Aeromonas can cause several diseases in humans, with gastroenteritis accounting for most cases. The role of Aeromonas as a pathogen in human enterocolitis has been questioned in recent years.
ObjectivesTo determine the incidence of gastrointestinal infection caused by Aeromonas in our area and its possible relationship to inflammatory bowel disease.
Patients and methodsThis was a retrospective observational study. All adult patients with a positive stool culture for Aeromonas were identified between January 2015 and December 2017 at Hospital Galdakao-Usansolo (Vizcaya, Spain).
ResultsNinety-eight patients were identified (median age 62 years; 51% women). Therefore, the incidence in our area was 32 cases per 105 inhabitants per year. Eleven per cent of them had been previously diagnosed with inflammatory bowel disease (four with ulcerative colitis and seven with Crohn's disease). Patients with inflammatory bowel disease more often received immunosuppressive therapy. Conversely, patients without inflammatory bowel disease suffered from more comorbidities. We also found comorbidity to be the risk factor most associated with Aeromonas infection.
ConclusionAeromonas infection is a common gastrointestinal infection that may occur in both immunocompetent and immunocompromised patients. Immunosuppression is a significant factor in inflammatory bowel disease patients, while comorbidity seems to confer a higher risk on patients without this disease.
Las Aeromonas son la causa de múltiples enfermedades en humanos, siendo la gastroenteritis la más frecuente. El papel de Aeromonas como patógeno en las enterocolitis y su relación con la enfermedad inflamatoria intestinal siguen siendo controvertidos.
ObjetivoDeterminar la incidencia de la infección porAeromonas en nuestro entorno y su posible relación con la enfermedad inflamatoria intestinal.
Pacientes y métodosEstudio retrospectivo y observacional de todos los pacientes adultos con al menos un aislamiento de Aeromonas en el cultivo de heces entre enero de 2015 y diciembre de 2017 en el Hospital Galdakao-Usansolo (Vizcaya).
ResultadosSe identificaron 98 pacientes con un cultivo de heces positivo paraAeromonas (edad media 62 años, 51% mujeres), estimando una incidencia de 32 casos/105 habitantes-año. El 11% tenían un diagnóstico previo de enfermedad inflamatoria intestinal (4 colitis ulcerosa y 7 enfermedad de Crohn). Estos pacientes se encontraban en tratamiento inmunosupresor con más frecuencia, aunque tenían menos comorbilidades que el grupo sin esta patología. También observamos que la comorbilidad era el factor que más se asociaba a la infección por Aeromonas.
ConclusionesLa infección porAeromonas tiene una incidencia de 32 casos/105 habitantes-año, y afecta tanto a pacientes inmunocompetentes como a inmunodeprimidos. La incidencia está influida por la inmunosupresión en pacientes con enfermedad inflamatoria intestinal, mientras que la comorbilidad parece conferir un mayor riesgo en pacientes sin esta patología.
Aeromonas is a genus of gram-negative and oxidase-positive facultative anaerobic bacteria. They are bacteria from an aquatic environment and are found all over the world. Bacteria of this genus are the cause of several conditions in humans, the most common being gastroenteritis, cellulitis, fasciitis and bacteraemia.1 Other infections described, although less frequently, are pneumonia, appendicitis and haemolytic uraemic syndrome.1–4 Diarrhoea is the most common symptom of digestive infection and is usually self-limited. Despite being isolated with relative frequency, its role as a pathogen remains controversial in enterocolitis,5,6 and for this reason it continues to be studied.4,7 Chronic infections8 and infections of varying severity have also been described. Despite the fact that most of the cases described have been identified in immunosuppressed patients, it is an infection also described in immunocompetent patients.2,5
Identification of the different Aeromonas strains is difficult due to their phenotypic heterogeneity.2 Currently, four species are isolated in 96% of cases: Aeromonas caviae (30%), Aeromonas dhakensis (26%), Aeromonas veronii (22%) and Aeromonas hydrophila (18%).2 Their virulence is multifactorial and is due to the synthesis of exotoxins (cytotoxic and cytotonic), proteases, haemolysins, lipases, adhesins, agglutinins and enterotoxins, in addition to other enzymes.7
The relationship between Aeromonas infection and inflammatory bowel disease (IBD) is a subject that has been studied in recent years.1 This infection can have important implications in IBD patients and can trigger an outbreak in some cases.1 The objective of our study was to ascertain the incidence of this infection in our area and to evaluate the influence of Aeromonas infection in patients with IBD.
