to evaluate the efficacy and safety of endoscopic vacuum therapy (EVT) in the management of perforations and anastomotic leaks of the upper gastrointestinal tract.
Patients and methodsThis is a retrospective observational study which included patients who underwent EVT due to any upper gastrointestinal defect between April 2017 and February 2019 in three Spanish Hospitals. To this end, we used the only medical device approved to date for endoscopic use (Eso-SPONGEr; B. Braun Melsungen AG, Melsungen, Germany).
Results11 patients were referred for EVT of an anastomotic leak after esophagectomy (n = 7), gastrectomy (n = 2), esophageal perforation secondary to endoscopic Zenker´s septomiotomy (n = 1) and Boerhaave syndrome (n = 1). The median size of the cavity was 8 × 3 cm. The median delay between surgery and EVT was 7 days. The median of EVT duration was 28 days. The median number of sponges used was 7 and the mean period replacement was 3.7 days. In 10 cases (91%), the defect was successfully closed. In 9 cases (82%) clinical resolution of the septic condition was achieved. 5 patients presented some adverse event: 3 anastomotic strictures, 1 retropharyngeal pain and 1 case of new-onset pneumonia. The median hospital stay from the start of EVT was 45 days. 1 patient died owing to septic complications secondary to the anastomotic leak.
ConclusionEVT was successful in over 90% of perforations and anastomotic leaks of the upper gastrointestinal tract. Moreover, this is a safe therapy with only mild adverse events associated.
evaluar la eficacia y seguridad de la terapia de vacío endoscópica (TVE) en perforaciones y dehiscencias del tracto digestivo superior.
Pacientes y métodosEstudio retrospectivo observacional donde se incluyeron todos los pacientes que presentaron algún defecto del tracto gastrointestinal superior y que fueron sometidos a TVE en el período comprendido entre abril de 2017 y febrero de 2019 en tres centros españoles. Para ello se utilizó el único sistema de terapia de vacío aprobado para uso endoscópico hasta la fecha (Eso-SPONGEr; B. Braun Melsungen AG, Melsungen, Germany).
ResultadosUn total de 11 pacientes fueron sometidos a TVE como tratamiento de una dehiscencia de sutura anastomótica tras esofaguectomía (n = 7), gastrectomía (n = 2), como tratamiento de una perforación esofágica tras septomiotomía endoscópica de Zenker (n = 1) y tras una perforación esofágica espontánea o síndrome de Boerhaave (n = 1). La mediana del tamaño de la cavidad fue de 8 × 3 cm. De mediana, la TVE se inició a los 7 días de la cirugía inicial. La mediana de duración de la TVE fue 28 días. La mediana de esponjas colocadas fue de 7 y el intervalo de recambio de las esponjas de 3,7 días. En 10 casos (91 %) se consiguió el cierre del defecto y en 9 casos (82%) la resolución clínica del cuadro infeccioso asociado. 5 pacientes presentaron algún evento adverso: 3 estenosis anastomóticas, 1 episodio de dolor retrofaríngeo y 1 caso de neumonía de nueva aparición. La mediana de estancia hospitalaria desde el inicio de la TVE fue de 45 días. 1 paciente falleció debido a complicaciones sépticas secundarias a la dehiscencia de sutura.
ConclusiónLa TVE tiene una eficacia superior al 90% en las perforaciones y fugas anastomóticas del tracto digestivo superior. Adicionalmente es una terapia segura, siendo los eventos adversos leves.
Oesophageal perforation and surgical wound dehiscence following oesophageal or gastric surgery are complications associated with higher rates of morbidity and mortality due to the associated onset of mediastinitis/peritonitis sepsis.1–6
Endoscopic vacuum therapy (EVT) is a novel treatment for closing upper gastrointestinal tract defects that is proving highly effective with a low rate of adverse events.7 We report the first experience at 3 Spanish centres with the use of EVT in patients with surgical wound dehiscence following oesophageal/gastric surgery or oesophageal perforation.
Material and methodsAll patients who presented any upper gastrointestinal tract defect and were referred for EVT between April 2017 and February 2019 at 3 Spanish centres — Hospital Clínic de Barcelona [Clinical Hospital of Barcelona], Hospital Universitari MútuaTerrassa [MútuaTerrassa University Hospital] and Clínica Teknon de Barcelona [Teknon Clinic of Barcelona] — were enrolled.
Defects were confirmed by a gastroscopy and a computed tomography (CT) scan with administration of oral contrast. If the patient presented a concomitant pleural effusion, mediastinal abscess or pneumothorax, a pleural drain was placed as part of the patient’s treatment.
