Cirrhotic patients often develop severe complications requiring ICU admission. Grade III–IV hepatic encephalopathy, septic shock, acute-on-chronic liver failure and variceal bleeding are clinical decompensations that need a specific therapeutic approach in cirrhosis. The increased effectiveness of the treatments currently used in this setting and the spread of liver transplantation programmes have substantially improved the prognosis of critically ill cirrhotic patients, which has facilitated their admission to critical care units. However, gastroenterologists and intensivists have limited knowledge of the pathogenesis, diagnosis and treatment of these complications and of the prognostic evaluation of critically ill cirrhotic patients. Cirrhotic patients present alterations in systemic and splanchnic haemodynamics, coagulation and immune dysfunction what further increase the complexity of the treatment, the risk of developing new complications and mortality in comparison with the general population. These differential characteristics have important diagnostic and therapeutic implications that must be known by general intensivists. In this context, the Catalan Society of Gastroenterology and Hepatology requested a group of experts to draft a position paper on the assessment and treatment of critically ill cirrhotic patients. This article describes the recommendations agreed upon at the consensus meetings and their main conclusions.
Los pacientes cirróticos presentan frecuentemente complicaciones graves de su enfermedad que requieren ingreso en la UCI. La encefalopatía hepática gradoIII-IV, el shock séptico, el fracaso agudo sobre crónico y la hemorragia variceal son descompensaciones que precisan un tratamiento intensivo específico en el paciente cirrótico. La mayor eficacia de los tratamientos empleados en cuidados intensivos y la generalización de los programas de trasplante hepático han mejorado de manera sustancial el pronóstico del paciente cirrótico crítico, hecho que ha facilitado su ingreso en las unidades de terapia intensiva. Sin embargo, el conocimiento de digestólogos e intensivistas sobre la patogenia, diagnóstico y tratamiento de estas complicaciones y sobre la evaluación pronóstica del paciente cirrótico crítico es limitado. Las alteraciones hemodinámicas y en la coagulación características de estos pacientes y la disfunción inmune que presentan aumentan la complejidad del tratamiento, el riesgo de presentar nuevas complicaciones y su mortalidad en comparación con la población general. Estas características diferenciales tienen implicaciones diagnósticas y terapéuticas clínicamente relevantes que deben ser conocidas por los intensivistas generales. En este contexto, la Sociedad Catalana de Digestología encomendó a un grupo de expertos la redacción de un documento de posicionamiento sobre la evaluación y el tratamiento del paciente cirrótico crítico. El presente artículo describe las recomendaciones acordadas en las reuniones de consenso y sus principales conclusiones.
The evaluation and treatment of patients with cirrhosis and severe complications of the disease have been evolving very rapidly in recent years. The spread of liver transplantation programmes (not only for patients with “stable” cirrhosis but also for those who present severe disease complications), improved efficacy of the intensive treatments used in critical care units, and early detection and treatment of severe complications of cirrhosis, among other factors, has led to substantial improvements in the prognosis of critically ill cirrhotic patients. Nevertheless, there are still many areas for improvement in terms of entities for which there is as yet no specific treatment (such as acute-on-chronic liver failure [ACLF]) or in which current treatment is only moderately effective (hepatic encephalopathy [HE] or hepatorenal syndrome [HRS]), and of current limitations on the admission of cirrhotic patients to intensive care units (ICU). It is also important that the medical team (gastroenterologists, internists, intensivists, surgeons) and nursing staff attending these patients have adequate knowledge of the severe complications that occur in cirrhosis. In this context, the Catalan Society of Gastroenterology and Hepatology requested a group of experts on the assessment and treatment of critically ill cirrhotic patients to draft a position paper. Although the critically ill cirrhotic patient often presents several decompensations simultaneously, in the interests of organisation and comprehension, this paper has been divided into 6 sections: (1) Severe sepsis and septic shock, (2) Acute renal failure, (3) Hepatic encephalopathy, (4) Gastrointestinal (GI) bleeding due to gastro-oesophageal varices, (5) ACLF, (6) Prognostic evaluation and futility rules. This article describes the recommendations agreed at the consensus meetings and their main conclusions.
Severe sepsis and septic shockSepsis is the result of the host's reaction to infection, and is characterised by the release of various inflammatory mediators, such as pro- and anti-inflammatory cytokines and procoagulant substances.1,2 These mediators are responsible for the systemic response to infection, which is stronger in patients with cirrhosis compared to patients with infection but no cirrhosis. This means a higher risk of developing sepsis, severe sepsis (i.e. organ failure or tissue hypoperfusion), septic shock (hypotension refractory to fluid resuscitation, and that requires vasopressors), multiple organ dysfunction syndrome and death.1–4 The hospital mortality rate in patients with cirrhosis and severe sepsis or septic shock is higher than in the general population, exceeding 50% and 80%, respectively.5
Initial resuscitationRapid initiation of sequential resuscitation measures in the acute phase of severe sepsis is essential in any patient. Resuscitation should be started as soon as possible once signs of peripheral hypoperfusion have been detected, without waiting until the patient is admitted to a critical care unit. In the general population, current clinical guidelines recommend that the following goals be achieved in the first 6h: mean arterial pressure ≥65mmHg, central venous pressure (CVP) between 8 and 12mmHg (12–15mmHg in ventilated patients), central venous oxygen saturation (ScvO2)≥70% and diuresis≥0.5mL/kg/h (early goal-directed therapy [EGDT]).6 Normalisation of the serum lactate concentration is another important clinical objective. These goals are achieved by the sequential administration of fluids, vasopressors, transfusions and, in certain patients, inotropic drugs. Two recent multicentre studies – also in the general population – failed to confirm that the package of measures included in EGDT improves survival in patients with severe sepsis, which suggests that the decreased mortality reported in previous studies could be related with early initiation of conventional therapy (early aggressive administration of fluids and antibiotics).7,8 Following early resuscitation in the emergency department or conventional hospital ward, guided or not by EGDT, patients should be transferred to an intensive or intermediate care area.
Although no studies have evaluated EGDTs in cirrhosis, routine clinical practice suggests that, due to their frailty, early resuscitation is at least as important in patients with cirrhosis. The goals, however, may differ from those of the general population, as patients with cirrhosis usually present lower arterial pressure, higher ScvO2 (hyperdynamic circulation), lower diuresis and haematocrit, and abnormal lactate metabolism.9,10
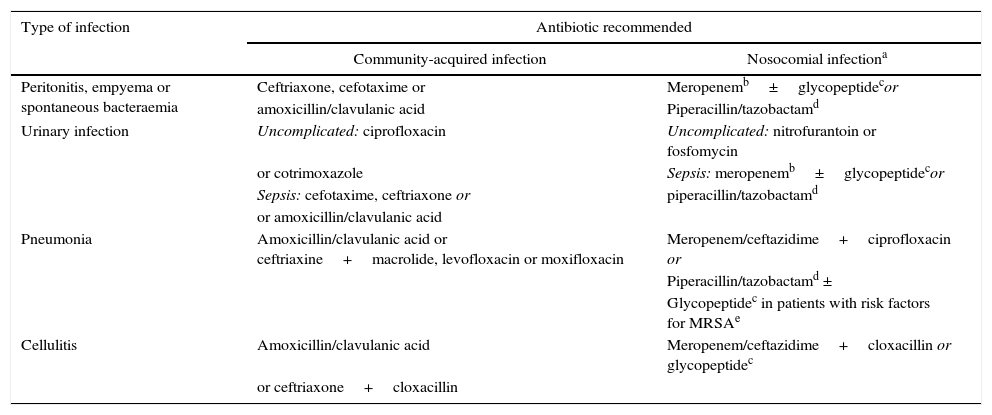
Early diagnosis and antibiotic treatmentDiagnosing the infection and starting intravenous (i.v.) treatment as soon as possible are key points in the management of these patients. The diagnosis of infection is not always easy, as patients often present non-specific symptoms and laboratory test abnormalities. Determination of serum C-reactive protein (CRP) (≥10ng/mL) is useful in establishing the suspicion of infection, but false positives and negatives are common.2,4 Systematic clinical assessment of the patient should be carried out, in order to identify the cause of the infection: thorough anamnesis and physical examination, blood cultures (2 or more), diagnostic paracentesis and ascitic fluid culture, urine sediment and culture, and chest X-ray. It is important to obtain samples for culture before administering broad spectrum empirical antibiotic treatment, which should always be started as soon as possible, preferably within the first hour after diagnosis. Appropriate early antibiotic treatment improves survival in severe sepsis in both the general population and in patients with cirrhosis.11,12 Mortality increases by 10% for each hour the start of appropriate antibiotic treatment is delayed. The initial empirical antibiotic treatment should cover the most likely pathogens, taking into account different variables: type of infection, whether it is a community-acquired infection, associated with the healthcare system or hospital-acquired (Table 1), and whether the patient has a history of recent antibiotic treatments or infections or colonisation by multiresistant bacteria. The resistance profile of each hospital should also be considered. The empirical antibiotic treatments recommended in community-acquired infections are third-generation cephalosporins or amoxicillin-clavulanic acid. A carbapenem or piperacillin tazobactam is recommended in nosocomial infections in areas of high and low prevalence of multiresistant bacteria, respectively, combined or not with a glycopeptide. Once the culture and antibiotic sensitivity results are known, treatment should be adjusted (de-escalated) to the most appropriate antibiotic(s).4
Empirical antibiotic treatment recommended in community-acquired and nosocomial bacterial infections in cirrhotic patients.
| Type of infection | Antibiotic recommended | |
|---|---|---|
| Community-acquired infection | Nosocomial infectiona | |
| Peritonitis, empyema or spontaneous bacteraemia | Ceftriaxone, cefotaxime or | Meropenemb±glycopeptidecor |
| amoxicillin/clavulanic acid | Piperacillin/tazobactamd | |
| Urinary infection | Uncomplicated: ciprofloxacin | Uncomplicated: nitrofurantoin or fosfomycin |
| or cotrimoxazole | Sepsis: meropenemb±glycopeptidecor | |
| Sepsis: cefotaxime, ceftriaxone or | piperacillin/tazobactamd | |
| or amoxicillin/clavulanic acid | ||
| Pneumonia | Amoxicillin/clavulanic acid or ceftriaxine+macrolide, levofloxacin or moxifloxacin | Meropenem/ceftazidime+ciprofloxacin or |
| Piperacillin/tazobactamd ± | ||
| Glycopeptidec in patients with risk factors for MRSAe | ||
| Cellulitis | Amoxicillin/clavulanic acid | Meropenem/ceftazidime+cloxacillin or glycopeptidec |
| or ceftriaxone+cloxacillin | ||
MRSA: Methicillin-resistant Staphylococcus aureus.