Material and methodsWe conducted a retrospective observational study of all adult patients with Aeromonas isolated from stool culture between 1 January 2015 and 31 December 2017 at the Galdakao-Usansolo Hospital (Vizcaya, Basque Country). The population coverage at the time of the study was 308,623 inhabitants (December 2017). The following variables were included in the patient analysis: age at diagnosis of infection, sex, smoking, comorbidities (Charlson index), admissions in the 6 months prior to diagnosis and antibiotics taken in the previous 3 months, symptoms at diagnosis, Aeromonas species isolated, other isolated enteropathogens, therapeutic attitude and characteristics of the IBD patients. Specific characteristics of IBD cases have been detailed, analysing the year of disease onset, the presentation of the clinical symptoms of the infection, changes in IBD treatment after infection and clinical evolution during follow-up. The infection was considered serious if it required hospital admission. Patients on treatment with an immunomodulator (thiopurines, methotrexate, mycophenolate mofetil, tacrolimus, cyclosporine or sirolimus), biologicals, corticosteroids at doses above 20 mg/day and/or any cytostatic drug were considered immunosuppressed.
Descriptive statistics were used to define the baseline characteristics of the sample, with means and standard deviation or with median and interquartile range depending on sample distribution. The characteristics of the groups of patients with and without IBD were compared using chi-square and non-parametric tests such as the Mann-Whitney test. Statistical significance was established at p < 0.05.
ResultsPatient characteristicsDuring the study period, 98 patients with Aeromonas isolated from stool culture were identified. The main characteristics of the patients are listed in Table 1, and the most relevant data of the patients with IBD are summarised in Table 2.
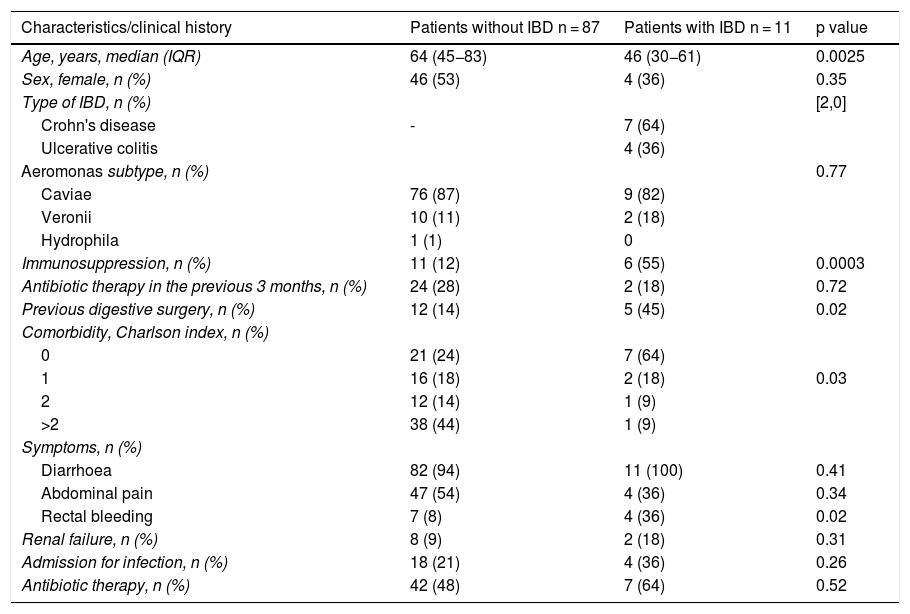
Patient characteristics.
