Hepatitis B virus (HBV) infection remains a global public health problem. HBV vaccination is the most effective tool to reduce the incidence of HBV disease. Despite there has not been new clinical developments for the treatment of chronic hepatitis B in the last few years, changing epidemiology and current insights on natural history, diagnostic tools and therapy indications make necessary an update of the former version of the consensus document of the Spanish Association for Study of the Liver on the treatment of hepatitis B infection published in 2012. The current document updates the management of chronic hepatitis B. The treatment of choice is the long-term administration of a nucleos(t)ide analogue with high barrier to resistance (entecavir, tenofovir or tenofovir alafenamide). Pegylated interferon may be an option in patients with non-advanced liver disease, but its applicability is limited due to the low efficacy and poor tolerability. All patients must be monitored for the risk of progression to advanced liver disease and development of hepatocellular carcinoma.
La infección por el virus de la hepatitis B (VHB) continúa siendo un problema global de salud pública. La vacunación frente al VHB, introducida en España en la década de 1990, constituye el método más eficaz para reducir la incidencia de la enfermedad. No obstante, la migración procedente de países donde la prevalencia de la infección es alta contribuye a que en nuestro país la tasa esté aún entre el 0,5 y el 1%. El espectro de la enfermedad crónica ocasionada por el VHB es muy variable, y abarca desde el portador inactivo a pacientes con hepatitis crónica, cirrosis y carcinoma hepatocelular (CHC). A pesar de que en los últimos años no se han producido grandes avances en el desarrollo clínico de nuevos fármacos para el tratamiento de la hepatitis crónica B, los cambios en la epidemiología, en el conocimiento de la historia natural, métodos diagnósticos e indicaciones de tratamiento, aconsejan la actualización del documento de consenso de la Asociación Española para el Estudio del Hígado (AEEH) sobre el tratamiento de la infección por el virus de la hepatitis B publicado en el año 2012. El documento de la AEEH revisa el tratamiento de la hepatitis crónica B y establece como mejor pauta la administración prolongada de un análogo de nucleós(t)ido con alta barrera genética a la resistencia (entecavir, tenofovir o tenofovir alafenamida). El interferón-pegilado constituye una alternativa en pacientes con enfermedad hepática poco avanzada, pero su aplicabilidad es limitada por su baja eficacia y efectos adversos frecuentes. En todos los pacientes se debe evaluar el riesgo de progresión a enfermedad hepática avanzada y de desarrollo de CHC. Determinados subgrupos de pacientes con infección crónica por VHB deben ser incluidos en programas de vigilancia para el diagnóstico precoz de CHC, que constituye en el momento actual la principal complicación de la enfermedad.
Although there have been no major advances in the treatment of chronic hepatitis B in recent years in terms of new drugs, there have been changes in both the epidemiology and the understanding of the natural history of hepatitis B, and in the diagnostic methods and treatment indications. Now therefore seemed to be the right time to update the 2012 consensus document on the treatment of hepatitis B virus (HBV) infection published by the Asociación Española para el Estudio del Hígado [Spanish Association for the Study of the Liver] (AEEH).1 For this task, the Board of Directors of the AEEH commissioned an expert panel to review and update the document, the final version of which was approved by said Board.
The recommendations in this document were based wherever possible on the available scientific evidence. However, in instances where information was insufficient or non-existent, recommendations were based on the opinions and personal experience of the experts themselves. The recommendations were classified according to a recommendation rating system used in different guidelines and consensus documents.2 The quality of the scientific evidence was classified into three levels: high (A), moderate (B) or low (C). The system provides two levels of recommendation: strong (1) or weak (2). This means that the higher the level of evidence, the stronger the recommendation, and vice versa.
Global epidemiology and epidemiology in SpainThe World Health Organization (WHO) estimates that 257 million people are infected with HBV worldwide, representing a prevalence of around 3.5% of the global population. The prevalence of hepatitis B is highest in the western Pacific region and in Africa, where about 6% of the adult population is infected. The regions of the eastern Mediterranean, Southeast Asia and Europe have estimated prevalences of 3.3%, 2.0% and 1.6%, respectively. The spectrum of chronic disease is highly variable, ranging from inactive carriers to patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma (HCC). In fact, the WHO estimates that in 2015 hepatitis B caused 887,000 deaths, mainly from cirrhosis and HCC.3
Spain has traditionally been classified among the countries with intermediate endemic rates, defined as a prevalence of HBV surface antigen (HBsAg) in the general population from 2% to 7%. However, after the vaccine was added to the Spanish Sistema Nacional de Salud [National Health System] vaccination schedule in the 1990s,4 with vaccination coverage above 95%,5 the incidence of hepatitis B decreased considerably, from two cases per 100,000 population in 2002 to 1.27 cases in 2005. In 2008, however, the incidence increased by 25% to 2.27 cases per 100,000 population, in the context of an increase in sexually transmitted infection and an increase in the migrant population from high-prevalence areas.6 After 2008, there was a further decline in incidence which continues to this day, with transmission rates in the range of 1.27 to 1.65 cases per 100,000 population. Spain is presently classed as a country with low endemicity, with a prevalence of HBsAg around 0.5%-0.8% of the general population.7–9
Apart from vaccination, other factors which contribute to reducing the incidence of acute infection are systematic control of blood donations and serological screening of pregnant women, as well as other preventive efforts with varying degrees of effectiveness aimed at avoiding or limiting high-risk behaviours such as parenteral drug use with non-disposable materials, unsafe sex without a condom, exposure to tattooing and body piercing under unhygienic conditions, and clinical activities termed "invasive procedures under unsafe conditions".
Hepatitis B was made a notifiable disease in Spain in 1995. Notification began in 1997 with the weekly aggregate declaration of acute hepatitis cases by the autonomous regions of Spain. Individualised declaration (demographic, clinical and vaccination variables) was added in 2005, sent annually to the Centro Nacional de Epidemiología [Spanish National Epidemiology Centre]. Since 2014, cases have been declared individually on a weekly basis, and the epidemiological survey has been expanded with variables for exposure and risk of infection. In addition, the case definition changed: suspected cases disappeared and probable and confirmed cases were reported instead.10 There has been a downward trend in the case notification rate in recent years, largely due to the impact of the vaccination programmes.
Recommendations:
- •
As it is a notifiable disease, cases of hepatitis B (probable or confirmed) should be sent to the Centro Nacional de Epidemiología (A1).
- •
Most people infected with HBV will not develop symptoms during the course of the infection, so serological screening is recommended in at-risk populations (A1).
Half of people infected with acute hepatitis B experience symptoms and 1% of those who develop jaundice will progress to fulminant hepatitis. However, in children and young people it tends to be asymptomatic. Age at infection is a predictive factor for progression to chronic disease: 90% after perinatal infection, 30% between the ages of one and five, and less than 5% in immunocompetent adults.
HBsAg positivity for more than six months is the characteristic marker of chronic HBV infection. Chronic hepatitis B is a dynamic process resulting from the interaction between virus replication and the host immune response.11 As HBV is a non-cytopathic virus and liver injury is caused by the host's immune response, increased transaminase levels, mainly alanine aminotransferase (ALT), are suggestive of liver inflammation (hepatitis). In contrast, in some cases transaminase levels are normal, indicating the presence of infection without inflammation and little or no immune response. Furthermore, in patients with chronic hepatitis B it is important to test for hepatitis B e-antigen (HBeAg) and its antibody, anti-HBe, as forms with positive versus negative HBeAg follow different courses and may require different treatment. It is also essential to quantify the virus's replication activity by measuring serum HBV DNA levels; to this end, various highly sensitive and precise techniques based on molecular biology procedures are available. It is also possible to quantify HBsAg in serum, identify the virus's genotype and detect certain mutations in the virus' genome.