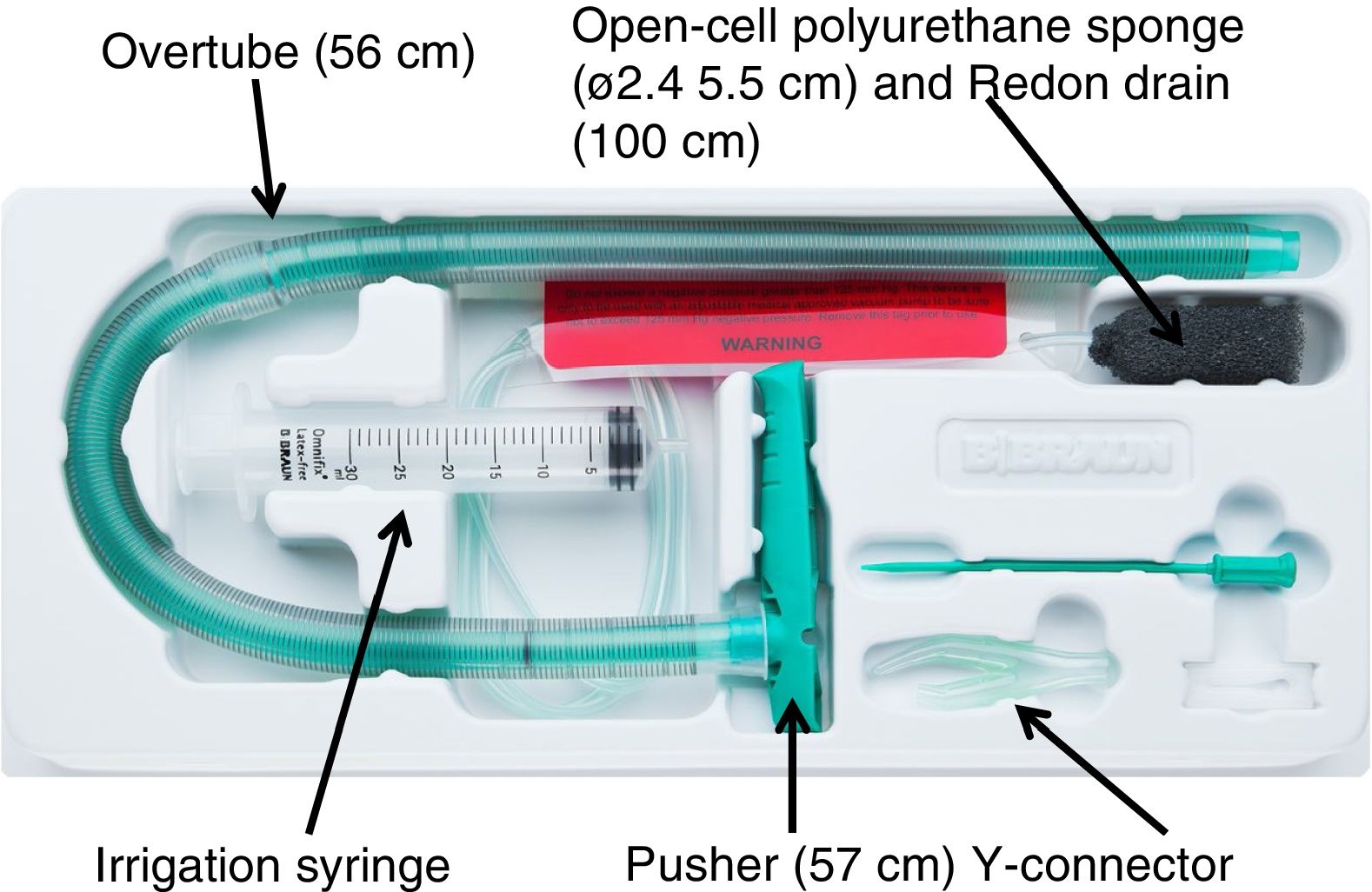
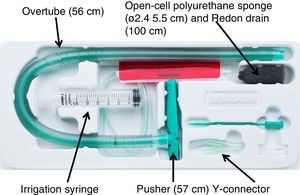
Vacuum therapy was performed using the only vacuum therapy system approved for endoscopic use to date (Eso-SPONGEr; B. Braun Melsungen AG, Melsungen, Germany). This system consists of an open-cell polyurethane sponge, measuring 5.5 cm in length by 2.4 cm in diameter, inserted in the end of a Redon drain, measuring 12 Ch and 100 cm in length. To facilitate endoscopic placement of the sponge, a silicone overtube with a length of 56 cm and an inner diameter of 13−15 mm, and a pusher measuring 57 cm in length were used (Fig. 1).
