Blastocystis hominis (B. hominis) is a protozoan commonly found in the gastrointestinal tract. There are doubts about its clinical significance. Metronidazole (MTZ) is the recommended first-line treatment.
Materials and methodsA retrospective review was carried out between 2011 and 2012. A total of 151 samples were randomly selected from 383 samples positive for B. hominis. Inclusion criteria were: suggestive symptoms, treatment indication and microbiological follow-up. A systematic review was performed of all studies that evaluated the effect of MTZ on B. hominis infection.
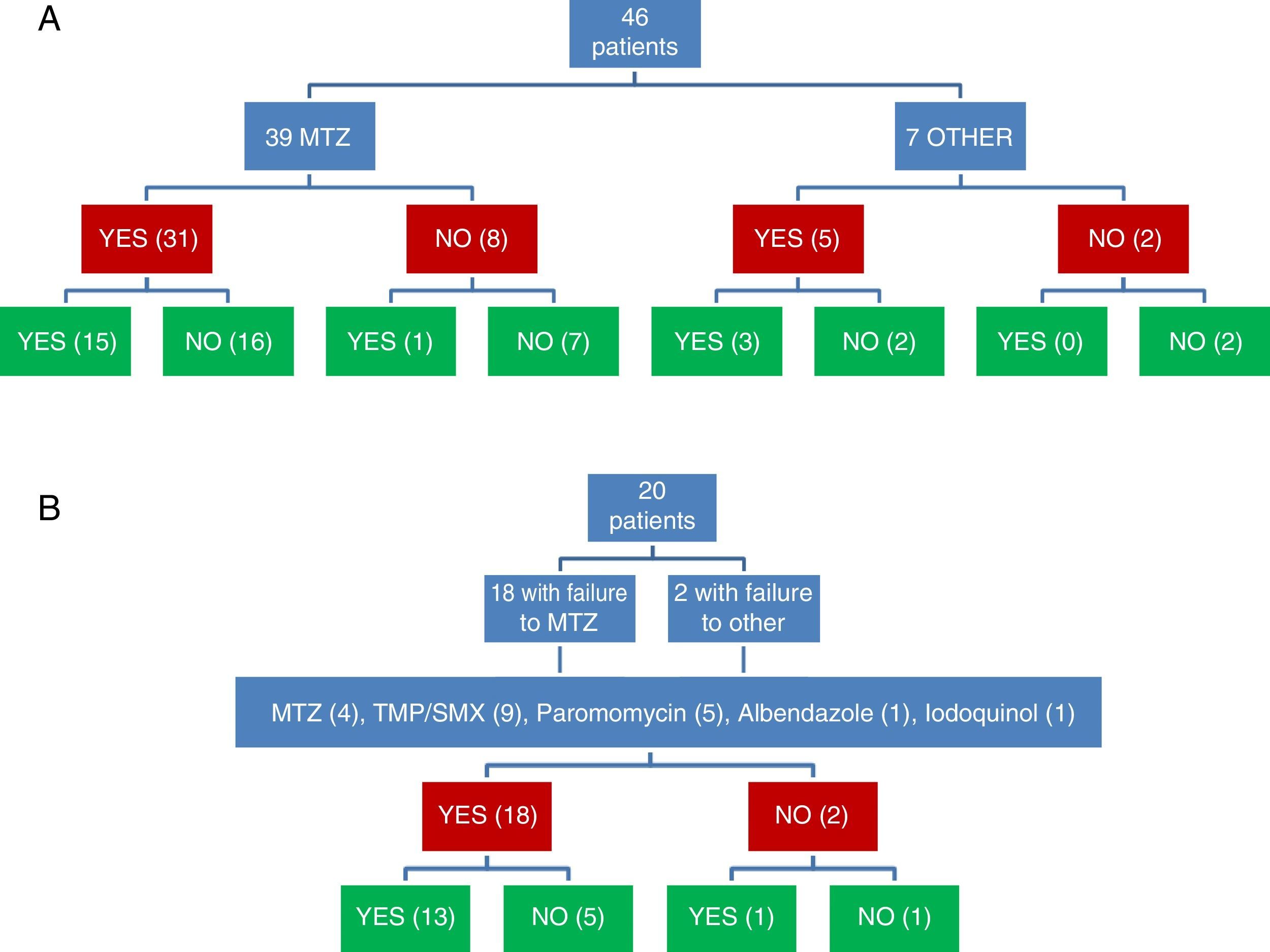
ResultsForty-six patients met the inclusion criteria (64% women; age, 44.2±2 years). MTZ was used in 39 patients, 31 of whom obtained a clinical response (79.5%) but only 15 a microbiological response (48.4%). No dose-effect relationship was observed. Twenty patients with no initial microbiological response received a second round of treatment (MTZ, cotrimoxazole, paramomycin, others), with a microbiological response in 70%. Overall, B. hominis was cured in 72% (95% CI: 57–83%). Of 54 treatments associated with a clinical response, a microbiological response occurred in 31 (57%), while in the remaining 12 with no clinical response, microbiological cure was observed in only 2 (17%) (P=.022). The eradication rate in the systematic review varied between 0% and 100%.