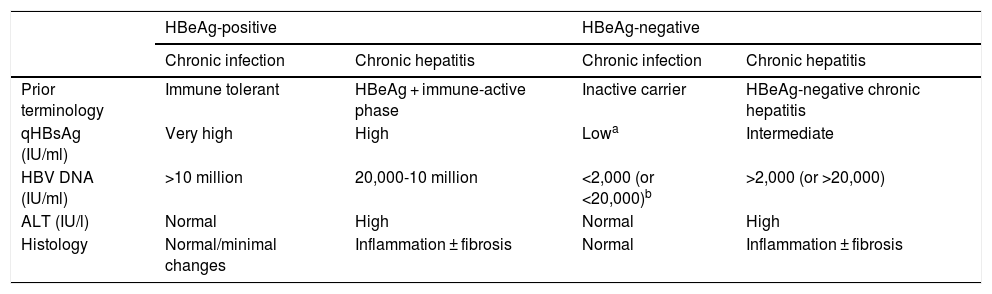
In the natural history of chronic hepatitis B, four different, not necessarily sequential, phases of active infection (Table 1) and a functional cure phase can be distinguished:
- 1
HBeAg-positive chronic infection phase (previously, immune tolerance phase,12) characterised by the presence of HBeAg, very high HBV DNA levels, normal ALT values, mild necroinflammatory activity or normal liver on liver biopsy, and slow or no progression of fibrosis. This phase is typical in young people (<40 years of age) who have acquired the virus by vertical transmission when the immune system is still immature, and can last 20 or 30 years. However, in patients who acquire the infection in adolescence or adulthood, it is short-lived. In this phase, the spontaneous seroconversion rate, defined as the loss of HBeAg with the development of anti-HBe, is very low. These patients are highly contagious, as they have a very high viral load.
- 2
Chronic hepatitis or HBeAg-positive immune active phase. This phase is characterised by the presence of HBeAg, a decrease in HBV DNA levels compared to those observed in the previous phase, high or fluctuating ALT values, and an increase in histological activity with progression of fibrosis. This phase has a more rapid onset in people infected in adulthood and can last for weeks to years. The annual rate of spontaneous seroconversion of HBeAg (without antiviral treatment) is 1%-20%. Patients who remain in this phase for years, especially if they have repeated flare-ups of necrosis, are at risk of developing cirrhosis and HCC.1,12
- 3
HBeAg-negative chronic hepatitis phase. In some patients with chronic HBV infection, inflammatory activity persists despite a lack of HBeAg positivity. In most cases, this phenomenon is due to infection by viruses with certain genomic mutations that prevent infected hepatocytes from producing HBeAg. HBeAg-negative chronic hepatitis, very common in the Mediterranean region and the East, is currently the most common form of chronic hepatitis B in Spain. Episodes of exacerbation can occasionally occur, with an increase in ALT values, preceded by an elevation in HBV DNA levels, followed by variable periods of remission. These episodes are associated with progression of fibrosis. In periods of remission, HBV DNA levels may be low (less than 2,000 IU/ml) and ALT values may be normal, simulating an inactive carrier phase or chronic HBeAg-negative infection. Therefore, frequent determinations (every 3-4 months) of ALT and HBV DNA levels for at least one year are necessary to properly categorise these patients.1,12
- 4
HBeAg-negative chronic infection phase (previously, inactive carrier phase). This is defined by HBeAg negativity and anti-HBe positivity, persistently normal ALT values and low HBV DNA levels (in general, less than 2,000 IU/ml, although some patients have levels ranging from 2,000 to 20,000 IU/ml). Measurement of HBsAg levels (quantitative HBsAg [qHBsAg]) may be useful to distinguish this phase from patients in the grey area (ALT normal or <1.5 times the upper limit of normal with fluctuating HBV DNA > 20,000 IU/ml). qHBsAg levels below 1,000 IU/ml are highly suggestive of HBeAg-negative chronic infection.13,14 In general, the long-term prognosis of patients in this phase is good,15,16 but reactivations of the infection with elevations in HBV DNA and ALT levels do occasionally occur, so it is advisable to monitor these parameters at least once a year. Loss of HBsAg with development of hepatitis B surface antibodies (anti-HBs) can occur spontaneously in 1%-3% of cases annually, generally after several years with undetectable HBV DNA levels.17
- 5
Functional cure phase after loss of HBsAg. This is defined by negative HBsAg, with or without anti-HBs, and positive hepatitis B core antibodies (anti-HBc), and is characterised by normal ALT values and usually undetectable levels of HBV DNA in serum. However, covalently closed circular DNA (cccDNA) can often be detected in the liver.12 Loss of HBsAg in patients without advanced fibrosis is associated with a minimal risk of cirrhosis, decompensation and HCC, and better survival. If cirrhosis has developed before loss of HBsAg, patients nevertheless remain at risk of developing HCC. It is important to remember that reactivation of HBV replication can occur in this phase and in the context of immunosuppression.18
Summary of the main characteristics of the phases of chronic hepatitis B virus infection.
| HBeAg-positive | HBeAg-negative | |||
|---|---|---|---|---|
| Chronic infection | Chronic hepatitis | Chronic infection | Chronic hepatitis | |
| Prior terminology | Immune tolerant | HBeAg + immune-active phase | Inactive carrier | HBeAg-negative chronic hepatitis |
| qHBsAg (IU/ml) | Very high | High | Lowa | Intermediate |
| HBV DNA (IU/ml) | >10 million | 20,000-10 million | <2,000 (or <20,000)b | >2,000 (or >20,000) |
| ALT (IU/l) | Normal | High | Normal | High |
| Histology | Normal/minimal changes | Inflammation ± fibrosis | Normal | Inflammation ± fibrosis |
The risk of progression to cirrhosis and HCC varies. Progression to cirrhosis is more common in HBeAg-negative chronic hepatitis (annual rate 8%-10%) than in HBeAg-positive chronic hepatitis (annual rate 2%-5%). In patients with compensated cirrhosis, five-year survival is 80%-86% compared to 14%-35% with decompensated cirrhosis.19 It is important to remember, as in other types of chronic hepatitis, that this is also associated with the development of HCC.
Recommendation:
- •
In the initial assessment of a patient with chronic HBV infection, serological and virological status should be assessed (HBsAg or preferably qHBsAg, HBeAg/anti-HBe and HBV DNA levels), as should degree of liver injury (ALT and fibrosis by non-invasive methods) (A1).
Chronic HBV infection is diagnosed by the presence of HBsAg in serum for over six months. It is a dynamic process associated with changes in HBV replication and in the immune response of the host. This means the disease spectrum is very broad, from patients with no liver injury or minimal liver injury to patients with liver cirrhosis or HCC. An accurate diagnosis is necessary to determine the current stage of the natural history of liver disease, in order to assess the likelihood of progression, the need for treatment and the follow-up regimen in untreated patients. In most patients, especially those with negative HBeAg, follow-up for six to 12 months is necessary for correct classification.
Diagnosis includes medical history, physical examination, biochemical parameters, HBV serological markers, HBV DNA determination, abdominal ultrasound, non-invasive fibrosis assessment methods and liver biopsy. In addition, all first-degree relatives, sexual partners and household members of patients with chronic HBV infection should be advised to undergo testing for HBV serological markers (HBsAg, anti-HBc and anti-HBs) and vaccination if they are negative for all markers.