Treatment also recommended in urinary infections and pneumonias associated with the healthcare system. Empirical treatment of spontaneous infections and cellulitis associated with the healthcare system should be decided based on the severity of the infection (patients with severe sepsis should receive the regimen recommended for nosocomial infections) and the local prevalence of multiresistant bacteria.
Current clinical guidelines in the general population recommend the use of crystalloids (physiological saline and Ringer's lactate solution) in the initial resuscitation of patients with severe sepsis. The recommended dose is at least 30mL/kg in the first 3h, although some patients may require higher doses.6 The use of hydroxyethyl starches is currently contraindicated in these patients. Their administration in patients with severe sepsis or septic shock has been associated with an increased incidence of renal failure and renal replacement therapy and, in some studies, with higher mortality.13,14
The use of albumin in resuscitation in severe sepsis and septic shock is controversial. Albumin is currently only recommended to reduce the risk of fluid overload and anasarca in patients who require substantial amounts of crystalloids.6 This risk is increased in patients with cirrhosis who often present significant hypoalbuminaemia, so their use could be more justified in patients with cirrhosis. A recent study in the general population showed no survival benefits for the combined administration of albumin and crystalloids compared to crystalloids alone in patients with severe sepsis.15 Although its usefulness in patients with cirrhosis and severe sepsis and septic shock should be specifically evaluated, there is clear evidence that administration of albumin in patients with spontaneous bacterial peritonitis, a minority with severe sepsis, prevents the development of HRS and improves in-hospital survival.16
Vasoactive supportCurrent guidelines recommend the use of noradrenaline as the vasoconstrictor agent of choice in patients with septic shock.6 Adrenaline (added to or as a substitute for noradrenaline) can be used when another vasopressor agent is needed to maintain adequate mean arterial pressure. Low-dose vasopressin (0.03U/min) and terlipressin infusion are alternatives to adrenaline in these types of patients.17 Dopamine is currently used in selected cases only, due to its high risk of inducing arrhythmias compared to noradrenaline.18 The administration of dobutamine in patients with cirrhosis and septic shock is rare, as these patients usually present high cardiac outputs. It is only recommended in patients with clinically relevant myocardial dysfunction.
However, we should take into account that hyporeactivity to vasopressor agents, and consequently a higher risk of refractory shock, is common in patients with cirrhosis. The presence of relative adrenal insufficiency significantly increases this risk.10
Relative adrenal insufficiency: steroid stress dosingRelative adrenal insufficiency is common in patients with cirrhosis and severe sepsis or septic shock (51–77%), and is associated with haemodynamic instability, renal dysfunction and high mortality (81% compared to 37% in patients without adrenal dysfunction).19–21 The efficacy of steroid stress dosing (50mg/6h i.v.) in patients with cirrhosis and septic shock is unclear. A small cohort study showed that the administration of steroids in patients with adrenal dysfunction improves shock reversal (96% vs 58%) and hospital survival.21 However, a recent randomised study showed no benefit in the administration of steroids.22 Current clinical guidelines in the general population only recommend steroid stress dosing in patients with refractory shock.6
Other support therapiesProtective mechanical ventilationICU mortality of patients with cirrhosis and severe sepsis who require mechanical ventilation is very high (around 70%).23 Protective ventilation (low tidal volumes: 6mL/kg of ideal body weight and plateau pressures<30cmH2O) improves survival in patients with adult respiratory distress syndrome (ARDS) in the general population.6 Cirrhotic patients with ARDS should be ventilated in accordance with these recommendations for the non-liver disease population.10
Sedation and analgesiaAs in the general population, protocols aimed at sedation and daily interruptions/decreases in continuous sedation should be used in liver cirrhosis patients. Drugs with a short half-life, such as propofol and remifentanil, are the preferred options. Benzodiazepines (midazolam) should be avoided in these patients, as their drug elimination capacity is reduced, thereby prolonging the mechanical ventilation time.10,24
Renal replacement therapiesContinuous renal replacement therapies and intermittent haemodialysis are equivalent in septic patients with acute renal failure. Continuous therapies are preferable in patients with haemodynamic instability. Current studies suggest that intensive renal support (35mL/kg/h in continuous techniques or daily intermittent haemodialysis) is not superior to conventional strategies (20–25mL/kg/h).6,10,25 There are no specific studies on renal replacement in patients with cirrhosis and severe sepsis or septic shock.
Blood glucose controlPatients with severe sepsis and significant hyperglycaemia (2 measurements>180mg/dL) who are admitted to the ICU should receive insulin therapy. Hyperglycaemia can act as a procoagulant, induce cell apoptosis and alter neutrophil function. However, strict blood glucose control (80–110mg/dL) in septic patients is not recommended, as it increases hypoglycaemic events and mortality compared with conventional blood glucose control.26 Less strict blood glucose control targets (140–180mg/dL) are currently recommended, and this also extends to patients with cirrhosis.6,10
Blood productsCurrent guidelines in the general population recommend a transfusion threshold of 7g/dL once tissue hypoperfusion has been resolved.6 A similar threshold (7–8g/dL) has been recommended in cirrhotic patients with bleeding due to portal hypertension (Baveno VI).27 Coagulation disorders should not be corrected in the absence of bleeding.10
Instrumentation, nutrition and other prophylactic strategiesPatients with septic shock and a significant percentage of patients with severe sepsis require insertion of a central line and a urinary catheter. Insertion of an arterial line is necessary in patients with septic shock and in patients with severe sepsis and respiratory failure. Placement of vascular catheters does not require correction of coagulation disorders, except in cases of severe thrombocytopenia (≤20×109L−1). Lines and catheters should be removed promptly after resolution of symptoms in order to prevent secondary nosocomial infections.
Patients with cirrhosis often present severe malnutrition that requires early nutritional support. Enteral nutrition is the modality of choice. Refeeding syndrome should be avoided, and vitamin and trace element deficiencies treated. Parenteral nutrition is not recommended in severe sepsis, except in severe malnutrition.
Stress ulcer prophylaxis using H2 or proton pump inhibitors is indicated in patients with cirrhosis and severe sepsis or septic shock. Thrombocytopenia and severe bleeding disorders contraindicate prophylaxis of deep vein thrombosis with low molecular weight heparin in many patients, in whom pneumatic compression stockings should be used.10
Renal failureDefinitionRenal failure is defined as a significant reduction in the glomerular filtration rate (GFR). Traditionally, renal failure in patients with liver cirrhosis is defined as serum creatinine>1.5mg/dL.28 However, this definition has limitations. Firstly, a creatinine value of 1.5mg/dL corresponds to a very low GFR (30mL/min), so the diagnosis is made when the renal failure is already very advanced. Furthermore, this definition does not take into account variations in creatinine with respect to previous values. The Acute Kidney Injury Network (AKIN) classification, initially described in critically ill patients in the general population, defines renal failure on the basis of small variations in the creatinine with respect to previous values, instead of a pre-determined cut-off point.29 This classification defines Acute Kidney Injury (AKI) as an increase in creatinine of ≥0.3mg/dL or of 50% with respect to the baseline value in 48h. Three stages of AKI are defined according to the severity of the renal failure (AKI 1, AKI 2 and AKI 3).
Recent studies in patients with cirrhosis suggest that the combination of both definitions–the AKIN and the traditional–is superior to both classifications separately, as it enables earlier diagnosis to be made and improves its predictive ability. In this respect, stage AKI 1 is divided into two subgroups depending on whether the creatinine at diagnosis of renal failure is less than or greater than 1.5mg/dL (AKI 1A and AKI 1B, respectively).30,31 Although this new classification (modified AKI; Table 2) is not described in clinical guidelines, the emergence of new information coupled with growing interest in its use in cirrhosis justify its description in this consensus. Most cirrhotic patients have renal failure at the time of admission. In order to establish whether they present renal failure based on AKI criteria on admission, a previous serum creatinine value must be used, which by consensus is the last value available in the previous 3 months (1 year if there is no value available in that period).
Proposed new classification for renal failure based on AKI criteria adapted to patients with liver cirrhosis.
| Stage | Criterion |
|---|---|
| AKI 1A | Increase in creatinine≥0.3mg/dL or 50% with respect to the baseline value with a final value ≤1.5mg/dL |
| AKI 1B | Increase in creatinine≥0.3mg/dL or 50% with respect to the baseline value with a final value >1.5mg/dL |
| AKI 2 | 2 to 3-fold increase in creatinine with respect to the baseline value |
| AKI 3 | >3-fold increase in creatinine with respect to the baseline value |
Patients with liver cirrhosis can present renal failure for numerous reasons.28 An aetiological diagnosis is essential, as the treatment and prognosis differ substantially. The most common causes of renal failure are:
- (a)
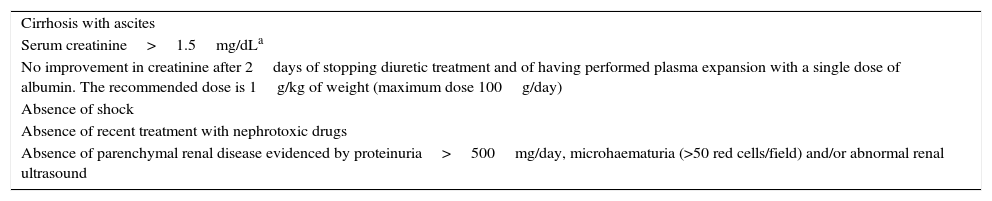
HRS: functional renal failure secondary to intense renal vasoconstriction. The diagnostic criteria for HRS are described in Table 3.32
Table 3.Diagnostic criteria for hepatorenal syndrome.