The procedure was performed under deep sedation or general anaesthesia in patients who were receiving mechanical ventilation. A conventional gastroscope and insufflation with CO2 were used whenever possible. In the initial endoscopy, the defect was identified and the distance to the dental arch, the percentage of the oesophageal or anastomotic circumference affected, and the size of the cavity were measured. Once the initial endoscopic assessment had been performed, the gastroscope was removed and inserted through the overtube outside the patient. The gastroscope was reinserted towards the cavity, using it as a guide to slide the overtube by sight inside the cavity (intracavitary therapy), or beside it in the gastrointestinal tract lumen (intraluminal therapy). In some cases in which the opening of the dehiscence was small in size, a decision was made to perform balloon dilation of up to 12−15 mm in order to be able to perform an intracavitary treatment. Once the overtube had been placed, the endoscope was removed and the sponge was advanced inside the overtube using the pusher until it reached the chosen site. Subsequently, the overtube and the pusher were jointly removed and the suitable placement of the sponge was confirmed endoscopically. Following this, a tube was inserted into the patient’s nose to make an exchange and end up removing the Redon drain of the sponge through the nose. Next, the drain was connected to an electronic vacuum pump at a constant negative pressure of 100−125 mmHg. If any of the cavities drained by EVT had any external drain inside, this was then removed. The sponges were changed by endoscopy with sedation every 2–5 days, to prevent adhesion of the tissue to the sponges which would make the change difficult. To do so, the drain for the continuous suction pump was disconnected and the sponge was irrigated with 10–20 cm3 of normal saline through the Redon drain, then carefully removed with gentle traction. In cases in which resistance to sponge removal was detected, endoscopic forceps were used to detach it from the tissue to which it was adhered and facilitate its removal. When the cavity appeared clean and was covered with granulation tissue, the sponge size was gradually reduced