- •
Medical history. A family history of cirrhosis or HCC, extrahepatic manifestations of HBV and any associated diseases the patient has, especially if related to metabolic syndrome, should all be assessed.
- •
Physical examination. Assess for signs of extrahepatic manifestations of HBV and the presence of liver cirrhosis or portal hypertension.
- •
Biochemical parameters. Transaminase levels are related to necroinflammatory activity, but normal values do not rule out advanced liver disease. However, determination of transaminase levels is helpful for treatment decision-making. Complete blood count, prothrombin activity, total bilirubin and albumin are used to assess portal hypertension and severity of liver damage.
- •
HBV markers. These include:
- a)
HBeAg and anti-HBe. They are essential for identifying the phase of chronic HBV infection.
- b)
HBV DNA. Levels indicate HBV replication. They should be measured by a technique with high sensitivity and a broad dynamic range, preferably real-time polymerase chain reaction (PCR). They are essential for identification of the phase of chronic HBV infection, treatment decision-making and follow-up of patients.
- c)
HBV genotype. Although this is not essential, it may be useful for screening patients with a high probability of response to treatment with pegylated interferon (PEG-IFN).1
- d)
qHBsAg. Levels reflect the amount and transcription activity of cccDNA located in the nucleus of HBV-infected hepatocytes, especially in HBeAg-positive patients. They are measured using an enzyme immunoassay technique and vary by HBV genotype, being higher in genotype A. In untreated HBeAg-negative patients, with HBV DNA < 2,000 IU/ml, qHBsAg values <1,000 IU/ml are indicative of chronic infection (inactive carrier) with a low risk of HCC, while qHBsAg values <100 IU/ml are associated with spontaneous loss of HBsAg. In patients treated with PEG-IFN, qHBsAg levels after 12 and 24 weeks of treatment have a high negative predictive value for response, and therefore are useful as stopping rules. Monitoring qHBsAg levels in patients treated with nucleos(t)ide analogues can be used to estimate the duration of treatment necessary to achieve loss of HBsAg and in particular to predict sustained response after treatment is stopped. Although the cut-off point has not been established, a level <100 IU/ml seems to predict sustained response. In future treatments aimed at achieving functional cure of HBV, monitoring qHBsAg levels will be essential.20
- e)
New HBV markers. HBV core-related antigen (HBcrAg) correlates with the transcription activity of cccDNA, especially in HBeAg-negative patients. It is determined by an electrochemiluminescence technique. Although further studies are needed, HBcrAg may be superior to qHBsAg in identifying patients with HBeAg-negative chronic infection (inactive carrier) regardless of genotype and in predicting viral relapse after stopping treatment with analogues.21,22 Circulating HBV RNA is the best indicator of cccDNA transcription activity, but there are still no standardised measurement methods. It can predict viral relapse after stopping treatment with analogues.23
- a)
- •
Associated diseases. Autoimmune, metabolic and alcoholic liver disease need to be ruled out, as do non-alcoholic fatty liver disease and co-infections with hepatitis D virus (HDV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV). Anti-hepatitis A virus (HAV) IgG antibodies should be determined and, if negative, hepatitis A vaccination should be recommended.
- •
Abdominal ultrasound. This is useful for identifying signs of liver cirrhosis and portal hypertension, as well as for diagnosing HCC. However, the absence of abnormalities on ultrasound does not rule out advanced liver disease.1
- •
Non-invasive methods for assessment of liver fibrosis.
- -
Liver elastography. In patients with chronic HBV infection, measurement of liver stiffness is useful to assess for significant fibrosis or advanced chronic liver disease or cirrhosis. HBeAg-negative patients with chronic infection (inactive carriers) have an elastography value similar to that of normal controls.24 A recent meta-analysis concluded that elastography has better diagnostic accuracy for ruling out than for confirming liver disease and that it is more accurate for diagnosing cirrhosis. There was great variation in the diagnostic cut-off points from one study to another, with medians of 7.2 kPa for significant fibrosis (F2-F4) and 12.4 kPa for cirrhosis (F4).25 The use of two cut-off points improves diagnostic accuracy as it assumes a grey area between the two values that requires further study. Recommended cut-off points are ≤6.2 kPa and >9.4 kPa for significant fibrosis and ≤9.4 kPa and >13.1 kPa for cirrhosis.26 Elastography values increase in patients with marked liver inflammation, and this is reflected in high transaminase levels. Therefore, elastography is not recommended with an ALT level more than five times the normal value. Raising diagnostic cut-off points in patients with ALT from one to five times the upper limit of normal has been proposed, but this strategy does not improve diagnostic performance.24
- -
Biomarkers. Several biomarkers for assessing liver fibrosis have been developed in recent years as an alternative to liver biopsy. APRI and Fibrosis-4 (FIB-4) scores are not suitable for use in chronic hepatitis B due to their moderate diagnostic precision and high degree of overlap.27,28 In a recent study, an FIB-4 cut-off point of <0.70 ruled out cirrhosis in 97% of cases over 30 years of age.29 Asian studies have found that the red cell distribution width (RDW) to platelet ratio (RPR) is superior to APRI and FIB-4 scores in predicting significant fibrosis and cirrhosis, and that the gamma-glutamyl transferase (GGT) to platelet ratio (GPR) is superior all other biomarkers, including RPR, in predicting advanced fibrosis (F3-F4), especially in HBeAg-negative patients with significant necroinflammatory activity (A3-A4).30,31 However, in another recent meta-analysis, the diagnostic accuracy of GPR was moderate and similar to that of FIB-4.28 These results need to be validated in the Caucasian population before recommending the use of these biomarkers.
- -
- •
Liver biopsy. In most cases liver biopsy is not essential for diagnosis or decision-making with regard to treatment. It is useful in cases with associated diseases, to assess the significance of each disease when considering treatment, and in cases with an elastography value in the grey area.
Recommendations:
- •
An accurate diagnosis is necessary to determine the current stage of the natural history of liver disease, based on the determination of HBeAg, anti-HBe, HBV DNA and ALT (A1).
- •
The determination of qHBsAg is useful for the diagnosis of HBeAg-negative chronic infection (B1).
- •
Liver elastography is the non-invasive method of choice for assessing liver injury due to its greater diagnostic precision, especially for ruling out advanced fibrosis (A1).
- •
Biomarkers seem to have lower diagnostic precision, so further studies are needed to expand their use (B1).
- •
Ultrasound is useful for diagnosing liver cirrhosis and HCC (A1).
- •
Liver biopsy is indicated in patients with associated liver disease and in whom the elastography value is in the grey area (B1).
Due to the persistence of cccDNA in the nucleus of the hepatocytes, eradication of HBV infection is not possible with the drugs that are currently available. Therefore, the ultimate goals of treatment in patients with chronic hepatitis B are to improve survival, prevent disease progression and prevent the development of decompensated liver disease or HCC, resulting in the patient's death or need for liver transplantation. As these events generally occur in the long term, the following intermediate goals have been set and are used as indirect measures of treatment efficacy:
- •
Sustained inhibition of virus replication.
- •
Negative results for HBeAg, with or without seroconversion to anti-HBe, in HBeAg-positive patients with chronic hepatitis B.
- •
Return to normal of ALT levels.
- •
Loss of HBsAg, with or without development of anti-HBs, interpreted as functional cure or resolution of the infection. This would be the ideal goal and is the closest point to curing the infection possible at present, but unfortunately is only achieved in a small proportion of patients.
- •
Other goals of treatment in HBV infection are to:
- o
Avoid vertical transmission of the virus in pregnant women.
- o
Avoid reactivation of the virus in patients receiving immunosuppressant treatment or requiring treatment for chronic hepatitis C.