Cirrhosis with ascites Serum creatinine>1.5mg/dLa No improvement in creatinine after 2days of stopping diuretic treatment and of having performed plasma expansion with a single dose of albumin. The recommended dose is 1g/kg of weight (maximum dose 100g/day) Absence of shock Absence of recent treatment with nephrotoxic drugs Absence of parenchymal renal disease evidenced by proteinuria>500mg/day, microhaematuria (>50 red cells/field) and/or abnormal renal ultrasound - (b)
Hypovolaemia (decreased intravascular volume): the most common causes are excessive doses of diuretics, GI bleeding and diarrhoea.
- (c)
Bacterial infections: these are the most common cause of renal failure, so an infection must be actively ruled out in all cirrhotic patients who present renal failure. It is important to emphasize that in the current definition of HRS, the existence of a bacterial infection is not considered an exclusion criterion in the absence of septic shock.
- (d)
Nephrotoxins: the drugs that most often cause renal failure are non-steroidal anti-inflammatory drugs and aminoglycosides, Antihypertensive drugs such as beta-blockers, angiotensin converting enzyme (ACE) inhibitors or angiotensin II receptor antagonists (ARA-II) can also cause renal failure in cirrhosis.
- (e)
Parenchymal nephropathy: patients with cirrhosis can present parenchymal nephropathy associated with hepatitis C virus (HCV), hepatitis B virus (HBV), alcoholic aetiology, diabetes or hypertension, among others. Its presence should be suspected in the case of abnormal urine sediment (major proteinuria and/or haematuria).
Renal failure is a common complication that is associated with significant morbidity and mortality in patients with cirrhosis. Prognosis depends on the severity and cause of the renal failure. With respect to severity, recent studies suggest that patients with AKI 1A have a good prognosis, similar to that of patients with no renal failure, while patients with AKI 1B present an intermediate prognosis (3-month survival of 68%).30,31 Patients with AKI 2 or 3 present a poor prognosis, with a 3-month survival of 30–40%.
The cause of the renal failure also has a prognostic impact.33 Patients with parenchymal nephropathy have a good prognosis, with a 3-month survival of 73%. Patients with renal failure due to hypovolaemia have an intermediate prognosis (46% at 3 months). Patients with renal failure associated with infections and HRS are those with a poorer prognosis (3-month survival of 31% and 15%, respectively).
Risk groupsPatients with liver cirrhosis are more susceptible to developing renal failure compared to the general population. Patients with a higher risk are those who present ascites and those admitted for acute complications of cirrhosis, basically bacterial infections, hyponatraemia and GI bleeding. Serum creatinine should be monitored every 48–72h in these patients for early detection of renal failure.
TreatmentGeneral measuresAll patients with renal failure require close clinical monitoring. Patients with severe renal failure, especially those on the liver transplant list, should be admitted to an intensive or intermediate care unit. Prompt detection of the onset of intercurrent complications is essential, especially bacterial infections, HE or GI bleeding.34
Placement of a urinary catheter and central venous line in critically ill cirrhotic patients is essential. In all other patients, it should be individualised, since although they provide stricter monitoring, they also increase the risk of infections.
As a general rule, diuretic treatment should be discontinued in all patients with acute renal failure. Patients with large-volume ascites should be treated with paracentesis with expansion with albumin (8g/L of ascites).34 In patients with acute renal failure secondary to hypovolaemia, this should be corrected by administering fluids. However, in most patients with cirrhosis and acute renal failure, sodium and fluid intake must be reduced, as they generally present increased extracellular volume with ascites, oedemas and dilutional hyponatraemia.
There are no definitive studies to contraindicate the use of beta-blockers in patients with acute renal failure. Nevertheless, it is likely that the administration of beta-blockers should be discontinued in these patients due to their potential negative effect: reduced cardiac output and secondary hypotension.
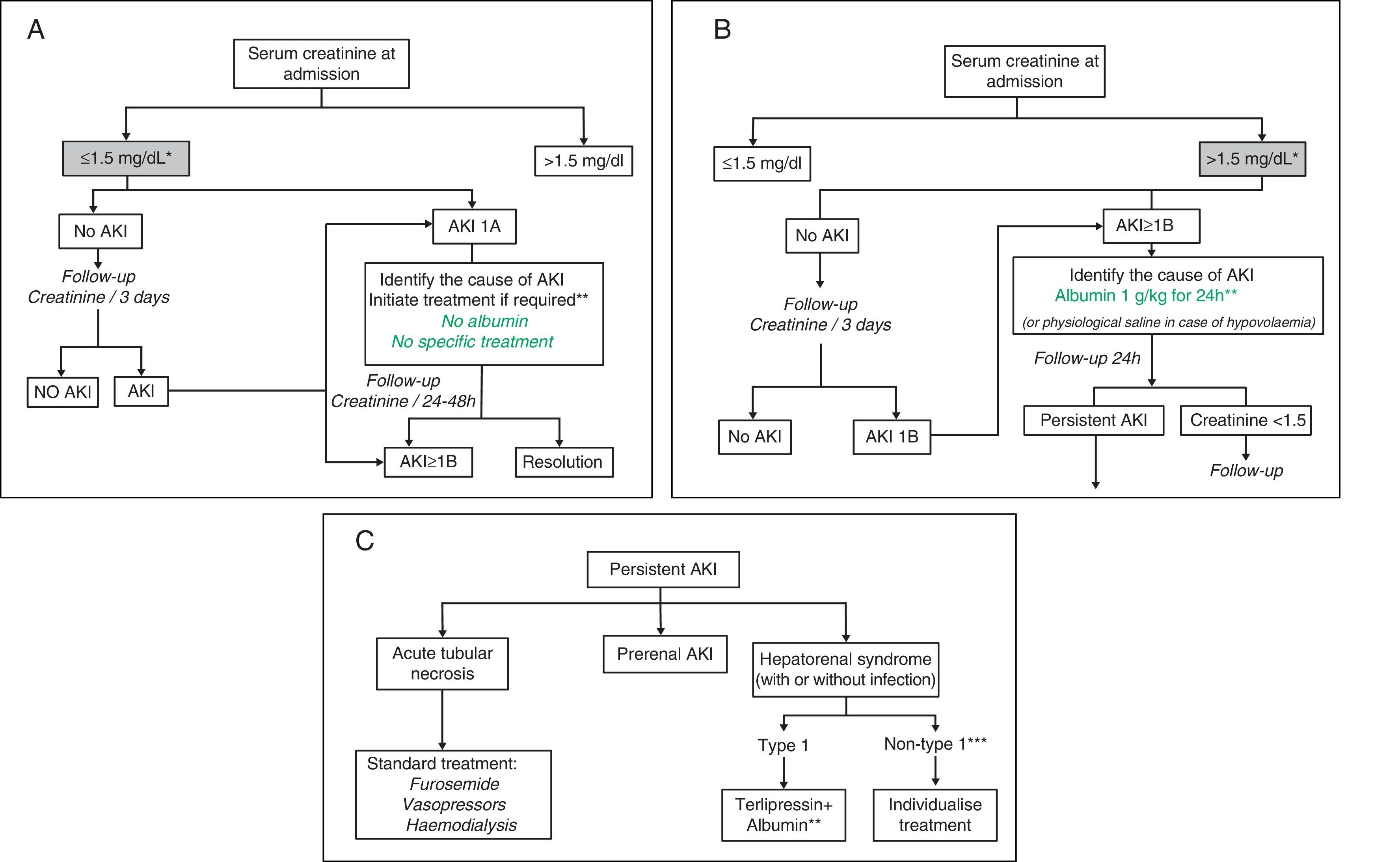
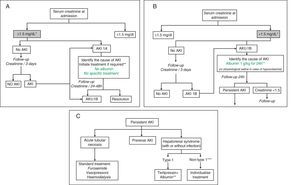
Treatment algorithmsFig. 1A–C shows the proposed evaluation and treatment algorithms for renal failure in cirrhosis according to serum creatinine on admission and the development of renal failure in accordance with modified AKI criteria.30,31 These algorithms should also be applied to patients who meet AKI criteria during their hospital stay.
Proposed diagnostic and therapeutic algorithm for acute renal failure in patients with cirrhosis according to the serum creatinine value at admission and development of renal failure: AKI classification adapted to patients with liver cirrhosis. (A) Patients with serum creatinine at admission ≤1.5mg/dL. * The baseline creatinine value used for the definition of AKI is the last creatinine available in the last 3 months prior to admission. ** The cause of the renal failure should be identified promptly to initiate treatment as soon as possible. Diuretic treatment should be discontinued in all patients with renal failure, and potentially nephrotoxic drugs eliminated. It is essential to rule out the existence of a bacterial infection as the cause of the renal failure. There are no studies on the efficacy of intravenous albumin in patients with AKI 1A. (B) Patients with serum creatinine at admission >1.5mg/dL. * The baseline creatinine value used for the definition of AKI is the last creatinine available in the last 3 months prior to admission. ** Intravenous albumin should be administered slowly to avoid volume overload. (C) Patients with persistent AKI. * Defined as patients who present gradual deterioration in renal function or those with AKI 1B in whom the serum creatinine has fallen <25% with respect to the creatinine of the previous 48h. ** Treatment with terlipressin is initiated at doses of 1mg/4h. If serum creatinine has not fallen by 25% with respect to the baseline value at 48h, the dose should be increased to 2mg/4h. A decrease in serum creatinine to values <1.5mg/dL is considered a complete response. Treatment should be maintained until a response is obtained or for a maximum of 14 days. If the creatinine has not fallen by >25% on day 7, treatment should be discontinued. Intravenous albumin should be concomitantly administered at doses of 20–40g/dL.7,8 *** Patients with HRS but who do not meet HRS type 1 criteria.