| Characteristics/clinical history | Patients without IBD n = 87 | Patients with IBD n = 11 | p value |
|---|---|---|---|
| Age, years, median (IQR) | 64 (45−83) | 46 (30−61) | 0.0025 |
| Sex, female, n (%) | 46 (53) | 4 (36) | 0.35 |
| Type of IBD, n (%) | [2,0] | ||
| Crohn's disease | - | 7 (64) | |
| Ulcerative colitis | 4 (36) | ||
| Aeromonas subtype, n (%) | 0.77 | ||
| Caviae | 76 (87) | 9 (82) | |
| Veronii | 10 (11) | 2 (18) | |
| Hydrophila | 1 (1) | 0 | |
| Immunosuppression, n (%) | 11 (12) | 6 (55) | 0.0003 |
| Antibiotic therapy in the previous 3 months, n (%) | 24 (28) | 2 (18) | 0.72 |
| Previous digestive surgery, n (%) | 12 (14) | 5 (45) | 0.02 |
| Comorbidity, Charlson index, n (%) | |||
| 0 | 21 (24) | 7 (64) | |
| 1 | 16 (18) | 2 (18) | 0.03 |
| 2 | 12 (14) | 1 (9) | |
| >2 | 38 (44) | 1 (9) | |
| Symptoms, n (%) | |||
| Diarrhoea | 82 (94) | 11 (100) | 0.41 |
| Abdominal pain | 47 (54) | 4 (36) | 0.34 |
| Rectal bleeding | 7 (8) | 4 (36) | 0.02 |
| Renal failure, n (%) | 8 (9) | 2 (18) | 0.31 |
| Admission for infection, n (%) | 18 (21) | 4 (36) | 0.26 |
| Antibiotic therapy, n (%) | 42 (48) | 7 (64) | 0.52 |
IBD: inflammatory bowel disease; IQR: interquartile range.
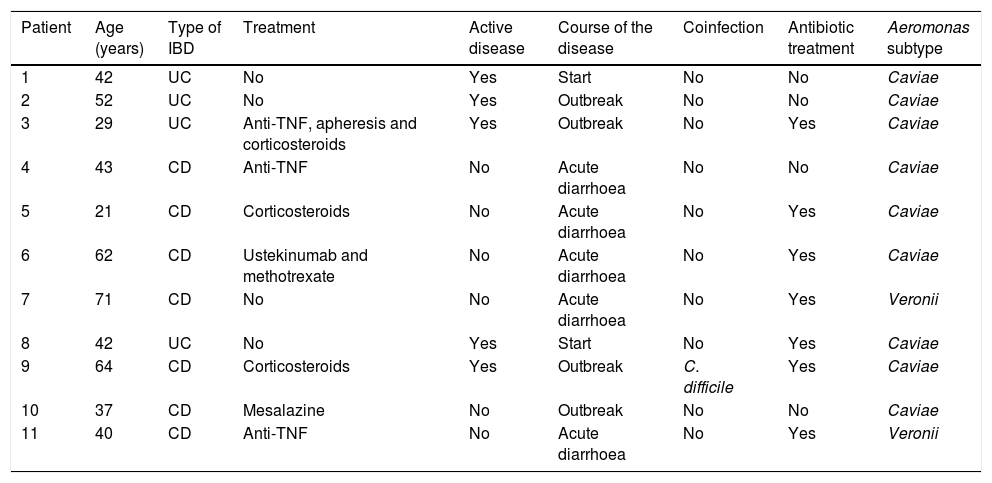
Characteristics of patients with inflammatory bowel disease.
| Patient | Age (years) | Type of IBD | Treatment | Active disease | Course of the disease | Coinfection | Antibiotic treatment | Aeromonas subtype |
|---|---|---|---|---|---|---|---|---|
| 1 | 42 | UC | No | Yes | Start | No | No | Caviae |
| 2 | 52 | UC | No | Yes | Outbreak | No | No | Caviae |
| 3 | 29 | UC | Anti-TNF, apheresis and corticosteroids | Yes | Outbreak | No | Yes | Caviae |
| 4 | 43 | CD | Anti-TNF | No | Acute diarrhoea | No | No | Caviae |
| 5 | 21 | CD | Corticosteroids | No | Acute diarrhoea | No | Yes | Caviae |
| 6 | 62 | CD | Ustekinumab and methotrexate | No | Acute diarrhoea | No | Yes | Caviae |
| 7 | 71 | CD | No | No | Acute diarrhoea | No | Yes | Veronii |
| 8 | 42 | UC | No | Yes | Start | No | Yes | Caviae |
| 9 | 64 | CD | Corticosteroids | Yes | Outbreak | C. difficile | Yes | Caviae |
| 10 | 37 | CD | Mesalazine | No | Outbreak | No | No | Caviae |
| 11 | 40 | CD | Anti-TNF | No | Acute diarrhoea | No | Yes | Veronii |
UC: ulcerative colitis; CD: Crohn's disease; TNF: tumour necrosis factor.
Incidence during the study period was 32 cases/105 inhabitants/year. Eleven patients (11%) had a previous diagnosis of IBD, 4 of them with ulcerative colitis (UC) and 7 with Crohn's disease (CD). One patient had immune-mediated colitis secondary to treatment with nivolumab that he was receiving for a lung neoplasm. The patients with IBD were younger and were more frequently on immunosuppression than those without this pathology (12% vs. 55%; p = 0.0003). However, the patients in the group without IBD had more comorbidities than those in the group with IBD (Charlson index 0 in 64% of the IBD group vs. Charlson index >2 in 44% of the non-IBD group; p = 0.0379).