- o
Reduce the risk of developing HCC in patients with a family history of this type of cancer in relation to HBV.
- o
Avoid progression to acute liver failure in patients with severe acute hepatitis B and avoid the need for liver transplantation in those with acute liver failure.
- o
Reduce the risk of infection recurrence after liver transplantation.
- o
Reduce the risk of cancer recurrence in patients with HCC.
- o
Manage or reverse systemic signs and symptoms of HBV.
- o
The indication for treatment is the same for individuals with HBeAg-positive and HBeAg-negative chronic hepatitis, and should be based on assessment of the following parameters:
- -
ALT levels.
- -
HBV DNA levels.
- -
Liver injury assessed by liver biopsy or non-invasive methods.
Treatment is indicated if at least two of the following three criteria are met: elevated ALT level, HBV DNA level above 2,000 IU/ml and presence of at least moderate necroinflammatory activity or fibrosis (grade A ≥ 2 or stage F ≥ 2 on the METAVIR scoring system).1,32–34 ALT levels are considered elevated if they are above the laboratory's upper limit of normal, which is usually lower for females than for males. The recommendations of the American Association for the Study of Liver Diseases consider lower limits for normal ALT (35 U/l for males and 25 U/l for females). However, the bulk of the scientific evidence is based on usual laboratory values.34 In patients with normal ALT levels and HBV DNA levels above 2,000 IU/ml, the severity of the liver injury will determine the indication for treatment, while in those with ALT levels more than twice the upper limit of normal and HBV DNA levels more than 20,000 IU/ml, treatment can be started regardless of liver injury. As a general rule, treatment is indicated in all individuals with chronic hepatitis B, both HBeAg-positive and HBeAg-negative, while the general recommendation in patients with chronic infection without liver injury is to monitor them periodically and customise treatment in special cases.
In newly diagnosed HBeAg-positive chronic hepatitis, treatment should be delayed for at least three months to provide the opportunity for spontaneous HBeAg seroconversion. In HBeAg-positive subjects with chronic infection (previously, immune tolerant phase) under the age of 30, with no other evidence of liver disease or family history of HCC and with non-invasive markers not indicative of advanced fibrosis, neither performing a liver biopsy nor starting treatment is indicated. These individuals should be followed up every three to six months, and a liver biopsy should be performed or an indication for treatment should be established when their ALT values rise or once they reach the age of 30. In HBeAg-positive patients with chronic infection, oral antivirals can reduce virus replication, but the chances of eliminating HBeAg and HBsAg completely are virtually non-existent despite prolonged treatment, so it is better to simply keep these patients under observation with periodic check-ups. HBeAg-positive patients with extrahepatic manifestations such as polyarteritis nodosa or membranous glomerulonephritis often have normal ALT levels, elevated HBV DNA levels and no liver injury. Treatment is indicated in these cases because it improves extrahepatic manifestations.
In patients with mild liver injury (A1F1 on the METAVIR scoring system), follow-up is essential and personalised treatment may be considered. In patients with compensated cirrhosis, treatment should be started when viraemia is detectable, regardless of HBV DNA or ALT levels.
Patients with HCC should be treated with nucleos(t)ide analogues, as in decompensated cirrhosis, as treatment has been shown to reduce the risk of recurrence of HCC and improve the prognosis of the liver disease.35,36 Treatment should also be considered in patients with a family history of HCC, in order to reduce their risk of developing HCC, even if they do not meet the criteria outlined above.12
HBsAg-positive patients co-infected with HCV who are to be treated with direct antivirals for hepatitis C, but in whom treatment for hepatitis B is not indicated, should be closely monitored (HBV DNA and ALT). If prophylaxis is required, it should consist of a nucleos(t)ide analogue during the hepatitis C treatment period and up to 12 weeks afterwards.12,37,38
Recommendations- 1
In individuals with chronic hepatitis B, treatment is indicated if ALT levels are elevated, HBV DNA levels are higher than 2,000 IU/ml and/or there is at least moderate necroinflammatory activity and/or fibrosis (A1).
- 2
Patients with compensated liver cirrhosis should be treated if HBV DNA is detectable, even if ALT levels are normal (A1).
- 3
Patients with decompensated cirrhosis should be treated without delay with potent nucleos(t)ide analogues, regardless of HBV DNA and ALT levels (A1).
- 4
Patients with a family history of HCC or extrahepatic manifestations may be treated even if they do not meet all the criteria (B2).
- 5
Patients with chronic hepatitis B not receiving treatment should be followed up every six months (A1).
Two types of drug are approved for the treatment of chronic hepatitis B: nucleos(t)ide analogues (lamivudine [LAM], adefovir dipivoxil [ADV], entecavir [ETV], telbivudine [TBV] and the two prodrugs of tenofovir, tenofovir disoproxil fumarate [TDF] and tenofovir alafenamide [TAF]) and interferons (the conventional IFN〈-2a and 〈-2b and the pegylated form of 〈-2a [PEG-IFN 〈-2a]). Among the analogues, LAM, ADV and TBV are not currently recommended due to their low genetic barrier to resistance.1 Among the IFNs, the pegylated form has replaced the conventional form due to its greater convenience of administration (one rather than three doses weekly), greater efficacy and better tolerance.1
Treatment with nucleos(t)ide analoguesThe recommended nucleos(t)ide analogues are ETV, TDF and TAF due to their high antiviral potency and high barrier to resistance. The main advantages of treatment with analogues are oral administration, high efficacy in inhibiting HBV replication, long-term safety and the fact they can be used in any situation, including decompensated cirrhosis, liver transplantation and, in the case of TDF, pregnancy. The main drawback is the need for prolonged treatment, which initially lasts indefinitely (Table 2). The inhibition of virus replication obtained by treatment with analogues significantly improves necroinflammatory damage and liver fibrosis, and can even reverse cirrhosis.39,40 As a result, treatment with analogues reduces the risk of complications of liver disease41,42 and increases survival, which becomes similar to that of the general population.43 Moreover, in patients in whom the treatment yields negative results for HBsAg, it yields a clinical benefit in addition to that achieved with inhibition of virus replication.44,45
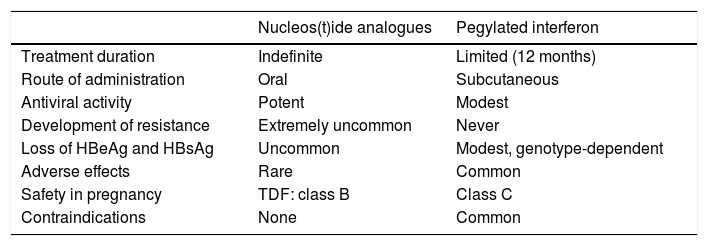
Advantages and disadvantages of treatment with nucleos(t)ide analogues or pegylated interferon.