by manual trimming. Once the cavity had an approximate size of around 2 × 1 cm, EVT was stopped (Fig. 2). In some cases, a decision was made to use an adjuvant endoscopic therapy once EVT had concluded to aid in the complete closure of the cavity. Resolution of dehiscence or perforation was defined as macroscopic re-establishment of the mucosa along with the absence of granulation tissue. In all cases, an endoscopy and a CT scan with oral contrast or an oesophageal–gastroduodenal transit study was performed in the first week following the removal of the final sponge to verify the closure of the leak. After the closure of the defect was confirmed with these tests, oral intake was started. Patients were followed up on an outpatient basis after they were discharged from hospital.
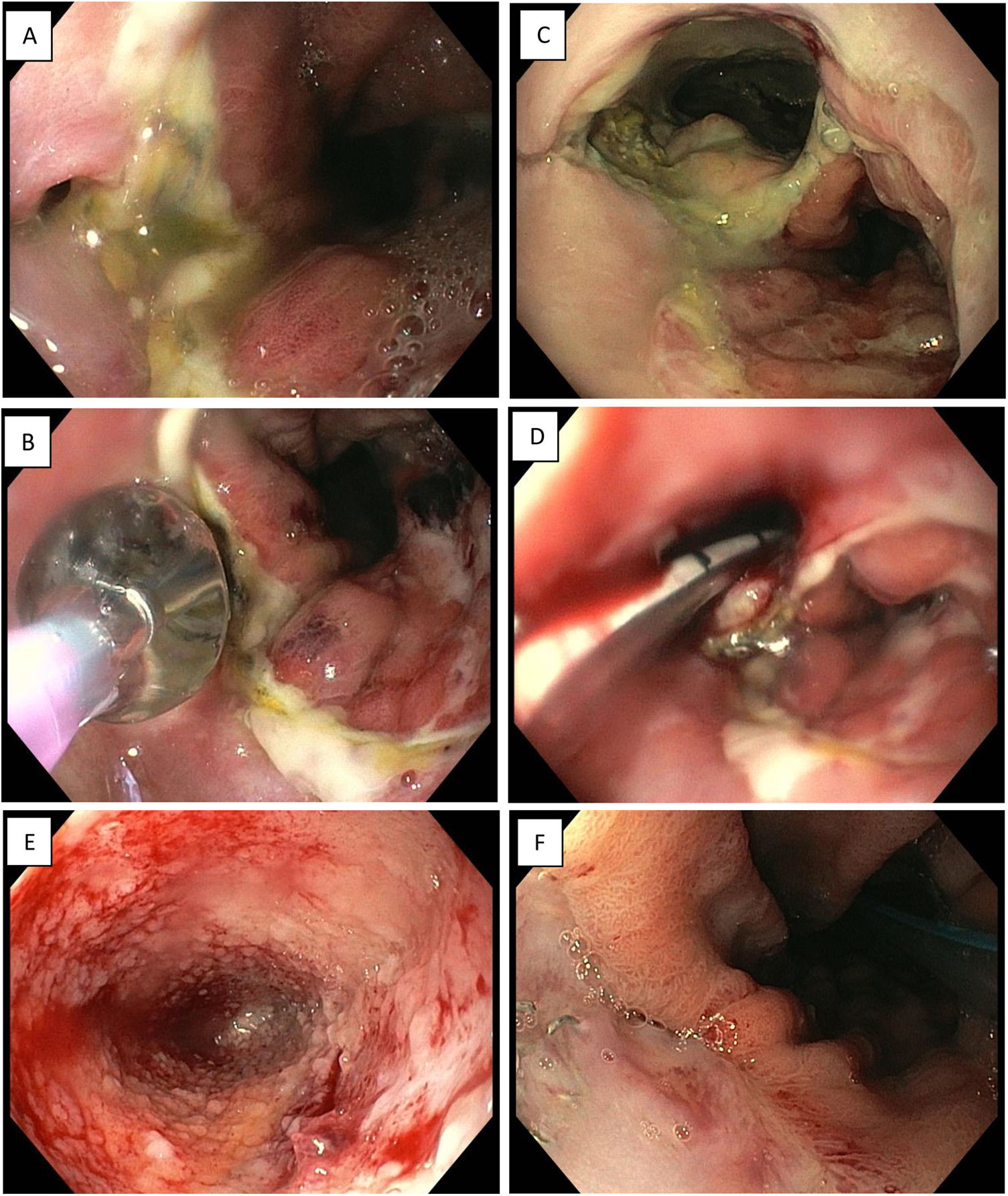
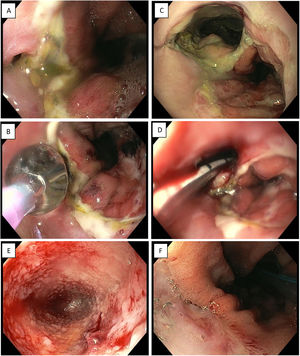
Endoscopic vacuum therapy in anastomotic surgical wound dehiscence following Ivor Lewis surgery. A) Small anastomotic defect. B) Balloon dilation of the opening of the dehiscence. C) Access to infected cavity. D) Insertion of Eso-SPONGE. E) Cleaning of the cavity and formation of granulation tissue (image following 3rd sponge change). F) Resolution of anastomotic dehiscence (after 6 sponges).
The data are expressed in terms of count (percentage) for categorical variables. Continuous variables are expressed in terms of mean ± standard deviation where they have a normal distribution, or median (range, minimum-maximum) where they do not. The software program used was the SPSS Statistical Package 2020.
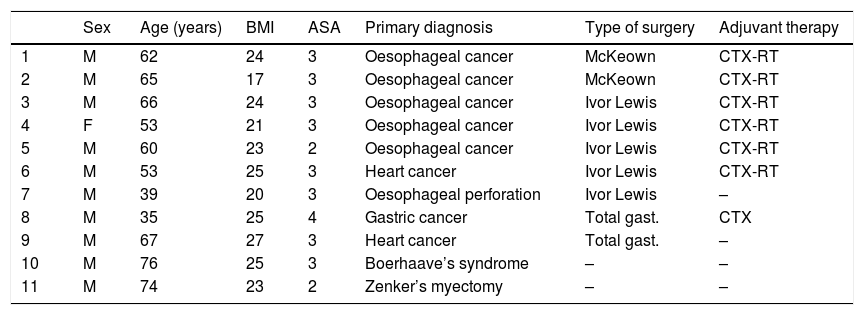
ResultsBetween April 2017 and February 2019, 11 patients (10 males and one female) with a mean age ± standard deviation of 59 ± 13 years underwent EVT to treat anastomotic surgical wound dehiscence following oesophagectomy (n = 7), gastrectomy (n = 2), to treat oesophageal perforation following endoscopic septal myectomy due to a Zenker’s diverticulum (n = 1) and following spontaneous oesophageal perforation or Boerhaave’s syndrome (n = 1). Seven of the patients had received neoadjuvant treatment with chemotherapy or radiochemotherapy prior to surgical resection. Individual patient characteristics are shown in Table 1.