ConclusionsThere seems to be a relationship between the clinical and microbiological response to B. hominis treatment. The microbiological response to MTZ treatment is insufficient in our geographical setting. The systematic review shows that the response to MTZ is very variable.
El Blastocystis hominis (B. hominis) es un protozoo comúnmente encontrado en el tracto gastrointestinal. Existen dudas sobre su significado clínico. El metronidazol (MTZ) es el tratamiento aconsejado de primera línea.
Material y métodosSe realizó una revisión retrospectiva entre 2011 y 2012. Se seleccionaron de forma aleatoria 151 de 383 muestras positivas para B. hominis. Los criterios de inclusión fueron: clínica sugestiva, indicación de tratamiento y realización de control microbiológico. Se realizó una revisión sistemática de los estudios que evalúan el efecto de MTZ sobre la infestación por B. hominis.
ResultadosCuarenta y seis pacientes cumplían criterios de inclusión (el 64% eran mujeres; edad, 44,2±2 años). Se utilizó MTZ en 39 pacientes, de los cuales 31 obtuvieron respuesta clínica (79,5%) pero solo 15 respuesta microbiológica (48,4%). No se apreció una relación dosis-efecto. Veinte pacientes sin respuesta microbiológica inicial recibieron una segunda tanda de tratamiento (MTZ, cotrimoxazol, paramomicina, otros), con una respuesta microbiológica del 70%. De forma global, se consiguió la curación de B. hominis en un 72% (IC95%: 57-83%). De 54 tratamientos asociados a respuesta clínica, se produjo respuesta microbiológica en 31 (57%); mientras que de los 12 que se siguieron de ausencia de respuesta clínica solo se observó la curación microbiológica en 2 (17%) (p=0,022). La tasa de erradicación en la revisión sistemática osciló entre 0 y 100%.
ConclusionesParece existir relación entre la respuesta clínica y microbiológica al tratamiento de B. hominis. En nuestro entorno geográfico la respuesta microbiológica al tratamiento con MTZ es insuficiente. La revisión sistemática muestra que la respuesta a MTZ es muy variable.
Blastocystis hominis (B. hominis) is a single-celled protozoan commonly found in the gastrointestinal tract. B. hominis parasitosis has a worldwide prevalence above 50%, although it varies by geographic area, being more common in developing countries.1B. hominis is one of the most commonly identified parasites in stool samples, together with Giardia lamblia (G. lamblia) and Dientamoeba fragilis. The mechanism of transmission is faecal–oral. It exists in granular, vacuolar, cystic and amoeboid forms; the latter is linked to the development of symptoms.2
Although numerous studies have been conducted, the pathogenic role of B. hominis remains controversial.3,4 There is no clear concept of its clinical significance, pathogenicity or need for treatment. This infection has been suggested to be commonly associated with gastrointestinal symptoms, such as abdominal distension and pain, nausea, vomiting, and diarrhoea. Recent data have indicated that it is also related to dermatological abnormalities, such as erythema and urticaria, although this form of presentation would be less common.1 Regarding the mechanism of pathogenic action, B. hominis has been reported to express cysteine proteases, which play an essential role in invasion of host cells, immune response and cell cycle regulation. Studies in vivo have shown that these proteases may inhibit the production of immunoglobulin A in the intestinal mucosa, thereby contributing to the survival of the parasite.5
B. hominis has broad genetic diversity. Around 17 subtypes have been identified through ribosomal RNA sequence analysis. The most common subtype in humans is Subtype 3. In Spain, the most common subtype is Subtype 4.6 The heterogeneity of the strains could account for differences in pathogenicity. However, more studies are required to clarify whether there is a relationship between the subtype and the pathogenicity or whether other factors may contribute.