| Nucleos(t)ide analogues | Pegylated interferon | |
|---|---|---|
| Treatment duration | Indefinite | Limited (12 months) |
| Route of administration | Oral | Subcutaneous |
| Antiviral activity | Potent | Modest |
| Development of resistance | Extremely uncommon | Never |
| Loss of HBeAg and HBsAg | Uncommon | Modest, genotype-dependent |
| Adverse effects | Rare | Common |
| Safety in pregnancy | TDF: class B | Class C |
| Contraindications | None | Common |
The formulation approved in chronic hepatitis B is PEG-INF 〈-2a. Theoretically, IFN has the advantage of dual antiviral and immunostimulant activity, so the aim of its use is to induce sustained immune control of the infection, with treatment of a limited duration. As a result, rates of negative results for HBeAg and HBsAg obtained with PEG-IFN are higher than those obtained with analogues. However, it has many drawbacks, including weekly subcutaneous administration, modest antiviral activity, very common adverse effects and a high number of contraindications (Table 2), which considerably restrict its use and have resulted in a marked reduction in the role of this option in chronic hepatitis B treatment in recent years. Nonetheless, the sustained response after stopping treatment is accompanied by histological improvement, a decrease in the risk of developing complications of liver disease and an increase in overall survival.1
Combining an analogue and PEG-IFN could in theory have synergistic effects. Therefore, various combination strategies have been tried, including initially administering combination treatment, adding PEG-IFN after a period of analogue treatment and switching from an analogue to PEG-IFN. Although a study comparing the de novo combination of PEG-IFN plus TDF to single-drug therapy with either drug showed a higher rate of negative results for HBsAg with the combination treatment, the beneficial effect was modest and limited to patients infected by genotype A.46 Another recent study showed no benefit of sequential treatment with an analogue followed by PEG-IFN over single-drug therapy.47 Therefore, there is no evidence at present to support this strategy compared to single-drug therapy.12,34
Recommendations- 1
Initial treatment of chronic hepatitis B, whether HBeAg-positive or HBeAg-negative, is based on single-drug therapy with a nucleos(t)ide analogue or PEG-IFN (A1).
- 2
The choice of one or the other strategy will depend on the stage of liver fibrosis, virological factors, the patient's comorbidity profile and the patient's own preferences (B1).
Response to antiviral treatment is classified as virological, serological, biochemical or histological. It can be assessed at different points in time during and after treatment. Definitions of virological response vary by therapeutic strategy used.
Types of virological response- •
Treatment with analogues
- o
Virological response during treatment: negative results for HBV DNA during treatment, measured by a sensitive PCR technique, with a limit of detection of 10 IU/ml.
- o
Partial virological response during treatment: reduction in HBV DNA by more than 1 log10 to a level that is nevertheless still detectable after 12 months of therapy in patients with good treatment adherence.
- o
Viral rebound during treatment: increase in HBV DNA levels by more than 1 log10 IU/ml from the lowest value obtained during treatment, confirmed in a second determination one month after the first determination. The most common cause of viral rebound when using ETV, TDF or TAF is poor adherence. Patients with good adherence who present viral rebound should be investigated for possible resistance, although the risk is extremely low with the currently recommended analogues. Viral rebound is usually followed by biochemical rebound, defined as elevation of ALT during treatment in a patient who had achieved an initial biochemical response.
- o
Sustained virological response after treatment: HBV DNA levels below 2,000 IU/ml for at least 12 months after stopping treatment.
- o
- •
Treatment with pegylated interferon
- o
Virological response during treatment: HBV DNA levels below 2,000 IU/ml after six months of treatment and on completion of treatment.
- o
Sustained virological response after treatment: HBV DNA levels below 2,000 IU/ml for at least 12 months after completing treatment.
- o
- •
Serological response in HBeAg-positive chronic hepatitis: negative results for HBeAg and development of anti-HBe. In patients treated with PEG-IFN this response may appear up to six months after the end of treatment.
- •
Resolution of the infection or functional cure: negative results for HBsAg, confirmed by a second determination, with or without development of anti-HBs.
Return to normal of ALT levels according to traditional values (∼40 IU/l).
Histological responseThis has classically been defined as a decrease in necroinflammatory activity (≥2 points in the histological activity index or the Ishak system), with no increase in liver fibrosis compared with pretreatment histological findings. However, at present, liver biopsies before and after treatment are not justified in most patients.
Relapse after discontinuation of analogue therapy- o
Virological relapse. Although not well defined, the most accepted criterion consists of HBV DNA levels in excess of 2,000 IU/ml after stopping treatment.
- o
Clinical relapse. This is defined as a combination of virological relapse and ALT levels more than twice normal values. Clinical relapse can be considered sustained when these criteria persist for more than three to six months and can be considered severe when ALT levels are more than ten times the normal values in two consecutive tests, when levels five to ten times the normal values are maintained for a month or longer, or when higher-than-normal levels are accompanied by an increase in direct bilirubin >1.5 mg/dl or an increase in INR ≥ 0.5 compared to baseline values.
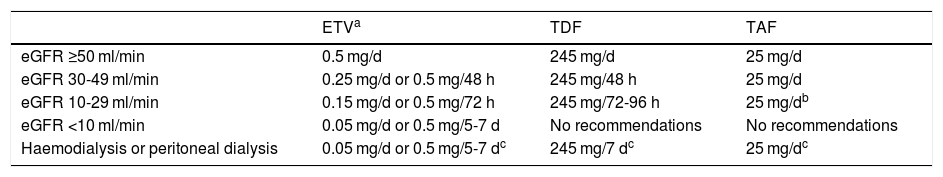
The recommended analogues are ETV, TDF and TAF. ETV is a cyclopentane administered orally at a dose of 0.5 mg/day (1 mg/day in case of resistance to LAM, although ETV is not the analogue of choice in this situation). TDF is a nucleotide analogue administered orally at a dose of 245 mg/day, while TAF is also a nucleotide analogue with a daily oral dose of 25 mg. TAF was approved for the treatment of hepatitis B by the European Medicines Agency in January 2017, so there is less experience with its use than with the other two analogues. At the time of writing (February 2020), TAF is not funded by the Spanish public health system for the treatment of hepatitis B, although it is funded when given in combination with other antivirals for the treatment of HIV infection. However, generic ETV and TDF formulations are available. Due to their renal elimination, ETV and TDF doses must be adjusted when the estimated glomerular filtration rate (eGFR) is <50 ml/min; this is not necessary with TAF (Table 3).
Dose adjustment of ETV, TDF and TAF based on estimated glomerular filtration rate (eGFR).
| ETVa | TDF | TAF | |
|---|---|---|---|
| eGFR ≥50 ml/min | 0.5 mg/d | 245 mg/d | 25 mg/d |
| eGFR 30-49 ml/min | 0.25 mg/d or 0.5 mg/48 h | 245 mg/48 h | 25 mg/d |
| eGFR 10-29 ml/min | 0.15 mg/d or 0.5 mg/72 h | 245 mg/72-96 h | 25 mg/db |
| eGFR <10 ml/min | 0.05 mg/d or 0.5 mg/5-7 d | No recommendations | No recommendations |
| Haemodialysis or peritoneal dialysis | 0.05 mg/d or 0.5 mg/5-7 dc | 245 mg/7 dc | 25 mg/dc |
eGFR: estimated glomerular filtration rate.