In both groups, the most frequent symptoms were diarrhoea (100% in patients with IBD vs. 94% in patients without IBD; p = 0.41) followed by abdominal pain (54% vs. 36%, respectively; p = 0.34). Rectorrhagia was more frequent in patients with what without IBD (36% vs. 8%, respectively; p = 0.02), with no statistically significant differences in the other symptoms. In our cohort, no extraintestinal complications were detected, except for renal failure due to digestive losses, tending to be more frequent in patients with IBD (18% vs. 8%, respectively; p = 0.31) One patient without IBD presented data of ischaemic colitis on diagnosis of the infection. One patient died of a colon adenocarcinoma unrelated to the infection.
Aeromonas infectionThe most frequently isolated species was A. caviae in both groups (82% in patients with IBD and 87% in patients without IBD). The second most frequent species was A. veronii (11% in the group without IBD vs. 18% in the group with IBD), while a single case of A. hydrophila (1%) was observed in a patient without IBD diagnosed with colon adenocarcinoma.
Risk factorsMost of the patients with IBD in our cohort did not have other comorbidities. However, the patients without IBD had comorbidities with greater frequency, frequently more than 2 comorbidities (Charlson index 0 in 64% of the IBD group vs. Charlson index >2 in 44% of the group without IBD; p = 0.03). In the group of patients with IBD, there was a higher rate of immunosuppressed patients (55% vs. 12%; p = 0.0003). Moreover, the patients without IBD tended to have received prior antibiotic therapy more frequently (28% vs. 18%; p = 0.72) and/or have recently been hospitalised (27% vs. 9%; p = 0.18).
Most of the infections were mild and did not require hospital admission. The patients with IBD required hospitalisation on a greater number of occasions (36% vs. 21%; p = 0.31) and were treated more frequently with antibiotic therapy (64% vs. 48%; p = 0.52), albeit without differences with the group without IBD. In general, 50% of the patients were treated with antibiotics, the most used ones being quinolones (73%), followed by other groups of antibiotics (18%) such as cephalosporins (8%), metronidazole (6%), vancomycin (4%), carbapenems (4%) and broad-spectrum penicillin (2%). In 4%, 2 types of antibiotics were combined. 8% of the treated cases had a recurrence, all of them without IBD.
Of the 98 cultures in our cohort, we only have antibiogram data on 6 (6%). In all of them, the microorganism was sensitive to ciprofloxacin and cotrimoxazole. In contrast, 3 of them (50%) were resistant to ampicillin and amoxicillin-clavulanate.
Patients with inflammatory bowel diseaseIn our series, two cases (18%) of UC onset associated with Aeromonas infection were identified. Five patients with IBD (45%) presented Aeromonas-induced acute gastroenteritis which did not alter the course of the disease. Four (4) of them required antibiotic treatment and one case was self-limited. In contrast, in another 4 patients (36%), Aeromonas infection caused a worsening of the disease activity, requiring a change in IBD treatment. Two of these patients required corticosteroids to control the disease, one of them required the initiation of biological anti-tumour necrosis factor (anti-TNF) treatment – α and another patient required a change of anti-TNF – α followed by switch of target to vedolizumab after the infection. This patient presented a refractory course, with subsequent superinfection by Clostridium difficile, and was finally diagnosed with 2 synchronous colon neoplasms, with clinical worsening and subsequent death.
Five of the patients in our cohort (5%) had a co-infection with another enteropathogenic microorganism, including Campylobacter jejuni (2 patients without IBD), Salmonella (one patient without IBD), Giardia lamblia (one patient without IBD) and C difficile (one patient with IBD). No differences were observed between the two groups in the rate of patients with coinfection (9% vs. 4%; p = 0.46). Aeromonas enterocolitis bacteraemia was not described in our cohort. However, one case coincided with beta-lactamase-producing Escherichia coli bacteraemia.
Apart from the patients with IBD, one patient with lung carcinoma diagnosed with colitis immune-mediated by nivolumab 4 months earlier presented acute diarrhoea caused by A. caviae, with persistent symptoms and evidence of active colitis despite treatment with metronidazole. Corticosteroid treatment with an anti-TNF biological was started, and after 10 months of treatment with infliximab the patient was switched to vedolizumab due to metastatic disease progression, remaining in clinical remission and without having presented new infections.