Although there are doubts as to the clinical significance of B. hominis and the extent to which it may be considered an intestinal coloniser, some studies have identified this parasite as a sole causative agent in gastrointestinal and dermatological infections in symptomatic patients.7 Therefore, there is an indication for eradication if symptoms persist, after ruling out other diagnoses. Spontaneous remission has been reported in 22% of cases in a cohort of individuals with B. hominis in stool samples,8 while another study conducted in Taiwan showed spontaneous remission in 91.2% after a year of follow-up.9
Different drugs have been used to eradicate B. hominis, including metronidazole (MTZ), nitazoxanide, trimethoprim/sulfamethoxazole (TMP/SMX), paromomycin, diiodohydroxyquinoline, ketoconazole and secnidazole, as well as probiotics.10 MTZ is considered a first-line treatment; however, its rate of eradication is highly variable and clearly insufficient in certain geographic areas.11 While the mechanism by which MTZ eradicates B. hominis is unknown, it has been reported to induce cell death by apoptosis in the parasite.12 The doses of MTZ used in the literature range from 250 to 750mg/8h for 10 days.13 It has been suggested that variability in response to MTZ may be secondary to pharmacokinetic properties, drug inactivation by the bacterial flora or the role of the different B. hominis subtypes in pathogenicity and antimicrobial susceptibility.14
The objectives of this study were: 1. To assess clinical and microbiological response to MTZ in Spain, and 2. To perform a systematic review of the published studies where MTZ has been used for B. hominis infection and thus determine its rate of efficacy.
Materials and methodsCase studyThe record of studies of parasites in stool samples of the Microbiology department of CATLAB (Viladecavalls, Barcelona) was evaluated. These corresponded to the health area of Hospital Universitari Mútua Terrassa (Terrassa, Barcelona). The health area of the hospital corresponded to a rural and urban environment and covered 215,214 inhabitants, according to the 2015 census. In 2011 and 2012, 9844 tests for parasites in stool were ordered; of these, 3.8% of samples (383 samples) were positive for B. hominis and 11.7% (1160 samples) were positive for G. lamblia. A sample of 151 adult patients was randomly selected from the B. hominis group, 100 from the Primary Care Centres (CAPs) in the area and 51 from the hospital itself. These patients made visits to gastroenterologists, both from primary care and from the hospital's outpatient department. The shared electronic medical history and the integrated electronic prescription system for the patients (SIRE; Ministry of Health, Regional Government of Catalonia) were reviewed retrospectively. All symptomatic patients in whom treatment to eradicate B. hominis was indicated and in whom microbiological monitoring had been performed to confirm the eradication of the parasite were selected. Cases with insufficient or unreliable information were excluded.
The form of clinical presentation, treatment used (drug, duration and dose), clinical response and microbiological response were recorded. Clinical response was considered the disappearance of symptoms, and microbiological response was considered a negative study of parasites in stool. Both were evaluated at least a month after completing treatment. As this was a retrospective study with anonymised data, it was not necessary to obtain patient consent.