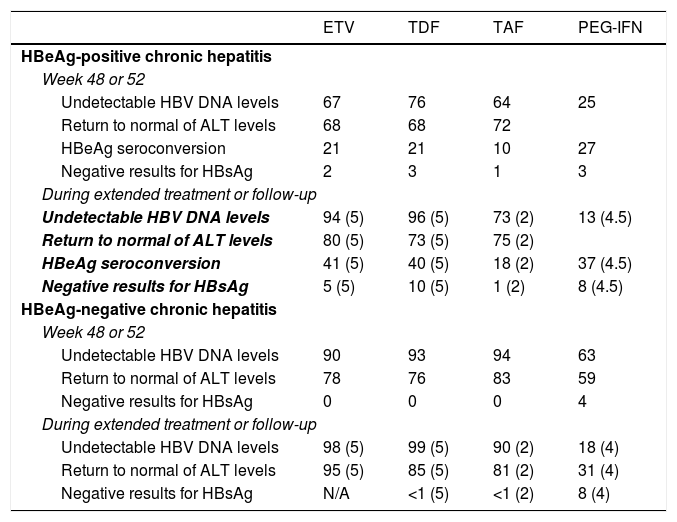
In terms of virological, biochemical and serological response and resolution of the infection, the three analogues all have similar efficacy (Table 4), although no studies have directly compared ETV to TDF or TAF. Some Asian studies have found a lower risk of developing HCC in patients treated with TDF than in those treated with ETV,48,49 but this effect was not identified in other studies also conducted in Asia or in other parts of the world.50–52 Therefore, there is insufficient evidence at present to support the notion that there are different clinical outcomes in terms of likelihood of developing HCC, need for liver transplantation and death depending on whether patients are treated with ETV or TDF.50
Virological, biochemical and serological response rates obtained with the drugs currently recommended.
| ETV | TDF | TAF | PEG-IFN | |
|---|---|---|---|---|
| HBeAg-positive chronic hepatitis | ||||
| Week 48 or 52 | ||||
| Undetectable HBV DNA levels | 67 | 76 | 64 | 25 |
| Return to normal of ALT levels | 68 | 68 | 72 | |
| HBeAg seroconversion | 21 | 21 | 10 | 27 |
| Negative results for HBsAg | 2 | 3 | 1 | 3 |
| During extended treatment or follow-up | ||||
| Undetectable HBV DNA levels | 94 (5) | 96 (5) | 73 (2) | 13 (4.5) |
| Return to normal of ALT levels | 80 (5) | 73 (5) | 75 (2) | |
| HBeAg seroconversion | 41 (5) | 40 (5) | 18 (2) | 37 (4.5) |
| Negative results for HBsAg | 5 (5) | 10 (5) | 1 (2) | 8 (4.5) |
| HBeAg-negative chronic hepatitis | ||||
| Week 48 or 52 | ||||
| Undetectable HBV DNA levels | 90 | 93 | 94 | 63 |
| Return to normal of ALT levels | 78 | 76 | 83 | 59 |
| Negative results for HBsAg | 0 | 0 | 0 | 4 |
| During extended treatment or follow-up | ||||
| Undetectable HBV DNA levels | 98 (5) | 99 (5) | 90 (2) | 18 (4) |
| Return to normal of ALT levels | 95 (5) | 85 (5) | 81 (2) | 31 (4) |
| Negative results for HBsAg | N/A | <1 (5) | <1 (2) | 8 (4) |
Data expressed as percentages. Years of treatment or follow-up in brackets.
In patients with HBeAg-positive chronic hepatitis, treatment with ETV for five years achieves a cumulative probability of virological response of 95%, a probability of HBeAg seroconversion of 45% and a probability of ALT levels returning to normal of 80%. Around 5% of patients achieve negative results for HBsAg after five years of treatment.1 In patients with HBeAg-negative chronic hepatitis, the cumulative probabilities of virological and biochemical response after five years are 98% and 95%, respectively.53,54
In patients with HBeAg-positive chronic hepatitis, treatment with TDF for five years achieves virological response in 96% of cases, biochemical response in 73%, seroconversion to anti-HBe in 40% and loss of HBsAg in 10%.40 In patients with HBeAg-negative chronic hepatitis, rates of virological response, biochemical response and resolution of the infection are 99%, 85% and <1%, respectively.1 After 10 years of treatment with TDF, 100% of HBeAg-negative and 98% of HBeAg-positive patients have a virological response, while biochemical response rates are 83% in the former group and 78% in the latter group.55
Currently available data with TAF is limited to 96 weeks of treatment. In HBeAg-positive chronic hepatitis, treatment with TAF for two years achieves virological response in 75% of patients, seroconversion of HBeAg in 10% of patients and resolution of the infection in 1% of patients, with no differences compared to the figures for TDF.56 In patients with HBeAg-negative chronic hepatitis, the efficacy of TAF was also similar to that of TDF, with virological response in 90% and loss of HBsAg in 1% after two years of treatment.56 Compared to TDF, both the HBeAg-positive and the HBeAg-negative groups treated with TAF had a higher rate of ALT levels returning to normal after 96 weeks of treatment,56 with no clear explanation found for this effect.
Risk of developing antiviral resistanceAfter five years of treatment, the likelihood of developing genotypic resistance to ETV in naive patients was 1.2%.1 For TDF, no resistance had been observed after eight years of treatment in HBeAg-positive or HBeAg-negative patients.57 However, a quadruple mutation in the gene encoding HBV reverse transcriptase which confers resistance on TDF was recently reported in two patients, suggesting that TDF has a very high but not insurmountable barrier to resistance.58 Although no long-term data are available, no resistance to TAF was observed after two years of treatment in the analysis that included both HBeAg-positive and HBeAg-negative patients.56
SafetyOne of the main advantages of analogue therapy is the long-term safety of these drugs.59,60 Long-term treatment with ETV is very safe, even in patients with comorbidities.61 However, while uncommon, long-term administration of TDF has been associated with deterioration in kidney function; tubular dysfunction, including Fanconi syndrome;40,62 and a decrease in bone mineral density.63 A meta-analysis showed no differences between patients treated with ETV and patients treated with TDF in the incidence of renal dysfunction,64 though that finding was attributed to the short treatment duration in the studies analysed. Studies that have compared the longer-term effects of ETV and TDF have shown greater deterioration in kidney function with TDF. However, this adverse effect only affects a very small number of patients65,66 and occurs mainly in those with baseline renal dysfunction, advanced age or comorbidities,67 and in those whose TDF dose was not adjusted based on their kidney function.
TAF is a prodrug which produces high levels of tenofovir in liver cells at a lower dose than TDF, resulting in lower plasma concentrations and less drug exposure on the part of the kidneys, bones and other organs. TAF's better theoretical safety profile compared to TDF has been confirmed in registry studies, where the use of TAF was significantly associated with less deterioration in kidney function and less bone mineral density loss after 96 weeks of treatment.56
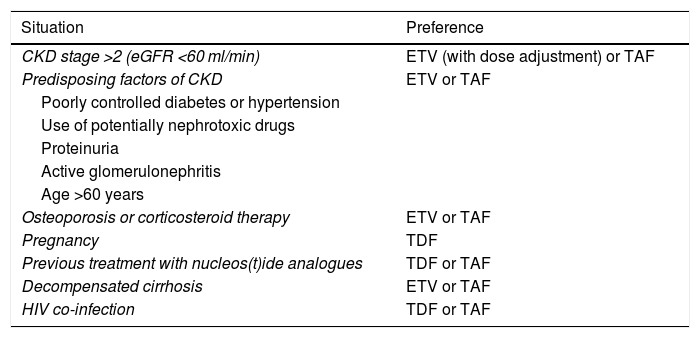
Renal dysfunction having developed during treatment with TDF has been found to improve when switching to ETV68 or TAF, although there is less experience with TAF and the data are mainly based on patients co-infected with HIV.69 Under certain circumstances, these safety-related aspects need to be taken into account when deciding between one analogue and another (Table 5).
Choice of analogue in special situations.
| Situation | Preference |
|---|---|
| CKD stage >2 (eGFR <60 ml/min) | ETV (with dose adjustment) or TAF |
| Predisposing factors of CKD | ETV or TAF |
| Poorly controlled diabetes or hypertension | |
| Use of potentially nephrotoxic drugs | |
| Proteinuria | |
| Active glomerulonephritis | |
| Age >60 years | |
| Osteoporosis or corticosteroid therapy | ETV or TAF |
| Pregnancy | TDF |
| Previous treatment with nucleos(t)ide analogues | TDF or TAF |
| Decompensated cirrhosis | ETV or TAF |
| HIV co-infection | TDF or TAF |
CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate.