Characteristics of the patients enrolled in the study.
| Sex | Age (years) | BMI | ASA | Primary diagnosis | Type of surgery | Adjuvant therapy | |
|---|---|---|---|---|---|---|---|
| 1 | M | 62 | 24 | 3 | Oesophageal cancer | McKeown | CTX-RT |
| 2 | M | 65 | 17 | 3 | Oesophageal cancer | McKeown | CTX-RT |
| 3 | M | 66 | 24 | 3 | Oesophageal cancer | Ivor Lewis | CTX-RT |
| 4 | F | 53 | 21 | 3 | Oesophageal cancer | Ivor Lewis | CTX-RT |
| 5 | M | 60 | 23 | 2 | Oesophageal cancer | Ivor Lewis | CTX-RT |
| 6 | M | 53 | 25 | 3 | Heart cancer | Ivor Lewis | CTX-RT |
| 7 | M | 39 | 20 | 3 | Oesophageal perforation | Ivor Lewis | – |
| 8 | M | 35 | 25 | 4 | Gastric cancer | Total gast. | CTX |
| 9 | M | 67 | 27 | 3 | Heart cancer | Total gast. | – |
| 10 | M | 76 | 25 | 3 | Boerhaave’s syndrome | – | – |
| 11 | M | 74 | 23 | 2 | Zenker’s myectomy | – | – |
ASA: anaesthesia risk classification; BMI: body mass index; CTX: chemotherapy; F: female; gast.: gastrectomy; M: male; RT: radiotherapy.
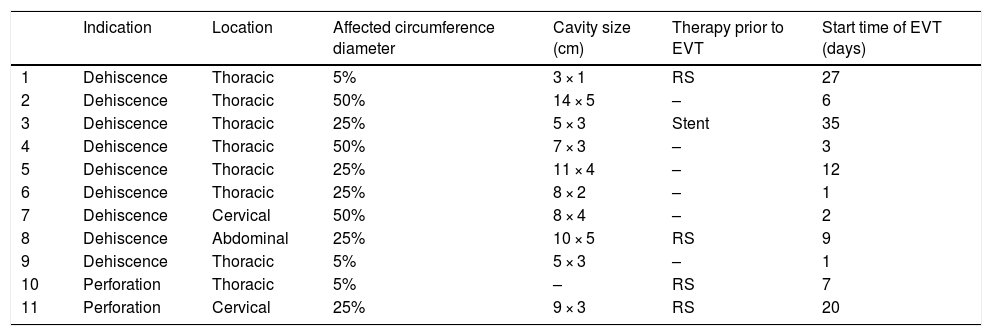
Anastomotic dehiscence was diagnosed a median (minimum/maximum) of 5 (1–28) days following surgery. Four (36%) patients underwent repeat surgery and one other patient had an oesophageal stent placed before undergoing EVT due to a lack of efficacy of these treatments. The characteristics of the defects and the endoscopic findings are shown in Table 2.
Type of defect and endoscopic findings.
| Indication | Location | Affected circumference diameter | Cavity size (cm) | Therapy prior to EVT | Start time of EVT (days) | |
|---|---|---|---|---|---|---|
| 1 | Dehiscence | Thoracic | 5% | 3 × 1 | RS | 27 |
| 2 | Dehiscence | Thoracic | 50% | 14 × 5 | – | 6 |
| 3 | Dehiscence | Thoracic | 25% | 5 × 3 | Stent | 35 |
| 4 | Dehiscence | Thoracic | 50% | 7 × 3 | – | 3 |
| 5 | Dehiscence | Thoracic | 25% | 11 × 4 | – | 12 |
| 6 | Dehiscence | Thoracic | 25% | 8 × 2 | – | 1 |
| 7 | Dehiscence | Cervical | 50% | 8 × 4 | – | 2 |
| 8 | Dehiscence | Abdominal | 25% | 10 × 5 | RS | 9 |
| 9 | Dehiscence | Thoracic | 5% | 5 × 3 | – | 1 |
| 10 | Perforation | Thoracic | 5% | – | RS | 7 |
| 11 | Perforation | Cervical | 25% | 9 × 3 | RS | 20 |
EVT: endoscopic vacuum therapy; RS: repeat surgery.