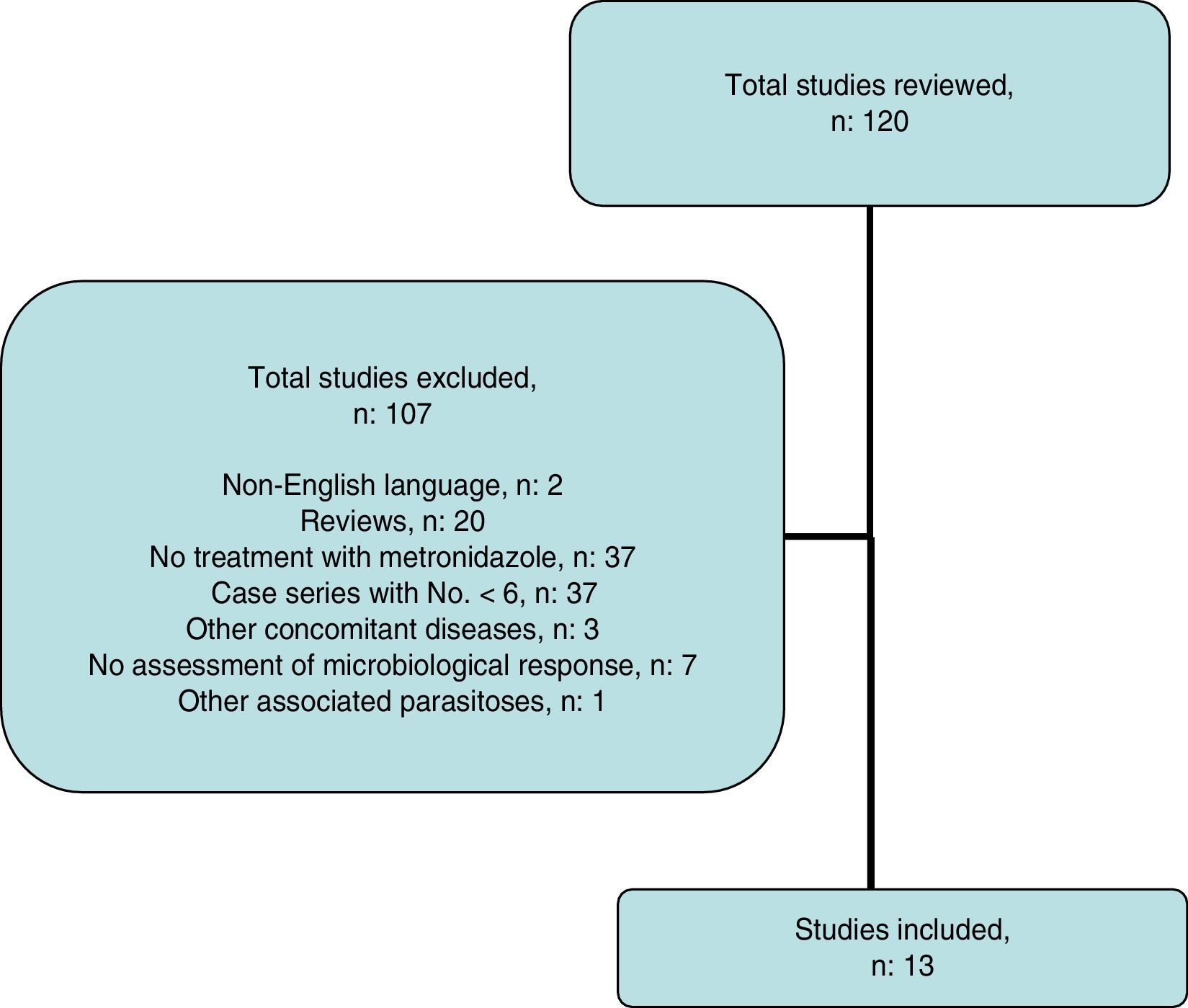
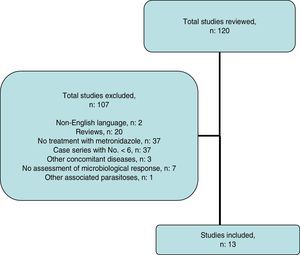
Systematic reviewA systematic literature search was performed to identify studies that evaluated the effect of treatment with MTZ on B. hominis infestation in children and adults. An electronic search was performed in PubMed and Scopus up to May 2016 using the following algorithm: (Blastocystis and treatment and human) OR (Blastocystis and metronidazole and human). The abstracts presented at the conferences of the American Gastroenterological Association (AGA) in the last 10 years were reviewed. The list of works cited in each article selected was also reviewed to identify other articles. The search, manuscript-selection and data-extraction process was performed independently by LB and FFB, and any discrepancies were resolved by consensus. Scientific reviews; case series with an N lower than 6, concomitant diseases (inflammatory bowel disease, states of immunosuppression, neoplasms, HIV infection, other associated parasitoses); and studies that did not assess microbiological response were excluded.
The sample size, MTZ dose, microbiological response to MTZ, year of publication and country in which the study was conducted were recorded.
Microbiological analysisFor each patient, 3 stool samples from different days were collected on a routine basis. All stool samples were sent to the microbiology laboratory after being fixed with a solution of sodium acetate, acetic acid and 10% formalin (SAF). B. hominis was detected using optical microscopy once the sample had been concentrated with the COPRO-PACK SAF fixation system (Biomedics, S.L., Germany).