Stopping treatment with analogues before the infection has resolved risks virus reactivation. Therefore, the safest strategy is to continue the analogue therapy until complete resolution is achieved.69,70,71 This is especially true in patients with cirrhosis, due to the potential adverse clinical consequences of reactivation. The ideal situation, however, would be to achieve resolution of the infection or at least a sustained virological response in the majority of patients with time-limited treatment. Therefore, the possibility of safely discontinuing treatment with analogues before achieving negative results for HBsAg has become one of the most debated matters in the current management of chronic hepatitis B.
In HBeAg-positive patients without liver cirrhosis, treatment can be stopped once virological response is detected and HBeAg seroconversion has occurred, confirmed by two determinations three to six months apart, after at least 12 months of consolidation therapy.1 When these conditions are met, one year after stopping treatment, most patients have a sustained serological response, but only 60% have a sustained virological response.72 Quarterly follow-up is therefore necessary in the first year to identify patients who will need to restart treatment. The indications for restarting treatment would essentially be the same as in treatment-naive patients.
In HBeAg-negative patients, most information about stopping analogue therapy before achieving negative results for HBsAg comes from Asian countries, as the Asian Pacific Association for the Study of the Liver decided in 2012 that discontinuation could be considered in patients with documented virological response on three separate occasions six months apart after at least 24 months of treatment.73 The goals of this recommendation were mainly cost savings and reduction of the adverse effects and antiviral resistance which can result from long-term therapy. Virological relapse rates after treatment discontinuation under these circumstances are around 55% after one year and 70% after two years, but are lower when consolidation therapy, i.e. continuing therapy after achieving negative results for HBV DNA, is maintained for more than 24 months.72 About 45% of patients develop a clinical relapse. If this happens on top of cirrhosis, it can cause decompensation of the disease.74 Rates of achieving negative results for HBsAg after discontinuing the analogue seen in Asian studies have generally been low, with annual incidences of less than 2%.75 This was probably because, in most cases, treatment was restarted early after virological relapse.76 A retrospective study that analysed the incidence and the predictive factors of achieving negative results for HBsAg after stopping of treatment in 691 patients found that, over a follow-up period of 3.2 years, among those individuals with a clinical relapse, negative results for the antigen were 7.3 times more likely in those who did not restart treatment than in those who did.75 A randomised study in Germany analysed the consequences of stopping or continuing TDF in 42 patients.77 The majority were of Caucasian origin, without advanced fibrosis, treated with TDF for at least four years and with sustained virological response for at least three and a half years. Restrictive criteria were used to restart treatment after clinical relapse in those in whom treatment was stopped. In this study, rates of achieving negative results for HBsAg were 9.5%, 14% and 19% at one, two and three years after discontinuation, respectively, while none of the patients who remained on treatment showed negative results for HBsAg during the follow-up period. Despite the restrictive criteria for restarting treatment, no unexpected adverse effects were detected, and after 144 weeks of follow-up, 38% of the patients had required reintroduction.77 These results confirm those published a number of years ago after discontinuation of ADV.78 They are also similar to those of other observational studies79,80 and raise the possibility of stopping treatment to achieve resolution of the infection through the immune response caused by virus reactivation.81 This strategy was included in the Clinical Practice Guidelines of the European Association for the study of the Liver12, stating that discontinuation of the analogue may be considered in selected patients without cirrhosis who have had sustained virological response for at least three years and are prepared to adhere to strict monitoring after discontinuation. Nevertheless, it would be prudent to have a better understanding of which patients may benefit from and which may be harmed by such a strategy, and to have a clear definition of the criteria for restarting treatment. Various studies have shown that the qHBsAg levels reached during treatment can be useful for determining the population which most benefits from discontinuation.75,80 However, as yet, no cut-off points enabling proper stratification have been established. A recent systematic review which included 11 studies, all Asian, concluded that a qHBsAg level of 100 IU/ml at the time of discontinuation is useful for predicting subsequent outcomes.82 Clinical relapse rates ranged from 15% to 29% in patients with qHBsAg <100 IU/ml and from 48% to 64% in patients with qHBsAg >100 IU/ml, while rates of achieving negative results for HBsAg ranged from 21% to 59% in the former group and from 3% to 7% in the latter group.82 Although it will most likely be possible in the near future to better identify these populations with the use of new HBV markers, such as HBcrAg,83 it does not seem prudent at present to recommend discontinuation of treatment in patients with qHBsAg levels >100 IU/ml, except within studies designed specifically to improve understanding of this strategy. If treatment is stopped prematurely, patients should be monitored monthly for the first six months in order to identify those who need to restart. The decision to restart treatment after clinical relapse is difficult, because early treatment can reduce the chances of achieving negative results for HBsAg, while a delay in starting treatment can be detrimental. In general, restarting would be indicated in severe or sustained clinical relapse, according to the above-mentioned definitions. Treatment in non-cirrhotic patients with clinical relapse under close monitoring achieves rapid virological and biochemical remission.72
Recommendations:
- •
The recommended nucleos(t)ide analogues are ETV, TDF and TAF (A1).
- •
In patients with chronic kidney disease (CKD) (eGFR <60 ml/min) or predisposing factors for CKD, including being over the age of 60, and in patients with or at risk of osteoporosis, ETV and TAF would be the nucleos(t)ide analogues of choice. In patients with eGFR <50 ml/min, TAF has the advantage over ETV that no dose adjustment is needed (B1).
- •
Initially, the duration of treatment with a nucleos(t)ide analogue is indefinite. The safest strategy for stopping treatment is based on doing so once negative results for HBsAg have been achieved; therefore, treatment should be stopped once that goal has been reached and confirmed to have been reached (B1).
- •
In patients with HBeAg-positive chronic hepatitis, treatment can be stopped before negative results for HBsAg have been achieved in case of virological response and HBeAg seroconversion confirmed by two determinations three to six months apart after at least 12 months of consolidation therapy (B2).
- •
In patients with HBeAg-negative chronic hepatitis, stopping treatment before achieving negative results for HBsAg can be considered in patients without advanced fibrosis or cirrhosis at the start of treatment, with a sustained virological response for at least three years, once HBsAg levels <100 IU/ml have been achieved, provided they are willing to be closely monitored after discontinuation (C2).
- •
In any event, treatment should not be stopped before the infection is resolved in patients with liver cirrhosis diagnosed at the start of or during treatment, patients with systemic manifestations of the disease, patients on immunosuppressant treatment and patients not willing to undergo close monitoring (B1).
- •
Patients who develop kidney disease during treatment with TDF should be switched to ETV, in which case their dose should be adjusted to suit their kidney function, or to TAF, which does not require dose adjustment. In case of previous treatment with LAM or TBV, the change should be made to TAF (B1).
PEG-IFN <-2a at a dose of 180 μg per week for 48 weeks is the recommended regimen in both HBeAg-positive and HBeAg-negative patients. Serological response and sustained virological response are achieved in approximately 25%-30% of HBeAg-positive patients.1,32 Additionally, in the majority of patients found to achieve negative results for HBeAg six months after completing treatment, this serological response is sustained on a medium- to long-term basis.1,32 Loss of HBsAg or functional cure is obtained in 3% of patients by six months after completing treatment, but these figures gradually increase, particularly in patients having achieved serological response, to 30% after three years of follow-up.1,32 The baseline factors that predict a favourable response to PEG-IFN therapy are essentially high ALT levels, low HBV DNA levels and genotype A or B infection.1,32
In patients with HBeAg-negative chronic hepatitis B, treatment with PEG-IFN for 48 weeks achieves sustained biochemical and virological response rates of 60% and 63%, respectively, six months after completion. However, unlike HBeAg-positive patients, their response rates decrease over time and are around 31% and 18%, respectively, four years after completing the therapy.1 Loss of HBsAg after PEG-IFN therapy is less common in HBeAg-negative than in HBeAg-positive patients, but rates do gradually increase after finishing treatment, from 3% at 6 months to 9% at three years and 12% at five years.1 No baseline factors have shown any utility for predicting response to PEG-IFN in HBeAg-negative patients.
