive monitoring of gastrointestinal symptoms in patients receiving this drug, in order to avoid delayed diagnosis and treatment of possible IBD.
FundingNo funding was received for this work.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Fernández-de la Varga M, del Pozo-del Valle P, Béjar-Serrano S, Garrido-Marín A, Bastida Paz G. Colitis ulcerosa inducida por secukinumab: abriendo la caja de Pandora de la inmunidad. Gastroenterol Hepatol. 2020;43:358–359.