Statistical analysisThe results were expressed as a mean±SEM and as proportions with a 95% confidence interval (95% CI) when deemed appropriate. The chi-squared test was used to analyse differences between qualitative variables. A p value<0.05 was considered statistically significant.
ResultsCase studyOf the 151 patients selected, only 46 met the inclusion criteria (64% women; mean age 44.2±2 [20–78 years]). In 90% of cases, there were symptoms of watery diarrhoea with no blood and/or abdominal pain, and diarrhoea was the predominant symptom in 71.7% of cases. The remaining 10% of cases had signs and symptoms of abdominal dyspepsia and/or bloating. None of the patients had fever or weight loss.
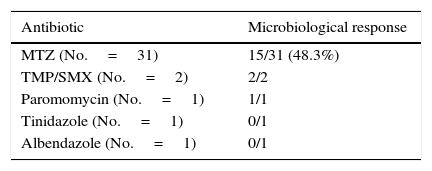
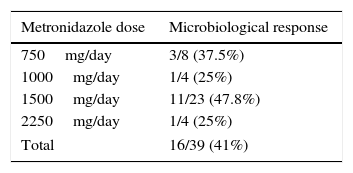
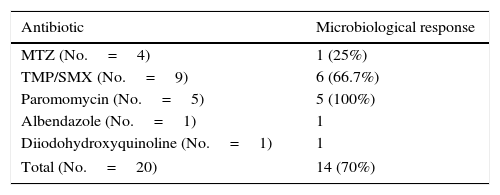
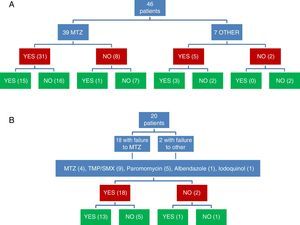
Clinical and microbiological response to initial treatment (39 patients treated with MTZ) is described in Fig. 1. In 36 patients (78.3%), clinical response was achieved (31 patients treated with MTZ; 79.5%). In 18 of these 36 patients (50%), microbiological response was achieved (15 treated with MTZ; 48.4%) (Table 1). No dose/effect relationship between the dose of MTZ used and the rate of microbiological response was seen (Table 2). Twenty patients with no microbiological response following initial treatment received a second round of treatment (18 had failed to respond to MTZ, and 2 had failed to respond to other antibiotics) (Table 3). Of these, 18 patients (90%) had clinical response. Microbiological response was achieved in 14 of 20 patients (70%), in 66.7% with TMP/SMX (dose: 800/160mg/12h) and in 100% with paromomycin (dose: 500mg/8h).
Microbiological response following a second round of treatment in patients with initial treatment failure (n=20).
| Antibiotic | Microbiological response |
|---|---|
| MTZ (No.=4) | 1 (25%) |
| TMP/SMX (No.=9) | 6 (66.7%) |
| Paromomycin (No.=5) | 5 (100%) |
| Albendazole (No.=1) | 1 |
| Diiodohydroxyquinoline (No.=1) | 1 |
| Total (No.=20) | 14 (70%) |
Joint assessment of clinical and microbiological response to the 2 rounds of antibiotic showed that clinical and microbiological response had been achieved following 31 of 66 treatments (Fig. 1). Of 54 treatments associated with clinical response, microbiological response occurred in 31 (57.4%), while of the 12 followed up in the absence of clinical response, microbiological cure was observed in only 2 (16.7%) (p=0.022).
Overall, B. hominis was cured in 33 of the 46 patients in whom clinical and microbiological response were assessed (71.7%; 95% CI: 57.4–82.7%). In 3 of 29 patients (10.3%) with clinical response and microbiological cure, recurrences of infestation diagnosed after symptoms reappeared were seen in follow-up. In the remaining 26 patients, no clinical recurrence was seen after a mean follow-up of 6.5±0.8 months.
Systematic reviewThe search strategy yielded 116 publications (Fig. 2); 103 were excluded after examining the title and abstract as they did not meet the inclusion criteria.