The first choice treatment in patients with type 1 HRS is the administration of vasoconstrictor drugs (terlipressin, noradrenaline or midodrine) and albumin.34,35 Most studies and information available refer to terlipressin administered in bolus form,36–38 so we consider first-line treatment of type 1 HRS in Spain to be terlipressin, where this drug is available, in combination with albumin. Recent studies suggest that continuous infusion of terlipressin could improve efficacy and reduce the side effects of the drug. Nevertheless, there is as yet insufficient evidence to allow us to firmly recommend this method of administration. In patients who are not candidates for liver transplant, the decision to treat type 1 HRS with vasoconstrictor drugs and albumin should be made on an individual basis.
Liver transplantLiver transplant is the definitive treatment for patients with type 1 and type 2 HRS.34,35 For this reason, all patients with HRS should be referred to a liver transplant centre. HRS is reversible after transplantation, so liver transplant only should be indicated in these patients. Double hepatorenal transplant should only be considered in patients who have required prolonged renal support for 6–8weeks, as the likelihood of reversal in these patients is very low.
Patients with type 1 HRS should be given priority on the transplant waiting list, due to the high risk of mortality. The implementation of the Model for End-Stage Liver Disease (MELD) waiting list prioritisation system has made it easier for these patients to receive a transplant. Several groups have proposed that the creatinine prior to the start of treatment be used to calculate the MELD score in these patients, thus giving them priority on the transplant list.39
Although there is no information from prospective studies, current clinical guidelines recommend that patients with type 1 HRS be treated with vasoconstrictors and albumin before the liver transplant, as improvement in renal function prior to the transplant may improve the prognosis in the post-transplant period.34,35
Renal replacement therapyNo studies have specifically evaluated the use of renal replacement therapies (RRT) in patients with type 1 HRS. RRT is not considered a first-line treatment, as it does not act on the pathophysiology of HRS. RRT, either continuous or intermittent according to the patient's haemodynamic tolerance, is only indicated in patients with type 1 HRS who do not respond to pharmacological treatment, and who develop indications for urgent dialysis (hypervolaemia, hyperkalaemia, metabolic acidosis, etc.).34,35 Nevertheless, clinical experience suggests that this situation is rare in patients with type 1 HRS.
Other alternative dialysis methods, such as the molecular adsorbent recirculating system (MARS) or fractionated plasma separation and adsorption (Prometheus) have been proposed as potential treatments for HRS.40,41 However, information on their usefulness in these patients remains limited and inconclusive.
Renal replacement therapy in renal failure in liver cirrhosisAs previously described for HRS, RRT in general is not a first choice treatment in patients with cirrhosis and renal failure. However, there are specific situations in which its use should be considered. With respect to the type of RRT, continuous or intermittent, the decision should be made on an individual basis according to the patient's haemodynamic stability and tolerance. Situations in which RRT should be indicated in patients with cirrhosis are: (a) type 1 HRS (see previous section) and (b) acute tubular necrosis: in patients who do not respond to standard treatment with furosemide infusion and/or vasopressors and who develop criteria for RST (hypervolaemia, metabolic acidosis, severe hyponatraemia, hyperkalaemia, etc.).
Indications for admission to intensive or intermediate care unitsAdmission to critical care units is indicated in patients with type 1 HRS who are candidates for transplant, and patients with renal failure associated with other severe complications of cirrhosis who require intensive monitoring (sepsis, GI bleeding, etc.).
Indications for contacting a transplant reference centreDuring the acute process, a transplant centre should be contacted in the case of patients with type 1 HRS or with any other type of renal failure if the patient is on the waiting list or is a potential candidate for transplant. In the case of patients with type 1 HRS, treatment should be initiated with terlipressin and albumin in accordance with the protocol and transplant centre contacted for transfer. At discharge, a transplant centre should be contacted in patients with type 2 HRS or with parenchymal renal failure who meet transplant criteria.
Hepatic encephalopathyDefinition, symptoms and classificationHE is a brain dysfunction caused by liver failure and/or portosystemic shunts.42 Other important elements in the pathophysiology of HE in patients with cirrhosis are alterations in the microbiota, hyperammonaemia and the inflammatory response.43 In most cases, there is a precipitating factor, mainly bacterial infections, constipation, GI bleeding and drugs such as diuretics and sedatives.42 HE manifests as a wide spectrum of neurological and/or psychiatric abnormalities which range from subclinical alterations (minimal HE, only detectable by neuropsychological or neurophysiological tests) to coma. As it is a continuous spectrum, it is not easy to classify. The most widely used classification continues to be the West Haven classification, which ranges from grade I (reduced attention span), grade II (disorientation for time and place, lethargy), grade III (gross disorientation, severe confusion, stupor, somnolence, but responsive to stimuli), and grade IV (coma).42 It is often difficult to differentiate between grades I and II. The International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) has recently established a new classification for HE in cirrhosis: “covert” HE, which includes minimal HE and HE grade I and “overt” HE, which includes grades II, III and IV.42,44
Prognosis and risk groupsHE is a common complication, since 30–40% of patients with cirrhosis present an episode of overt HE at some time during the course of their illness.42,45 It also determines a poor prognosis, as mortality within 1 year of an episode of HE is approximately 50%.46 It is a cause of frequent readmissions and severe deterioration in the quality of life of patients and their family, and is not only a considerable family burden, but also a social and economic burden for the community.42,47,48 Furthermore, recent studies have shown that repeated episodes of HE can leave permanent cognitive sequelae in patients.49 Some predisposing factors for overt HE are previous HE, minimal HE, hyponatraemia, renal failure, grade of hepatic failure and presence of large calibre portosystemic shunts.42,43,50,51
DiagnosisThe diagnosis of HE is based on the patient's history and the clinical examination. The presence of asterixis (flapping tremor) is common but not pathognomonic.42,42 Imaging tests such as brain computed tomography (CT) are often necessary to rule out other causes of neuropsychological alterations, such as a brain haemorrhage, ischaemic stroke or brain tumour.42,43 Patients with cirrhosis are reported to have a 5 times higher risk of brain haemorrhage compared to the general population,52 although this finding has not been confirmed by other authors.53
Blood ammonia determination is of little use, as it does not contribute to the diagnosis, staging or prognosis of HE. Furthermore, in order for the results to be reliable, a series of technical requirements must be followed (sample must be kept cold and analysed shortly after extraction). However, normal ammonia levels call into question the diagnosis of HE.42
Treatment of acute hepatic encephalopathyTreatment of HE requires rapid intervention. The goals of this intervention are (a) to promptly identify and treat the precipitating factor, which can be potentially serious, such as an infection or GI bleed, and (b) to prevent progression of the HE and the complications derived from a decreased level of consciousness, such as pulmonary aspiration.42,43,47
The treatment of patients with cirrhosis and HE will depend on the grade of HE. Patients with HE grade I or II can often be discharged from the emergency department if they improve during their stay. In contrast, patients with more severe grades (grade III–IV) require admission to an ICU and orotracheal intubation.23,42 Patients with grades I or II are usually admitted to conventional hospital wards.
In patients with HE grade III or IV, a nasogastric tube should be inserted to administer medication and nutrition after intubation, until their level of consciousness allows resumption of oral feeding.42,47
The most important point in the treatment of HE is the systematic search for and subsequent treatment of the precipitating factor: bacterial infections, GI bleeding, constipation, renal failure, electrolyte disorders, or drugs such as diuretics and sedatives.42,43,47
Another important aspect of the treatment of acute HE is decreased blood ammonia, acting especially on the microbiota through non-absorbable disaccharides or antibiotics.42,43 First-line treatments are non-absorbable disaccharides, lactulose and lactitol. They are usually administered orally (HE grade I and potentially II), but also by nasogastric tube (grades II–III–IV) or in enema form when the patient's condition does not permit oral administration.42,43,47,54 As an alternative to disaccharides, non-absorbable antibiotics can be used (neomycin, metronidazole and, more recently, rifaximin at a dose of 400mg/8h).42 However, there is insufficient evidence on the usefulness of rifaximin in combination with disaccharides in the treatment of an episode of HE.47 Rifaximin, added to lactulose, prevents recurrence of HE.55 Other treatments that decrease blood ammonia, such as l-ornithine-l-aspartate (LOLA) or ornithine phenylacetate, are not used in routine clinical practice because they are either not available or still in the research phase.
Flumazenil can temporarily improve the level of consciousness in patients with HE, especially in patients with a history of previous benzodiazepine ingestion. It can be tested in certain situations to confirm the diagnosis of HE triggered by benzodiazepines, by confirming the reversibility of clinical symptoms.42
Albumin dialysis systems (MARS) improve the level of consciousness in patients with HE grades III and IV who do not respond to conventional treatment.56 This treatment however, aside from being complex, costly and not widely available, has not been show to improve survival, and as such is rarely used.47,56
Nutritional support without protein restriction must be maintained in patients with HE. Low protein diets, which lead to loss of muscle mass, are not justified and can worsen the patient's nutritional status and increase blood ammonia. Intravenous branched-chain amino acids are not useful in the treatment of HE, but oral supplements can help improve the nutritional status.42,43,57
Indications for admission to intensive or intermediate care unitsAs previously mentioned, patients with HE grade III or IV require admission to an intermediate or intensive care unit for strict monitoring and mechanical ventilation, if required.42,54 Orotracheal intubation is considered essential in patients scoring less than 8 on the Glasgow coma scale.54 Sedatives should be avoided in these patients.54 When they are essential, it is preferable to use those with a short half-life and limited liver metabolism, such as remifentanil and propofol.
Liver transplantationConsidering the poor prognosis of patients who have presented an episode of HE, most should be evaluated for liver transplantation and referred to a reference centre.42 This evaluation may be postponed in patients with preserved liver function, as they have a better prognosis and might benefit from other treatments, such as embolisation of portosystemic shunts in patients with a low MELD score (<12).51
The aforementioned measures allow HE episodes to be treated in a hospital without requiring highly complex techniques. However, an episode that is difficult to control due to its clinical course or the precipitating factors (GI bleeding, severe sepsis) in a patient awaiting transplantation should prompt their physician to contact the transplant centre to assess their transfer.