EVT was started a median of 7 (1–35) days from the initial surgery, 20 (7–35) days in cases in which a treatment prior to EVT was performed, and 2 (1–12) days in cases in which EVT was the initial therapy. In 10 cases, intracavitary therapy was performed, and in one case, intraluminal therapy was performed. The median number of sponges placed was 7 (1–14), and the period of time elapsed between sponge changes was 3.7 (2–5) days. The median duration of EVT was 28 (5–48) days for surgical wound dehiscence, 5 days for Boerhaave’s syndrome and 26 days for perforation following endoscopic septal myectomy for a Zenker’s diverticulum (Table 3).
Characteristics of EVT, efficacy and adverse events.
| Type of EVT | No. of sponges | Duration of EVT (days) | Hospital stay (days) | Closure of defect | Clinical success | Adverse events | |
|---|---|---|---|---|---|---|---|
| 1 | IC | 2 | 12 | 59 | Yes | Yes | – |
| 2 | IC | 7 | 34 | 90 | Yes | Yes | Stenosis |
| 3 | IC | 13 | 46 | 49 | Yes | No | – |
| 4 | IC | 6 | 30 | 41 | Yes | Yes | Stenosis |
| 5 | IC | 9 | 29 | 31 | Yes | Yes | – |
| 6 | IC | 6 | 20 | 34 | Yes | Yes | – |
| 7 | IC | 14 | 35 | 45 | No | No | Stenosis |
| 8 | IC | 14 | 49 | 75 | Yes | Yes | – |
| 9 | IC | 3 | 10 | 26 | Yes | Yes | Empyema |
| 10 | IL | 1 | 5 | 10 | Yes | Yes | – |
| 11 | IC | 7 | 26 | 54 | Yes | Yes | Pain |
EVT: endoscopic vacuum therapy; IC: intracavitary; IL: intraluminal.
Of the 11 patients, 3 received parenteral nutrition, 7 received nutrition through a jejunostomy and one received nutrition through an enteral feeding tube. Four patients had to be admitted to the intensive care unit (ICU) during treatment, and 3 of them required mechanical ventilation. Nine (81%) patients required placement of a pleural drain as an additional treatment for empyema.
The median hospital stay from the start of the EVT was 45 (10–90) days for all patients taken together. Broken down, the mean hospital stay was 45 (26–90) days in cases of anastomotic dehiscence, 10 days in the case of Boerhaave’s syndrome, and 54 days in the case of perforation following septal myectomy for a Zenker’s diverticulum (Table 3).
In 10 (91%) cases, closure of the defect was achieved; in 7 (63%) cases, it was achieved following treatment with EVT alone; and in 2 (18%) cases, it was achieved by adding a treatment to the end of the EVT. In these 2 patients, once EVT treatment had been performed and the cavity showed a clean appearance and granulation tissue but remained quite large with a small opening that precluded intracavitary sponge placement, a decision was made to place a double-pigtail plastic stent along with an OVESCO clip and a nasogastric tube, respectively, to maintain internal drainage until the cavity closed completely.
In one (9%) patient, EVT did not achieve resolution of the dehiscence. This patient underwent intracavitary therapy with the placement of a total of 14 sponges over a period of 35 days, with near-complete resolution of the dehiscence; there was a residual cavity measuring around 2 × 1 cm which followed a good clinical course. However, a week after the patient’s EVT concluded, an endoscopy revealed growth of the cavity and a passage with a small-calibre leak. In this patient, closure of the leak was achieved following placement of 2 consecutive oesophageal stents.
ComplicationsFive (45%) patients had some sort of adverse event, and none had a serious or fatal adverse event; 3 (27%) patients had anastomotic stenosis a median of 116 (50–207) days after having finished EVT. In 2 cases stenosis was resolved following a single dilation session; the third patient is currently on a regular dilation regimen. The patient with septal myectomy for a Zenker’s diverticulum presented moderate retropharyngeal pain during treatment which resolved with analgesics and was probably linked to the perforation itself. Another patient presented pneumonia and pleural empyema 10 days after starting EVT, which was managed with antibiotics and placement of a pleural drain (Table 3).
Hospital mortality was 17% (n = 2). One patient presented resolution of the cavity following placement of 13 sponges in the course of 46 days, confirmed by gastroscopy and CT with oral contrast. However, this patient followed a poor clinical course and died 48 h from the removal of the final sponge as a result of septic complications (pneumonia and respiratory failure) related to surgical wound dehiscence. This case also showed local progression of the neoplasm diagnosed by endoscopic biopsy. Another patient with a medical history of heart disease died as a result of an acute cardiac ischaemia event 9 days after having finished EVT. The autopsy identified a complete closure of the oesophageal surgical wound dehiscence with no local or systemic inflammatory signs.