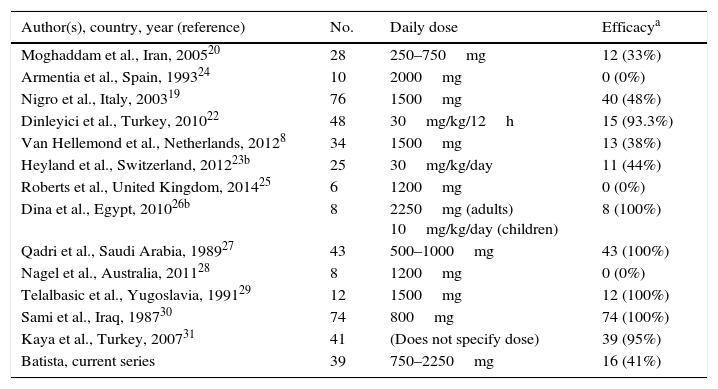
Ultimately, 13 studies were included. These are described in Table 4, together with the data for this study. Overall, data were obtained for 452 patients with B. hominis infestation treated with MTZ. Drug doses were highly variable with no dose/response effect being seen. The rate of eradication was also highly variable, ranging from 0% to 100% (overall rate: 62.6–50% for European studies only). The heterogeneity of the results obtained precluded a meta-analysis of the studies selected.
Rates of efficacy in the eradication of B. hominis using metronidazole reported in the literature.
| Author(s), country, year (reference) | No. | Daily dose | Efficacya |
|---|---|---|---|
| Moghaddam et al., Iran, 200520 | 28 | 250–750mg | 12 (33%) |
| Armentia et al., Spain, 199324 | 10 | 2000mg | 0 (0%) |
| Nigro et al., Italy, 200319 | 76 | 1500mg | 40 (48%) |
| Dinleyici et al., Turkey, 201022 | 48 | 30mg/kg/12h | 15 (93.3%) |
| Van Hellemond et al., Netherlands, 20128 | 34 | 1500mg | 13 (38%) |
| Heyland et al., Switzerland, 201223b | 25 | 30mg/kg/day | 11 (44%) |
| Roberts et al., United Kingdom, 201425 | 6 | 1200mg | 0 (0%) |
| Dina et al., Egypt, 201026b | 8 | 2250mg (adults) 10mg/kg/day (children) | 8 (100%) |
| Qadri et al., Saudi Arabia, 198927 | 43 | 500–1000mg | 43 (100%) |
| Nagel et al., Australia, 201128 | 8 | 1200mg | 0 (0%) |
| Telalbasic et al., Yugoslavia, 199129 | 12 | 1500mg | 12 (100%) |
| Sami et al., Iraq, 198730 | 74 | 800mg | 74 (100%) |
| Kaya et al., Turkey, 200731 | 41 | (Does not specify dose) | 39 (95%) |
| Batista, current series | 39 | 750–2250mg | 16 (41%) |
In this study, a determination of parasites in stool was ordered in patients with gastrointestinal signs and symptoms, with no warning signs. B. hominis was detected in 4% of the samples studied. This percentage was lower than the frequency of G. lamblia infestation. A study conducted in Madrid recorded a prevalence of B. hominis infection of 6.9%.15 Another study in A Coruña showed a prevalence of 4.5% in a paediatric population.16 Therefore, the prevalence detected in Spain was similar to that previously reported. Clinical and microbiological response to treatment was achieved in 72% of patients. The fact that clinical response was significantly higher in patients with eradication of the parasite supported the hypothesis that B. hominis may cause these symptoms. However, controlled clinical trials are needed to confirm this statement.
There is controversy with respect to first-line treatment to eradicate B. hominis. This parasite is only present in the lumen of the colon and does not interact with the mucosa. Therefore, the ideal drug should reach high concentrations in the lumen of the colon, have a short transit time in the small bowel and resist inactivation by the intestinal flora.17 Currently, as already mentioned, MTZ is considered a first-line drug.
However, its reported effectiveness ranges from 0% to 100% (Table 4). One of the main reasons for this variability may be differences in the degree of resistance to the drug. In fact, the effectiveness of MTZ has been suggested to depend on the B. hominis subtype.18 Therefore, due to geographic differences with respect to the variability in the sensitivity of B. hominis to antibiotics, efficacy studies at a local level must be available.