Prevention of recurrenceEducation of patients and their family is very important: identification of early signs and symptoms, nutritional advice, treatment adherence and modification, avoiding constipation and administration of enemas.42,47 Non-absorbable disaccharides are recommended to prevent recurrence after the first episode of HE, adding rifaximin after the second episode.42,55 These treatments are not routinely recommended after placement of a transjugular intrahepatic portosystemic shunt (TIPS).58 Embolisation of portosystemic shunts detected by ultrasound or CT scan can be effective in the prevention of recurrent HE in patients with preserved liver function.51 Patients with TIPS and recurrent HE can consider reducing the TIPS lumen.59
Gastrointestinal bleeding due to gastro-oesophageal varicesDefinitionsA gastrointestinal bleed in a patient with cirrhosis is considered to be due to gastro-oesophageal varices in any of the following 3 situations: (a) active bleeding from an oesophageal or gastric varix; (b) presence of signs of recent bleeding in a varix (fibrin clot or adherent clot); or (c) presence of blood in the stomach in the absence of other potentially bleeding lesions.
Onset of bleeding (time zero) is considered to be the time at which medical treatment commenced, or the time at which bleeding appears if it presents in an inpatient.
The bleeding period has a duration of 120h (5days). Most early rebleeding and complications associated with the bleed occur within this interval.
Therapeutic failure is considered as the death of the patient or the need to switch treatment, defined as: (a) haematemesis or nasogastric aspirate of more than 100mL of fresh blood in 2h, despite having initiated pharmacological and/or endoscopic treatment; (b) onset of hypovolaemic shock; or (c) decrease of 3g or more in haemoglobin levels (9% of haematocrit) over a period of 24h without transfusion.
Early rebleeding is bleeding that occurs in the first 5days, with an incidence of 10–15%.27,60
Prognostic factorsWith current treatment, which consists of a combination of vasoactive drugs, endoscopic treatment and antibiotics, control of an acute bleed can be achieved in 80–90% of cases. Despite prognostic improvement in recent years, current mortality is still 15–20% in the first 6 weeks after the initial episode.27,60
Rebleeding occurs in up to 15–20% of patients treated during the first 6weeks. Approximately 40% of these new bleeds appear in the first 5 days after the bleeding episode. Moreover, initial treatment failure occurs in 10–20% of cases, with a mortality rate that can exceed 50%.27
In previous studies, the finding of an active bleed in urgent endoscopy, as well as visualisation of large varices, were risk factors for rebleeding.27 However, recent studies on the current effectiveness of endoscopic treatment (band ligation) have shown no differences in therapeutic failure in patients with active bleeding at the time of the endoscopy, compared to those without.61
An HVPG of ≥20mmHg during the first 48h has been associated with an elevated risk of rebleeding and death. However, other clinical variables, such as grade of hepatic failure, determined by the Child–Pugh or MELD score, also have an important predictive value for treatment failure at 5days. Thus, patients with Child C or MELD≥18 have a high likelihood of recurrence or death.
The presence of portal vein thrombosis, bacterial infections, and renal failure are also associated with a greater risk of treatment failure at 5days. Antibiotic prophylaxis has been shown to improve prognosis in these patients by reducing the rebleeding rate and mortality. Renal failure–present in up to 10% of patients–is an independent factor for mortality.60 Patients with baseline creatinine ≥1.35mg/dL have a higher 6-week mortality.
Risk groupsThe aim of stratifying patients into risk groups is to identify those individuals with a higher probability of treatment failure or with a poorer prognosis who might therefore benefit from a more aggressive therapeutic strategy. Recent cohort studies have evaluated different predictive models that identify the factors that define patients with a higher risk of treatment failure.
Thus, patients with HVPG≥20mmHg within the first 24h of admission have a higher risk of failure and a higher likelihood of associated complications than patients with HVGP<20mmHg. Determination of HVPG has been shown to be one of the best predictors of treatment failure and long-term survival; hepatic vein catheterisation also enables early decompression of the portal venous system to be performed by implantation of a TIPS.
Child–Pugh B patients with active bleeding at the time of the endoscopy or Child–Pugh C patients also have a high risk of treatment failure and, therefore, a poor prognosis. Despite this, questions have been raised as to whether Child–Pugh B patients with active bleeding are really a high risk group.61
Due to the fact that HVPG measurement cannot be performed in all hospitals, and that certain models use subjective variables (Child–Pugh, active bleeding at endoscopy), a more objective model has been proposed based on the MELD score. In this study, patients with MELD≤11 presented a 5% risk of mortality (low risk), while patients with MELD≥19 had a 20% risk (high risk).62 The only predictive risk stratification model that has been validated in external cohorts is the one based on the MELD score. Further studies are therefore necessary to validate these stratification models and to be able to correctly identify patients who would benefit from a different therapeutic strategy.
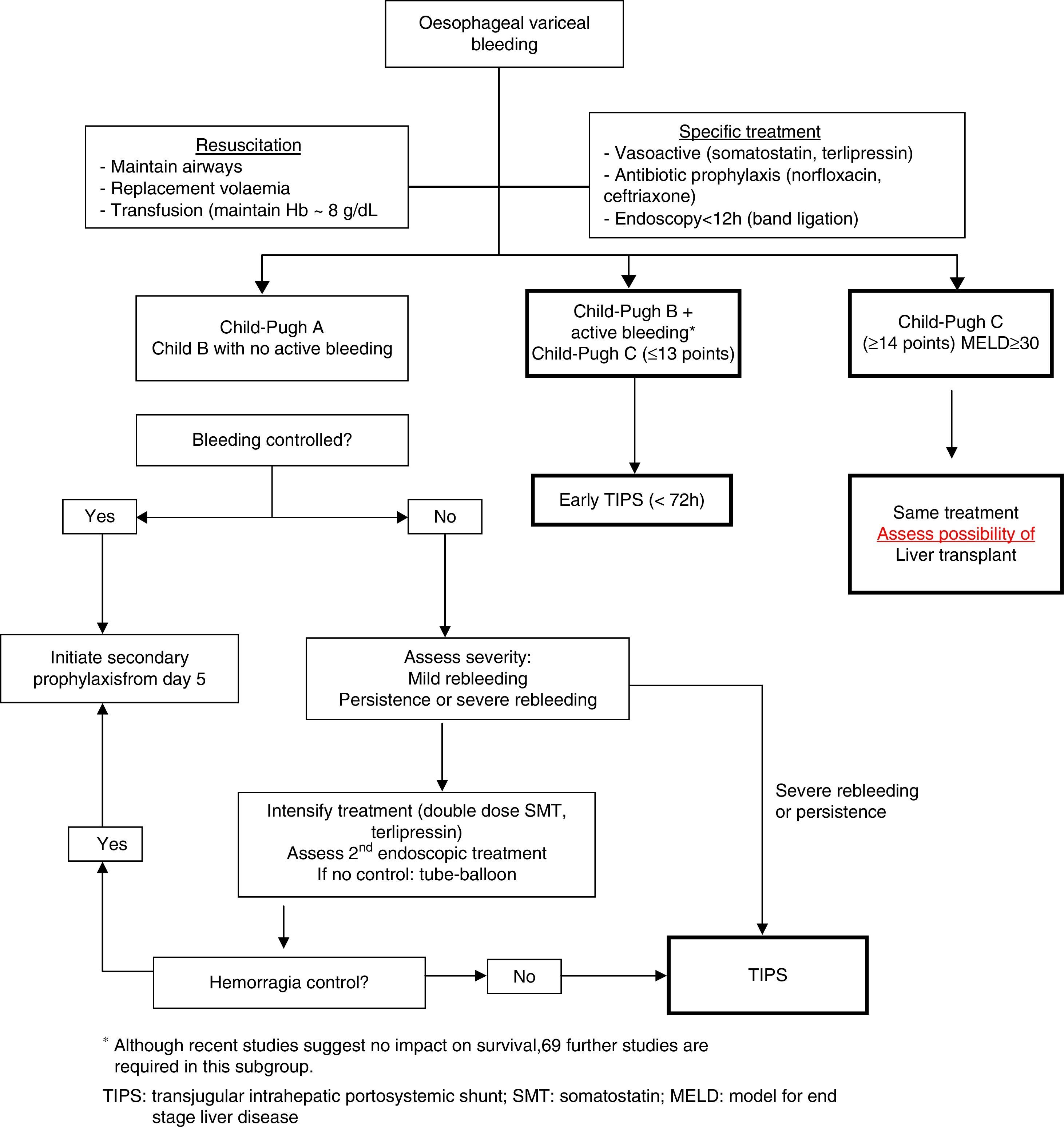
TIPS implantation within the first 72h of the bleeding episode (ideally within the first 24h) could benefit patients previously defined as high risk.63 However, in the study that described HVPG to define high risk patients, current standard treatment was not used in the medical treatment arm, which could explain why the results in this group were poorer than expected.64
Other special situations, such as the presence of advanced hepatocarcinoma, haemorrhagic shock, hepatic coma or sepsis, can worsen the prognosis of cirrhotic patients with variceal bleeding.
TreatmentGeneral measuresThese comprise the initial resuscitation and airway protection measures. The risk of respiratory complications and bronchoaspiration is high in patients with massive bleeding or presence of HE, and for this reason the need for orotracheal intubation must be assessed.