DiscussionAeromonas species are relatively frequently isolated bacteria in cases of infectious enterocolitis, but their role as a pathogen in these cases remains controversial. It is generally considered that the virulence of Aeromonas is multifactorial, conditioned by both pathogen and host factors, and that only some strains of Aeromonas can cause gastroenteritis in humans.2 The growing interest in this microorganism has led recent studies to address its potential pathogenicity and even its possible relationship with cases of IBD.1,9 However, this aspect is controversial since between 1-4% of asymptomatic subjects are known to be possible carriers of Aeromonas at digestive level.5
Most Aeromonas infections have been described in immunosuppressed patients, although it can also affect immunocompetent patients.2,5 In our study, only 17% of the patients with this infection were immunosuppressed and 23% had a previous diagnosis of a neoplasm. We also observed that patients without IBD with an Aeromonas infection present more associated comorbidity than patients with IBD according to the Charlson index.
In recent years, reported case series have also analysed the possible relationship between Aeromonas species and IBD. Aeromonas infection has been described as having different implications in patients with this pathology.1,10–14 On the one hand, Aeromonas infection has been shown to be a possible triggering factor for the onset of IBD, both UC10,11 and CD.1 Moreover, it has been described as associated with a reactivation of the inflammatory activity of the disease1,12 and with acute gastroenteritis symptoms, having no effect on the course of the disease.1,13,14 In our series, the course of the disease after infection was also variable, with symptoms presenting at the beginning of the disease in some cases, in others as acute diarrhoea and even as an outbreak of the disease that required treatment escalation. It is important to note that 36% of patients required a change in IBD treatment after infection and so these patients should therefore be closely monitored due to the risk of clinical worsening.
Although the most frequent symptom secondary to this infection is acute diarrhoea, this microorganism can be the cause of chronic diarrhoea, as described in cases with diarrhoea coursing for more than one month.1,8,15 In our study, this microorganism was isolated in 7 patients (7%) in a study of chronic diarrhoea that did not remit despite empirical antibiotic treatment in 71%. This finding supports the possible isolation of Aeromonas in carrier and non-pathogenic subjects.
It has also been observed that Aeromonas can present in 14% of patients as coinfection with another enteropathogen such as C. jejuni, C. difficile, Salmonella or Blastocystis hominis, among others, as described in the study by Nolla-Salas et al.2 In our series, there is a trend towards a higher rate of coinfection in cases with IBD (9% vs. 4% patients without IBD; p = 0.46), which could be explained by the higher proportion of patients on immunosuppressive treatment in this cohort. There may be other exceptional complications such as Aeromonas bacteraemia in patients with gastroenteritis.16
A recent study showed that more than 90% of Aeromonas had high sensitivity to third- or fourth-generation cephalosporins, aminoglycosides, aztreonam and tigecycline, indicating that these antibiotics could be the first empirical therapeutic option for Aeromonas infections.2 Nevertheless, given the study's limitations, it should be evaluated according to the geographical area. However, in our cohort, according to the antibiograms described, all isolates were sensitive to fluoroquinolones and cotrimoxazole and 50% were resistant to beta-lactams.
The limitations encountered in this study are, on one hand, the low number of patients identified with Aeromonas infection and IBD, as well as the usual limitations of retrospective studies, subject to multiple biases such as the lack of participation of asymptomatic patients and homogeneous management or systematic patient follow-up. Another limitation was the small number of antibiograms performed from cultures, which made it difficult to interpret relevant information regarding sensitivity to different antibiotics.
In conclusion, we observed that gastrointestinal infection by Aeromonas is relatively frequent in our setting, with an incidence of 32 cases/100,000 inhabitants per year. The main risk factor for this infection is the presence of comorbidities, although immunosuppression seems to play a more important role in patients with IBD. This infection plays a relevant role in the natural history of IBD in up to one third of IBD patients, whereby close monitoring of these cases is necessary.
FundingThis study has not received any specific funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Elorza A, Rodríguez-Lago I, Martínez P, Hidalgo A, Aguirre U, Cabriada JL. Infección gastrointestinal por Aeromonas: incidencia y su posible relación con la enfermedad inflamatoria intestinal. Gastroenterol Hepatol. 2020. https://doi.org/10.1016/j.gastrohep.2020.04.014