A placebo-controlled study conducted in Italy in 76 patients with B. hominis infection demonstrated clinical resolution a month after treatment in 88% of patients treated with MTZ vs 14% of patients in the placebo group. Microbiological response to MTZ was 80%, while response to placebo was 3%. A follow-up was performed at 6 months where 75% of patients treated with MTZ were asymptomatic. However, microbiological response was maintained in only 48%.19 According to our results, most patients (79.5%) achieved clinical response with MTZ. However, just 38.5% achieved microbiological response. This figure was clearly lower than that provided in the above-mentioned Italian study.19 In any case, the difference between initial microbiological response and that maintained in the above-mentioned Italian study was striking and indicated that the initial microbiological study may have been performed too early. Moreover, the differences between the rate of clinical response and the rate of microbiological response may have been due to several possibilities: the number of parasites may have decreased, resulting in clinical improvement but not managing to eradicate the parasitosis definitively, or MTZ could have acted indirectly by inhibiting other unidentified pathogenic micro-organisms responsible for the patient's signs and symptoms. In addition, an initial placebo effect could not be ruled out. In any case, it is clear that assessing initial clinical response to treatment alone is insufficient to consider the patient cured.
When a concomitant analysis of clinical and microbiological response was performed, microbiological cure was found to be significantly higher when clinical response was achieved. This observation supported the notion that the parasite plays a causative role in the pathogenesis of patients’ symptoms.
Reports of therapeutic failures with MTZ have motivated a search for alternative drugs. There is no consensus on which treatment to offer to patients with therapeutic failure with MTZ. Other drugs have been proposed as first-line treatment, including TMP/SMX and paromomycin, with rates of eradication of 70–95%. In our study, a second treatment with MTZ was ineffective (microbiological response: 25%). By contrast, microbiological response to TMP/SMX or paromomycin was good (67% and 100%, respectively), although the number of patients treated was limited.
The data in the literature with respect to the effect of TMP/SMX are controversial. In a Turkish study in a cohort of 53 symptomatic patients who received treatment with this drug (children, doses of 6mg/kg TMP and 30mg/kg SMX; adults, 320mg TMP, 1600mg SMX for 7 days), parasite cure rates of 93.3% in adults and 94.7% in children were achieved; the rate of clinical response was 73%.21 However, a study by Swiss authors indicated that TMP/SMX only reduces the number of B. hominis parasites and does not permanently eradicate it.23 This study also demonstrated that TMP/SMX does not appear to be superior to placebo in treating paediatric patients with recurrent abdominal pain and B. hominis infection. Eradication was achieved in 35% of patients with TMP/SMX and in 44% of patients with MTZ. Spontaneous clearance occurred in 29% of the placebo group. In a controlled study with MTZ conducted in Iran, response to TMP/SMX (1 tab 3 times daily for 10 days) was 22%, while response to MTZ was 33%.20 As already mentioned, differences in B. hominis subtype and pathogenicity or degree of resistance to antibiotics could account for variations in response to TMP/SMX in different geographic areas of the world. In this regard, it seems important to have data particular to different geographic environments.
Another second-line drug is paromomycin. This broad-spectrum antimicrobial has bactericidal activity. This drug has been reported to be effective in cases of B. hominis infection and chronic urticaria.24 To date, studies conducted in small series of patients have reported cure rates above 75%. The longest series corresponded to a Dutch study with a population of 52 patients and a rate of eradication of 77% (dose 500mg/8h for 7–10 days). In this same study, response to MTZ was 38%.8 Although in our study paromomycin was used in only 5 patients, all had microbiological cure. These results seem promising.
In conclusion, there appears to be a relationship between clinical response and microbiological response to B. hominis treatment. This shows the parasite's causative role. In Spain, microbiological response to treatment with MTZ is clearly insufficient. A systematic review showed that response to MTZ is highly variable, ranging from 0% to 100%. Controlled studied are needed to evaluate the efficacy of other drugs such as TMP/SMX and paromomycin as first-line treatments. Such studies will help to confirm the pathogenic role of this infestation.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Batista L, Pérez Jove J, Rosinach M, Gonzalo V, Sainz E, Loras C, et al. Escasa eficacia de metronidazol en la erradicación de Blastocystis hominis en pacientes sintomáticos: serie de casos y revisión sistemática de la literatura. Gastroenterol Hepatol. 2017;40:381–387.
This study was presented at the conference of the Spanish Association of Gastroenterology held in Madrid in 2014, and was published as an abstract in Gastroenterología y Hepatología in 2014.