Volume replacement must be started promptly to maintain systolic blood pressure between 90 and 100mmHg. Colloids are the solutions of choice as they cause fewer alterations in haemostasis and anaphylactic reactions than dextrans. There is no specific recommendation on the use of fresh frozen plasma and platelets in these patients, although it seems reasonable to transfuse patients with active bleeding and severe coagulopathy. The use of coagulation factors, such as recombinant factor VII, is not recommended. Despite the fact that recent recommendations advocate maintaining haemoglobin levels between 7 and 8g/dL, these are based on expert opinions.27 These recommendations have been validated and confirmed in the only study published to date, in which patients with restrictive transfusion (transfusion if Hb<7g/dL, to maintain levels between 7 and 9g/dL) had a lower rebleeding rate and better survival than patients with liberal transfusion (transfusion if Hb<9g/dL, to maintain levels between 9 and 10g/dL). This benefit was observed in Child–Pugh A and B patients, with no significant differences in Child–Pugh C patients.65 This study reinforces the recommendation to avoid overtransfusion in patients with gastro-oesophageal variceal bleeding, although the final decision must be made on an individual basis according to the patient's age and associated comorbidities, such as ischaemic heart disease and heart failure, among others.
A recent meta-analysis confirms that antibiotic prophylaxis is associated with a significant reduction in mortality, rebleeding rate and hospitalisation time.66 Although norfloxacin continues to be the standard therapy, in patients with advanced liver disease (Child–Pugh B and C), in those who are on prophylaxis with quinolones and in settings with a high prevalence of quinolone resistance, i.v. ceftriaxone is the antibiotic of choice. Treatment duration is 7days.
Specific treatmentCombined treatment with vasoactive agents and endoscopic therapy is the primary haemostatic treatment, achieving initial control of the bleeding episode in between 80% and 90% of cases.
Vasoactive drugsThese should be administered as early as possible, and before endoscopy, since this strategy improves initial control of the bleeding, facilitates performance of endoscopic therapy and reduces the risk of early recurrence. The usual regimens are:
- (a)
Somatostatin: 250μg/h in continuous infusion. Boluses of 250μg/h (can be repeated up to three times in the first 3h). Patients with active bleeding can benefit from double doses (500μg/h).
- (b)
Terlipressin: 2mg/4h i.v. bolus (1.5mg in patients weighing between 50–70kg, 1mg in patients weighing less than 50kg) during the first 48h, and 1mg/4h until day 5. There is no evidence to recommend the use of both vasoactive drugs combined.
The current recommendation is to maintain vasoactive treatment for 2–5days.
Endoscopic treatmentEndoscopic variceal ligation (EVL) is the treatment of choice in acute episodes.27 Sclerotherapy is only indicated when ligation is not technically possible. EVL should generally be done as soon as possible, preferably in the first 6–12h, once the patient has been stabilised. For endoscopy sedation, propofol is preferable to other sedatives due to its pharmacokinetic characteristics.
Transjugular intrahepatic portosystemic shuntThe main indication for TIPS has traditionally been as rescue treatment following combined endoscopic and pharmacological treatment failure. TIPS controls bleeding in more than 95% of cases, despite it being associated with a high mortality rate. This is because patients with standard treatment failure have more severe liver disease (Child–Pugh C), have presented rebleeding and have a severe deterioration in liver function, all of which lead to higher mortality when TIPS is implanted. Early TIPS has been proposed as a means of avoiding treatment failure in high risk patients and thus improving survival. A preliminary study showed that early placement of uncovered TIPS in high risk patients (identified with HVPG>20mmHg) was associated with a lower rate of early rebleeding and longer survival.64 A recent study explored this concept in patients at high risk of failure (Child–Pugh B with active bleeding and Child–Pugh C≤13). Patients with early TIPS had a lower rate of rebleeding or failure to initially control the bleeding (3% vs 45%; p=0.001) and lower mortality (12% vs 39%; p=0.01) with respect to the control group, with no differences observed as regards the presence of HE.63 Despite the fact that its efficacy in the initial control of bleeding and in the prevention of early rebleeding have been confirmed in a real clinical practice setting,67,68 its real impact on survival requires further confirmation.69
Balloon tamponade and oesophageal stentsIn patients with massive bleeding or refractory haemodynamic instability, bridging treatment should be started to control the bleeding episode before the implementation of more definitive treatment, such as TIPS. The Sengstaken–Blakemore tube and the Linton tube (for oesophageal and gastric varices, respectively) achieve haemostasis in almost 90% of cases, despite the fact that their use is associated with a high rate of serious complications such as pneumonia, oesophageal ulcers and even perforation. It is highly advisable to intubate the patient before the procedure, and to maintain the tamponade for the least time possible, not more than 24h. To avoid the drawbacks of the abovementioned techniques, the efficacy of self-expandable oesophageal stents in these patients has recently been explored in small case series, reporting bleeding control rates of 100%.69 The advantages of the stents are better bleeding control, a significant reduction in serious adverse effects, and in the fact that they can be left in the oesophagus for up to 7–10days, allowing the patient to be fed. Even so, randomised controlled clinical trials are needed to determine their real role in the management of severe bleeding.
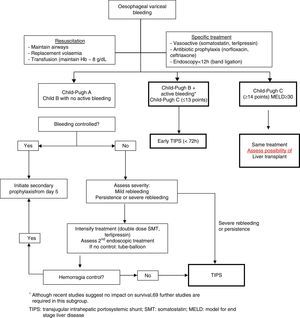
Fig. 2 shows a treatment algorithm for upper GI bleeding due to gastro-oesophageal varices in liver cirrhosis.
Criteria for admission to intensive and intermediate care unitsPatients with cirrhosis who require admission to the ICU have higher mortality than any other type of critically ill patient, since their prognosis depends not only on the severity of their liver disease, but also on the dysfunction of other organs.10 Despite this, acute variceal bleeding, as a complication of portal hypertension, has a better prognosis than other complications of cirrhosis, such as severe sepsis or HRS. Although prognostic indices are very useful, they should not be used categorically to exclude patients who require super-specialised care, but as tools to aid clinical decision-making. Recent studies have shown that early admission to the ICU is one of the strategies that improves survival in these patients.70
Admission to the ICU should be assessed in different situations: (a) severe upper GI bleeding or uncontrolled upper GI bleeding that requires the use of a balloon tamponade; (b) patients with criteria for transplantation, regardless of the severity of the bleeding; and (c) patients with treatment failure in the first 5days.
Despite the recommendations, each case must be considered on an individual basis and referral to a reference centre evaluated early on (preferably within the first 24–48h) for assessment of TIPS placement and/or liver transplantation.
Futility situationsAlthough treatment must be individualised according to each patient's situation and specific characteristics, futility situations must be considered: advanced liver failure (Child–Pugh≥14), in which TIPS is contraindicated, although it can be attempted to optimise the initial treatment; multifocal hepatocarcinoma (stage D of the Barcelona clinic liver cancer [BCLC] classification); acute haemorrhage in patients with MELD≥30, and patients with ACLF type 3 (failure of 3 organs).
Acute-on-chronic liver failureApproximately 30% of patients admitted as a result of decompensation of cirrhosis present ACLF. This recently defined syndrome is characterised by the development of organ failure(s), with or without a precipitating factor, and has a high short-term mortality (33% at 1 month and 51% at 3months).71,72 This high mortality calls for early diagnosis of the syndrome and its potential precipitating factor, admission to the ICU or intermediate care facility in many cases, and referral of the patient to specialised centres.
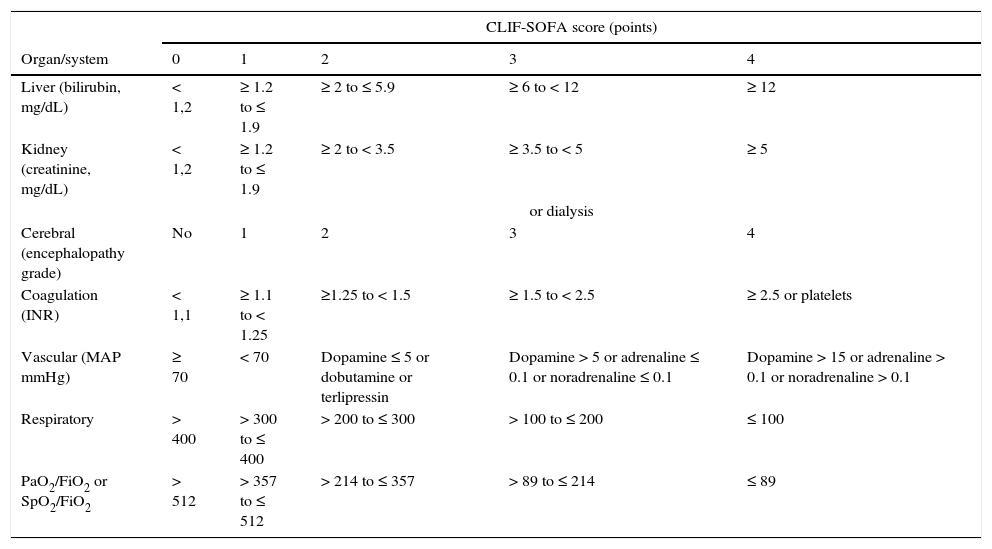
The criteria for organ failure used to define ACLF derive from an adaptation of the sepsis-related organ failure assessment (SOFA) for patients with cirrhosis (Table 4). Liver failure is defined based on a serum bilirubin≥12mg/dL; renal failure as a creatinine≥2mg/dL; cerebral failure as HE grade 3 or 4; coagulation failure based on an INR≥2.5 or a platelet count≤20×109L–1; circulatory failure on the need to use vasopressors to maintain arterial pressure, and respiratory failure on a PaO2/FiO2≤200 or SpO2/FiO2≤214.71 Active alcoholism and bacterial infections, especially spontaneous bacterial peritonitis, bacteraemia and pneumonia, are the most common triggers of ACLF. In 40–50% of patients, no precipitating factor can be identified. ACLF is not a syndrome typical of patients with terminal cirrhosis, but a complex entity frequently observed in young patients (mean age 56±12years vs 58±12years in patients without ACLF), which in many cases is the first manifestation of their liver disease (27%). The short term mortality (28days) of patients admitted with ACLF with no previous decompensations is higher than that observed in previously decompensated patients (42% vs 30%).71,72
Definition of organ failure in patients with liver cirrhosis.