In long-term follow-up, 2 patients died due to progression of their neoplastic disease 156 and 345 days after being discharged, respectively. The mean overall follow-up period for the sample was 87 days (3–455 days).
DiscussionOesophageal perforations, whether spontaneous of iatrogenic, are linked to a high mortality rate, which may be up to 20%.1 The incidence of anastomotic leaks following oesophageal or gastric surgery ranges from 3% to 30% and 3% to 11%, respectively,2–4 and is associated with a high postoperative mortality rate — up to 50%, depending on the series.5,6 The treatment thereof remains a challenge, considering that repeat surgery is associated with high rates of morbidity and mortality (20%–30%).5,6 With the advancement of digestive endoscopy, multiple less invasive endoscopic treatments have been used to treat these patients. Endoscopic clips used through the working channel (TTS) are useful for closing acute perforations of a small size, whereas clips premounted on a tip (OVESCO clips) are used in perforations with everted margins and in anastomotic leaks of a small size. Endoscopic suture systems are technically complex and have only been used in selected cases.7 Placement of fully or partially covered metal stents is the endoscopic therapy with the greatest efficacy and applicability for the closure of anastomotic defects of the upper gastrointestinal tract, and endoscopic treatment is considered to be the treatment of choice in these patients. However, the efficacy of stents remains limited, with a rate of closure of the anastomotic leak ranging from 44% to 80%.8,9 In addition, they are associated with a non-negligible rate of adverse events (15%-50%) such as migration, stenosis, bleeding and, more rarely, perforation secondary to necrosis induced by the stent itself.8–10
Successful EVT for the closure of defects of the upper gastrointestinal tract was first reported in 2008.11 It consists of endoscopic placement of a sponge inserted in the end of a multiperforated drain, which in turn is connected to a negative-pressure pump with continuous suction. EVT offers as a primary theoretical advantage over endoscopic stents the fact that it not only "seals" the cavity but also drains it, such that it manages to clean the infection, stimulate the development of granulation tissue and close the gastrointestinal defect by secondary intention. Another advantage it has over stents is that it can be used in defects located in the cervical oesophagus. Furthermore, it does not require the use of fluoroscopy, making it more accessible to patients on an intensive care unit. A recent review12 that included all observational studies with more than 5 patients found an overall efficacy rate of 87% (60%-100%) in a total of 422 patients having undergone EVT for upper gastrointestinal tract defects. Most patients presented surgical wound dehiscence or oesophageal perforation, whereas a small proportion received EVT to treat perforation or surgical wound dehiscence located in the stomach or duodenum. Also, recently it has been used successfully as a preventive measure in cases in which the anastomosis features at least one risk factor, such as ischaemia, or even to promote proper healing during surgery.13
In our study, we achieved closure of the defect in 10 of the 11 patients enrolled. One of these 10 patients died 48 h after finishing EVT as a result of septic complications related to surgical wound dehiscence. Therefore, although closure of the defect was achieved, it can be concluded that clinical success was achieved in 9 of the 11 cases enrolled (82%); this would be consistent with the published data. It should be noted that in 2 cases internal drainage was performed by placement of a nasogastric tube and a double-pigtail plastic stent along with an OVESCO clip following completion of EVT. Some studies have reported the use of clips or placement of internal drains as a first-line treatment for closure of defects or as a complementary treatment following completion of treatment with EVT.14–16 The usefulness of adding these therapies once the cavity has been completely drained and the formation of granulation tissue has been achieved is not well-established, and in most cases closure of the cavity by secondary intention is achieved, regardless of cavity size, by EVT alone, with no need to add other endoscopic therapies.
In our study, all patients but one received intracavitary therapy. In 2 of the 3 patients with small openings, a decision was made to dilate the defect with a balloon in order to then perform intracavitary therapy; in the other patient, intraluminal therapy was performed. The theoretical benefit of intracavitary therapy would be better drainage of the collection and improved stimulation of the formation of granulation tissue, such that it would be especially indicated in cases in which an infected extraluminal cavity is seen. In any case, there are not enough data to affirm that intracavitary therapy is superior to intraluminal therapy.11,12,17 It should be noted that in many of the published cases in which intraluminal therapy was performed, self-made sponges measuring up to 12 cm in length were used. This could lead to greater ease of application and greater efficacy in intraluminal therapy than through the use of Eso-SPONGEr (B-Braun) which has a smaller length (5.5 cm). A recently published study that enrolled a total of 16 patients reported the use of new thinner open-cell drainage materials of a small calibre that could facilitate EVT by being put through small openings with no need to dilate the opening.18
The median duration of EVT in our study was 28 days; this was slightly higher than that seen in the literature (i.e. 17 days) but was within the published ranges (11–36 days).12,17 As in all techniques, there is a learning curve that could account for this fact. One factor linked to greater efficacy of EVT is an early start to therapy.7,19 This was also seen in our study, where the mean delay in application of EVT in patients in whom the therapy was not successful was 18 days, whereas in patients in whom closure was achieved it was 9 days.