| CLIF-SOFA score (points) | |||||
|---|---|---|---|---|---|
| Organ/system | 0 | 1 | 2 | 3 | 4 |
| Liver (bilirubin, mg/dL) | < 1,2 | ≥ 1.2 to ≤ 1.9 | ≥ 2 to ≤ 5.9 | ≥ 6 to < 12 | ≥ 12 |
| Kidney (creatinine, mg/dL) | < 1,2 | ≥ 1.2 to ≤ 1.9 | ≥ 2 to < 3.5 | ≥ 3.5 to < 5 | ≥ 5 |
| or dialysis | |||||
| Cerebral (encephalopathy grade) | No | 1 | 2 | 3 | 4 |
| Coagulation (INR) | < 1,1 | ≥ 1.1 to < 1.25 | ≥1.25 to < 1.5 | ≥ 1.5 to < 2.5 | ≥ 2.5 or platelets |
| Vascular (MAP mmHg) | ≥ 70 | < 70 | Dopamine ≤ 5 or dobutamine or terlipressin | Dopamine > 5 or adrenaline ≤ 0.1 or noradrenaline ≤ 0.1 | Dopamine > 15 or adrenaline > 0.1 or noradrenaline > 0.1 |
| Respiratory | > 400 | > 300 to ≤ 400 | > 200 to ≤ 300 | > 100 to ≤ 200 | ≤ 100 |
| PaO2/FiO2 or SpO2/FiO2 | > 512 | > 357 to ≤ 512 | > 214 to ≤ 357 | > 89 to ≤ 214 | ≤ 89 |
The CLIF-SOFA includes sub-scores from 0 to 4 for the 6 organs/systems evaluated; the greater the organ dysfunction, the higher the score. The catecholamine doses are shown in μg/kg/min.
FiO2: fraction of inspired oxygen; MAP: mean arterial pressure; PaO2: partial pressure of arterial oxygen; SpO2: oxygen saturation by pulse oximetry.
The shaded area defines the organ failures.
The pathogenesis of ACLF is unknown. Studies conducted in the 1990s showed that decompensated liver cirrhosis involves a major systemic inflammatory response, with an increase in plasma pro-inflammatory cytokines (IL-6, TNF-alpha) and oxidative stress.73,74 It has been suggested that this inflammatory response – moderate in decompensated cirrhosis – is more intense in patients with ACLF, leading to the involvement of other organs or systems.72 The severity of the ACLF is correlated with the degree of systemic inflammation. Patients with more severe forms of ACLF have a higher blood leucocyte count and CRP levels than patients with less severe forms. Individual susceptibility/tolerance to organ failure is another factor that could be implicated in its pathogenesis.
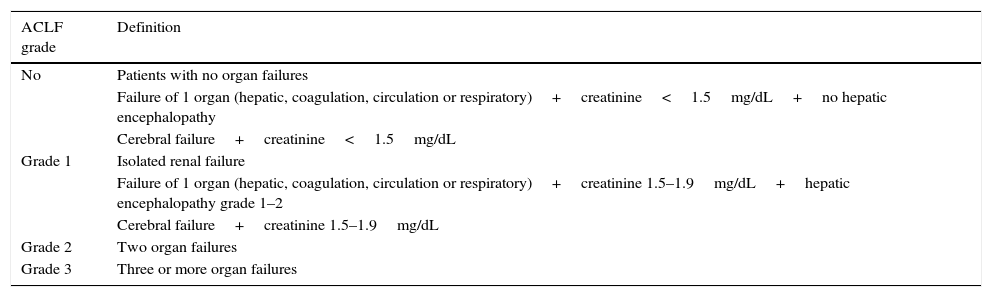
ACLF gradesThe CANONIC study classifies patients with ACLF into three grades based on the number of organ failures at the time of diagnosis of the syndrome and associated mortality (Table 5). The no ACLF group includes: (a) patients with no organ failure; (b) patients with a single “non-kidney” organ failure (i.e. single failure of the liver, coagulation, circulation, or respiration) who have a serum creatinine level<1.5mg/dL and no HE, or (c) patients with single cerebral failure who have a serum creatinine level <1.5mg/dL. These patients have a very low 28-day mortality (1.9%). Patients with ACLF grade 1 are those who present single kidney failure or single organ failure with serum creatinine≥1.5mg/dL and/or HE grade 1–2. ACLF grade 2 is defined based on the presence of two organ failures, and ACLF grade 3 when there are 3–6 organ failures. In the CANONIC study, 51% of patients with ACLF were grade 1, 35% were grade 2 and 14% were grade 3.71
Definition of ACLF and grades.
| ACLF grade | Definition |
|---|---|
| No | Patients with no organ failures |
| Failure of 1 organ (hepatic, coagulation, circulation or respiratory)+creatinine<1.5mg/dL+no hepatic encephalopathy | |
| Cerebral failure+creatinine<1.5mg/dL | |
| Grade 1 | Isolated renal failure |
| Failure of 1 organ (hepatic, coagulation, circulation or respiratory)+creatinine 1.5–1.9mg/dL+hepatic encephalopathy grade 1–2 | |
| Cerebral failure+creatinine 1.5–1.9mg/dL | |
| Grade 2 | Two organ failures |
| Grade 3 | Three or more organ failures |
As previously mentioned, the prognosis for patients with ACLF depends on the number of organ failures, as defined by the CLIF-SOFA score (Table 4). The overall 28-day mortality is remarkable – around 30% – and varies between 22% in patients with ACLF grade 1, 32% in patients with ACLF grade 2, 68% in patients with 3 organ failures and 89% in patients with 4 or more organ failures at ACLF diagnosis.71
A recent study showed that ACLF is a dynamic process; as such, the prognosis can be more accurately determined after analysing the patient's clinical course.75 ACLF resolves or improves to grade 1 in almost 50% of patients and worsens in around 20% of cases. The 28-day transplant-free mortality was relatively low (6–18%) in patients with a non-severe early course (final no ACLF or ACLF-1) and high (42–92%) in patients with a severe early course (final ACLF-2 o -3), irrespective of the initial ACLF grade. Independent predictors of course severity (final ACLF-2 or -3) were the CLIF-Consortium ACLF score (CLIF-C ACLFs) and presence of liver failure (total bilirubin≥12mg/dL) at ACLF diagnosis. Most patients reached the final ACLF grade during the first week after diagnosis, so evaluation at 3–7days can predict their short-term mortality more accurately than the initial evaluation. Without transplant, patients with 4 or more organ failures at 3–7days after diagnosis or a CLIF-C ACLFs>64 had a 28-day mortality of 100%. In contrast, patients with ACLF grade 2–3 who underwent early transplant had a 1-year survival rate of 75%.75 These data suggest that in patients with ACLF, the prognosis and the resulting therapeutic decisions must be established not when the syndrome is diagnosed, but after evaluating the patient's response to treatment in the short term (3–7days).
TreatmentThe high mortality in patients with ACLF calls for early diagnosis of the syndrome and admission of many of these patients to intensive or intermediate care units. This is necessary if there is vascular failure (shock), respiratory failure or cerebral failure (encephalopathy grade III-IV), and is advisable in patients with renal failure.10 In the CANONIC study, 50% of patients with ACLF were admitted to the ICU (86% of patients with ACLF grade 3).71
If liver transplantation is not contraindicated because of the patient's age or associated comorbidities, patients should be referred to a transplant centre, as this is currently the only treatment that improves survival in these patients. Patients who are actively drinking should also be referred to specialised units, as they may benefit from potential investigational therapies.
A key aspect in the management of patients with ACLF is to treat the precipitating factor early and appropriately (correct antibiotics in infected patients, steroids in patients with severe acute alcoholic hepatitis, etc.).10 Artificial liver support systems have also become available in recent years, and use dialysis techniques to remove both water- and fat-soluble substances from the plasma (dialysis with albumin). The most widely used of these systems is the MARS, although there are 2 other similar systems: the PROMETHEUS and Single-Pass Albumin Dialysis (SPAD).76,77
Several studies have shown that the MARS system: (a) significantly reduces serum levels of total and conjugated bilirubin, bile acids, ammonia, aromatic amino acids, benzodiazepines, fatty acids, copper, urea, creatinine and lactate; (b) improves systemic haemodynamics, and (c) reduces portal pressure. Two randomised studies have also shown that MARS is effective in the treatment of cerebral failure (HE grade 3–4).56,76,77 However, dialysis with albumin (MARS or PROMETHEUS) does not improve survival in patients with ACLF. Two recent studies support this claim.40,41 Survival at 28days in patients who were treated with liver support devices was comparable to that observed in patients who received standard medical treatment (61–66% vs 60–63%, respectively). However, a subanalysis of the study that evaluated the PROMETHEUS system suggested that this treatment could improve survival in the subgroup of patients with MELD>30points (57% vs 42%; p=0.02).41
A small randomised placebo-controlled study suggested that the administration of granulocyte colony-stimulating factors in patients with ACLF improves 60-day survival (66% vs 26%, p=0.001).78 These interesting results should be confirmed in other studies.
The heterogeneity of the patients included in all these studies and of the definitions of ACLF used, the diversity of the precipitating events and lack of treatments that promote liver regeneration in patients with cirrhosis make it extremely difficult to evaluate the efficacy of a single therapeutic strategy. Technical improvements are therefore necessary in the artificial liver support systems, as well as new controlled trials that re-evaluate indications, treatment time points, other treatments such as plasma exchange or cell therapy and combined therapies.
Prognosis for the critically ill cirrhotic patientPatients with cirrhosis often require intensive care for severe decompensations of their liver disease. Regardless of the reason for admission, cirrhosis adds complexity and a poor prognosis to the critically ill patient, although the prognosis has improved in recent years.10,79 Recent studies show an ICU mortality of 35–48%, which depends basically on two variables: the reason for admission and number of organ failures. Patients with variceal bleeding or HE have an excellent prognosis (survival of 87–90% and 74%, respectively) compared to patients admitted for septic causes (ICU mortality: 64%).80,81 Moreover, the presence of single organ failure (SOFA criteria) is associated with a mortality of 30–48%, increasing to 65% and 70–89% in patients with two or three organ failures, respectively.82 Other factors that worsen the prognosis are the need for mechanical ventilation or RRT and the presence of sepsis. Fifty-nine percent of patients with cirrhosis who require mechanical ventilation die during their stay in the ICU, rising to 70% if the ventilatory support is secondary to severe sepsis.23 The need for 3 or more support treatments (dialysis, mechanical ventilation, vasoactive support or MARS) raises ICU mortality to 96%.80 This mortality is higher than that observed in critically ill patients without cirrhosis. Most deaths occur during the first week, mainly caused by multiple organ dysfunction syndrome (ACLF).81,83 The lack of efficient artificial liver support systems and the cascade of events that lead the patient in a vicious circle – deterioration in liver function that causes the onset of other organ failures that in turn accentuate the deterioration in liver function – explain this poor prognosis in critically ill patients with cirrhosis. Accurately identifying patients who might survive admission to the ICU is therefore necessary, avoiding a futile admission whenever possible.10,83
Although prognosis in critically ill cirrhotic patients is relatively poor, there is renewed interest in these cases following the increased use of the MELD system as a criterion for allocating organs (“sickest first”), which prioritises transplantation in patients with a higher MELD score. In fact, the number of patients with cirrhosis admitted to ICUs has been rising in recent years.79
Limitations of the MELD and Child–Pugh scoresThe MELD score, based on serum bilirubin, INR and creatinine values, is an excellent predictor of 3-month mortality in non-critically ill patients with cirrhosis. However, MELD assesses only the liver, kidney and coagulation function, and does not consider other key organs in critically ill patients (vascular, cerebral and respiratory). Although patients with a higher MELD score on admission to the ICU have higher mortality, this index does not adequately discriminate the population of patients with a reasonable likelihood of survival in the ICU. The MELD-Na, Refit MELD and Refit MELD-Na do not improve its predictive ability.80,82,83 In contrast, the integrated MELD, or iMELD, based on the MELD, age and encephalopathy grade, has excellent predictive value in patients with ACLF whose precipitating factor is a hepatic event (hepatotropic viruses, hepatotoxic drugs, alcohol).84 The limitations of the MELD are applicable to the Child–Pugh score.
Usefulness and limitations of intensive care unit general prognostic indicesVarious ICU general indices have been proposed to evaluate the severity of the disease and its course (Acute Physiology and Chronic Health Evaluation [APACHE] II, APACHE III, Simplified Acute Physiology Score [SAPS] II and Mortality Probability Model [MPM]) or to evaluate patients according to the number of organ failures (Logistic Organ Dysfunction Score [LODS], Multiple Organ Dysfunction Score [MODS] and SOFA). The APACHE II and SOFA indices are the most widely used to assess the prognosis of patients admitted to general ICUs, with the SOFA the most widely used in critically ill patients with cirrhosis.80,82,83
Several studies have compared hepatic indices (Child–Pugh and MELD) with general ICU indices (APACHE II, SAPS II and SOFA) in critically ill cirrhotic patients (Table 6). These studies show that the SOFA score is the most useful in predicting ICU mortality in these patients, probably because it includes markers of brain, cardiovascular, kidney, respiratory, haematological and liver function. The modified version of this index, excluding assessment of platelets (mSOFA or non-haematological SOFA), seems to be the best general prognostic index in the population of critically ill cirrhotic patients. Its precision increases when re-evaluated on the second or third day of admission.81,85 The prognostic limitations of the general ICU indices in the population with cirrhosis are probably related with inappropriate evaluation of liver function, central in the prognosis of these patients.10
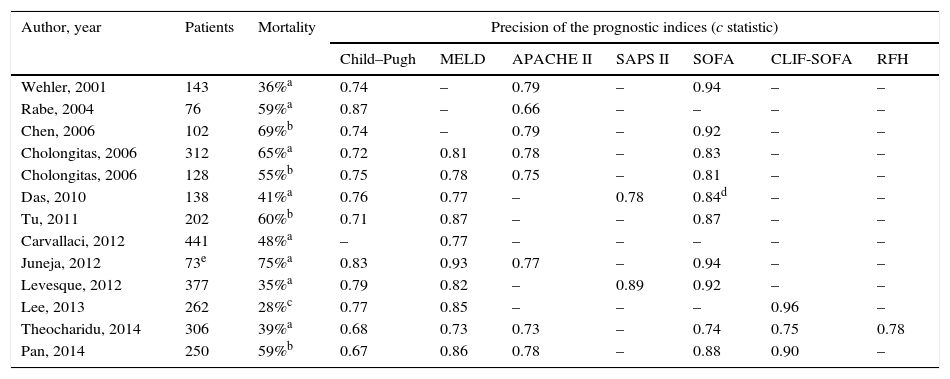
Ability of different prognostic indices to predict mortality in critically ill cirrhotic patients admitted to the ICU.
| Author, year | Patients | Mortality | Precision of the prognostic indices (c statistic) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Child–Pugh | MELD | APACHE II | SAPS II | SOFA | CLIF-SOFA | RFH | |||
| Wehler, 2001 | 143 | 36%a | 0.74 | – | 0.79 | – | 0.94 | – | – |
| Rabe, 2004 | 76 | 59%a | 0.87 | – | 0.66 | – | – | – | – |
| Chen, 2006 | 102 | 69%b | 0.74 | – | 0.79 | – | 0.92 | – | – |
| Cholongitas, 2006 | 312 | 65%a | 0.72 | 0.81 | 0.78 | – | 0.83 | – | – |
| Cholongitas, 2006 | 128 | 55%b | 0.75 | 0.78 | 0.75 | – | 0.81 | – | – |
| Das, 2010 | 138 | 41%a | 0.76 | 0.77 | – | 0.78 | 0.84d | – | – |
| Tu, 2011 | 202 | 60%b | 0.71 | 0.87 | – | – | 0.87 | – | – |
| Carvallaci, 2012 | 441 | 48%a | – | 0.77 | – | – | – | – | – |
| Juneja, 2012 | 73e | 75%a | 0.83 | 0.93 | 0.77 | – | 0.94 | – | – |
| Levesque, 2012 | 377 | 35%a | 0.79 | 0.82 | – | 0.89 | 0.92 | – | – |
| Lee, 2013 | 262 | 28%c | 0.77 | 0.85 | – | – | – | 0.96 | – |
| Theocharidu, 2014 | 306 | 39%a | 0.68 | 0.73 | 0.73 | – | 0.74 | 0.75 | 0.78 |
| Pan, 2014 | 250 | 59%b | 0.67 | 0.86 | 0.78 | – | 0.88 | 0.90 | – |
RFH: Royal Free Hospital.
Despite the fact that the SOFA score has better predictive ability than the Child–Pugh and MELD scores, its components do not take into account the pathophysiological and clinical characteristics of cirrhosis. For this reason, the European Association for the Study of the Liver CLIF-Consortium modified that score, creating a new index called the CLIF-SOFA, adapted to liver patients (Table 4).71 Its main changes are the substitution of the platelet count and the Glasgow scale for the INR and West-Haven score to assess coagulation and cerebral failures, respectively. This recently created prognostic index has been shown to have a predictive ability superior to the SOFA and APACHE III in some studies, especially if assessed 48h after admission to ICU (area under the receiver operating curve [AUROC]: 0.85–0.90).86
Two new prognostic indices that are potentially applicable to critically ill patients with cirrhosis have recently been created: the Royal Free Hospital (RFH) score and the CLIF-C ACLFs score.87,88 The RFH score is complex and includes bilirubin, INR, lactate, alveolar-arterial O2 pressure gradient, urea and reason for admission to ICU (sepsis, variceal bleeding or others). The CLIF-C ACLFs simplifies and improves the predictive ability of the CLIF-SOFA score, reduces the number of subscores to define organ dysfunction from 5 to 3, and includes age and blood leucocyte count. Its predictive ability is superior to that of hepatic indices in patients with ACLF and improves if re-evaluated at 48h and 3–7days. Its predictive ability seems to be inferior to the iMELD in patients with ACLF triggered by hepatic events. The real clinical usefulness of these new scores in critically ill cirrhotic patients should be evaluated in new studies.
Futility criteriaAs previously mentioned, the overall prognosis for patients with cirrhosis admitted to the ICU is relatively poor. However, at the individual level, the likelihood of survival that either justifies or rules out admission to the ICU it is still a topic of discussion. The decision to provide intensive treatment depends on a series of factors, including the patient's short- and long-term prognosis, the possibility of performing “rescue” transplantation and the availability of resources. Moreover, several studies have shown good outcomes in selected critically ill cirrhotic patients. Therefore, the reluctance to admit these patients to the ICU should be put into context. In general, any patient with a potentially fatal acute complication with a low MELD score should be admitted to ICU before they develop more serious complications. In contrast, in patients with advanced cirrhosis (MELD>30), 3 or more organ failures and no prospect of “rescue” transplantation, intensive management is questionable. A practical approach is to provide these patients with unrestricted intensive support for 3days.10 In accordance with this policy, the presence of 3 or 4 non-haematological organ failures should not contraindicate admission to ICU. However, persistence of failure (3 or more failures) after 3days of intervention should be considered a criterion to establish a limitation on intensive treatment, as nearly all cases result in death. More recently, another futility criterion has been suggested based on the evolution of patients with ACLF at 3–7days after initiation of treatment. According to the results of this study, patients with 4 or more organ failures, or with a CLIF-C ACLFs>64points at 3–7days after ACLF diagnosis have a very high 28-day mortality (100% in the study), so that if there is no possibility of a “rescue” transplant, limiting the therapeutic effort must be considered.75
Conflict of interestsThe authors declare that they have no conflict of interests.
Please cite this article as: Fernández J, Aracil C, Solà E, Soriano G, Cardona MC, Coll S, et al. Evaluación y tratamiento del paciente cirrótico crítico. Gastroenterol Hepatol. 2016;39:607–626.
Catalan Society of Gastroenterology and Hepatology position paper.