The published overall rate of adverse events in relation to EVT is 0%-14%.16,20–25 Of all adverse events, the most common is stenosis, with a mean frequency of 7.6% (3-14%).16,21,25 The causality relationship of treatment with EVT and onset of stenosis is not well-established, as it has also been reported in other patients who have developed anastomotic dehiscence resolved by means of surgery or other endoscopic techniques.21 In our study, 3 (27%) patients presented anastomotic stenosis. Interestingly, these 3 patients were the ones who presented a higher percentage of impairment (50%) of the anastomotic circumference. Two of the cases were resolved following a single dilation session, and the other presented refractory stenosis. The latter patient was the one who did not respond to EVT and the one in whom the leak was resolved following placement of 2 stents, despite the theoretical preventive effect of stents on the onset of stenosis. Therefore, it seems quite likely that there is an individual component and other poorly understood factors that may predispose patients with an anastomotic leak to developing this complication beyond the endoscopic therapy used.
To date, an overall rate of severe and/or fatal bleeding of around 1% secondary to treatment with EVT has been published.16,24,26 This would occur due to the local inflammatory reaction of the sponge which would induce erosion and eventual rupture of the wall of a vascular structure of a small or even a large calibre, such as the aorta or the heart. None of our 11 patients presented any episode of bleeding in the total of 82 sponges placed. Although there is no completely safe way to prevent this complication, some authors have recommended performing a CT scan after placing the first sponge to ensure that the end of the sponge is not close to or adjacent to any mediastinal vascular structure. In addition, EVT is considered to be contraindicated in patients with clotting abnormalities, patients with severe thrombocytopenia and patients being treated with anticoagulants and/or antiplatelets. In this regard, the recent appearance of new thinner open-cell drainage materials of a small calibre18 could decrease adverse events, such as bleeding secondary to damage to neighbouring vascular structures, though studies will need to be conducted to verify this hypothesis.
There are insufficient data as to how these patients are to be nourished during EVT. It seems reasonable to think that enteral nutrition via a nasal feeding tube should be the method of choice. However, it should be noted that this may increase the discomfort caused by the EVT tube itself, and therefore many of the published cases, as in the patients in our series, were nourished through a jejunostomy or parenteral nutrition. Some authors incorporate an enteral feeding tube into the sponge tube or use 3-lumen tubes to facilitate nutrition.27,28 It is also unknown whether oral nutrition would be possible in these patients as EVT could prevent saliva and food from getting inside cavity; therefore, this specific matter should be evaluated in future studies.
To date, just 4 studies15,20,22,23 enrolling a total of 163 patients (71 in the EVT group and 92 in the endoscopic stent group) have retrospectively compared EVT to placement of endoscopic stents to close anastomotic leaks following oesophagus or stomach surgery, with clinical success being significantly greater in the group undergoing EVT (89% versus 59%). These studies also showed a lower rate of serious adverse events and even a lower rate of intrahospital mortality in favour of EVT in a recent systematic review and meta-analysis.29 However, these data should be taken with caution as they correspond to retrospective, observational, uncontrolled studies with markedly different characteristics and few patients. Undoubtedly, randomised clinical trials are needed to determine the true role of EVT compared to implantation of metal stents.
ConclusionEVT is a safe, effective treatment for perforations and anastomotic leaks of the upper gastrointestinal tract and in particular of the oesophagus. Early application of EVT could improve its effectiveness. Clinical trials must be conducted in order to determine the true role of this therapy compared to other therapies, especially the leading endoscopic therapy to date; i.e. implantation of metal stents.
Conflicts of interestDr Oriol Sendino is a consultant for the B. Braun company.
Dr Dulce Momblán is a consultant for the B. Braun company.
There are no other conflicts of interest.
Please cite this article as: Sendino O, Loras C, Mata A, Momblán D, Andujar X, Cruz M, et al. Eficacia y seguridad de la terapia de vacío endoscópica para el tratamiento de perforaciones y dehiscencias anastomóticas del tracto digestivo superior. Gastroenterol Hepatol. 2020;43:431–438.