SafetyTreatment with PEG-IFN causes frequent side effects. These are sometimes serious, and require dose reduction in approximately 25% of patients and early discontinuation in 5%.1 It is contraindicated in patients with severe psychiatric disease, poorly controlled epilepsy, severe heart disease, autoimmune disease or cytopenia. It is also contraindicated during pregnancy and in patients with decompensated cirrhosis or severe acute hepatitis. It should be used with caution in patients with compensated cirrhosis, especially those with portal hypertension, due to the risk of decompensation resulting from immune-mediated hepatocellular necrosis.
Treatment durationIn patients with HBeAg-negative chronic hepatitis B, prolongation of PEG-IFN therapy to 96 weeks was studied,84 and a significant increase in response rates compared to the standard 48-week treatment was found. However, post-treatment follow-up was short, so at present the recommended duration is 48 weeks.
If a lack of response can be anticipated in the course of the PEG-IFN therapy, it should be cut short in order to avoid adverse effects and unnecessary costs. In HBeAg-positive patients, qHBsAg levels of more than 20,000 IU/ml in week 12 of treatment (for patients with genotype B or C infection) and the absence of a decrease compared to baseline (for patients with genotype A or D infection) predict a very low probability of serological response85 and can be used as indicators for stopping treatment early. In HBeAg-negative patients, a combination of the absence of a decrease in qHBsAg levels at week 12 of treatment and a decrease by <2 log10 in HBV DNA levels, both compared to baseline, has a very high predictive capacity for lack of response in patients with genotype D infection1 and can also be used as a rule for stopping treatment early.
Recommendations:
- •
The recommended duration of PEG-IFN therapy is 48 weeks (B1).
- •
In HBeAg-positive patients with genotype A or D infection who, after 12 weeks of treatment, have qHBsAg levels >20,000 IU/ml and the absence of a decrease compared to baseline, treatment can be stopped due to lack of efficacy (B1).
- •
In HBeAg-negative patients with genotype D infection who, after 12 weeks of treatment, show the absence of a decrease in qHBsAg levels combined with the presence of a decrease in HBV DNA by <2 log10, both compared to baseline, treatment can be stopped due to lack of efficacy (B1).
This may occur with any analogue, especially in patients with high HBV DNA levels at the start of treatment. In most cases, prolonging treatment with the same analogue achieves virological response.1 However, HBV DNA kinetics should be assessed and, if levels continue to drop as of week 48 of treatment, the same drug can be continued, since the risk of developing resistance is extremely low. However, if HBV DNA levels remain stable, a change in analogue or combination therapy with ETV + TDF or TAF should be considered, particularly in patients with advanced liver disease in whom continued virus replication can have adverse clinical consequences.86 If combination therapy is chosen, once virological response has been achieved, a return to single-drug therapy may be considered after a consolidation period.87
Viral reboundWhen ETV, TDF or TAF is used, the most common cause of viral rebound is poor adherence to treatment. In treatment-naive patients, the risk of developing resistance to ETV is very low1 and development of resistance to TDF is rare.58. However, up to 51% of patients with previous resistance to LAM develop resistance to ETV after five years of treatment.1,32 Therefore, ETV should not be used in patients with resistance to LAM or TBV or in cases of uncertainty about a patient's history of treatment with nucleos(t)ide analogues. Resistance to TDF has not been reported when TDF is used as rescue treatment in patients with previous failure of other analogues,88 nor has it been reported in naive patients treated with TAF.56 Although there are no data on the use of TAF in patients with resistance to other analogues, it is assumed that its genetic barrier to resistance is similar to that of TDF. In patients who develop resistance to ETV, switching to TDF or TAF is recommended; TAF is preferable in patients with kidney disease, bone disease or predisposing factors for either. Single-drug therapy with TDF has been shown to be effective as rescue treatment in patients with resistance to LAM, ADV or ETV or multidrug resistance88 and in simplifying treatment after achieving virological response with combination therapy in patients with resistance to LAM.89 In the hypothetical case of resistance to TDF or TAF, the recommendation is to switch to ETV or add ETV to TDF or TAF in case of a history of resistance to LAM, though this recommendation is unsupported by scientific evidence.
Recommendations:
- •
In any situation involving the absence of virological response to analogue therapy, the degree of adherence to treatment should be investigated (B1).
- •
In patients with partial virological response, HBV DNA kinetics should be analysed as of week 48 of treatment. If HBV DNA levels continue to decline, the same analogue should be continued; if they do not, switching to another analogue or administering combination therapy with ETV + TDF or TAF should be considered (B2).
- •
In patients who develop resistance to ETV, switching to TDF or TAF is recommended (A1).
- •
In the hypothetical case of resistance to TDF or TAF, the recommendation is to switch to ETV or add ETV to TDF or TAF in case of a history of resistance to LAM.
Follow-up during treatment will depend on the drug used. Patients on treatment with ETV, TDF or TAF should undergo laboratory tests three months after starting therapy, including tests of kidney function (creatinine, eGFR, serum phosphate levels), ALT and HBV DNA.90–93 In HBeAg-positive cases, determination of HBeAg should be added. These laboratory tests should be repeated every three months until HBV DNA is undetectable. Subsequently, tests are recommended every six months to check kidney function, ALT and HBV DNA. If the drug used is TDF, the twice-yearly checks should include serum phosphate levels. In subjects treated with TAF, no dose adjustment in relation to eGFR is required as long as it remains ≥15 ml/min.
In HBeAg-positive patients, measurement of HBeAg and anti-HBe every six months is recommended. In case of HBeAg loss, the results need to be confirmed in a consecutive sample. In all cases, HBsAg or, preferably, qHBsAg should be determined annually. In cases in which negative results for HBsAg are achieved, they must be confirmed by further testing. qHBsAg is particularly useful in HBeAg-positive subjects, as a significant drop in qHBsAg levels in the first 24 weeks of treatment is associated with HBeAg loss and greater chances of HBsAg clearance.
Patients with liver cirrhosis should be included in a periodic surveillance programme for early diagnosis of HCC, with an ultrasound every six months.
Patients receiving PEG-IFN require closer monitoring during the 48 weeks of treatment. In addition to the tests already mentioned, follow-up of these patients also requires determination of haemoglobin, leucocytes and platelets at baseline, one month after treatment and every three months in order to adjust IFN doses, and measurement of thyroid stimulating hormone (TSH) every three to six months during treatment. In these patients, measurement of qHBsAg titres 12 weeks after treatment is useful to identify those who will not respond. In HBeAg-positive patients infected by genotype A or D, the absence of a drop in qHBsAg levels after 12 weeks of PEG-IFN treatment predicts lack of response. In HBeAg-negative patients infected by genotype D, the requirements for stopping treatment due to lack of response are a combination of a drop by less than 2 log10 IU/ml in HBV DNA and the absence of a decrease in qHBsAg at week 12.85,94,95
Recommendation:
- •
During treatment, periodic testing should be done to check ALT, HBV DNA and qHBsAg levels. In patients treated with nucleos(t)ide analogues, kidney function and serum phosphate levels should be periodically monitored, while in those treated with PEG-IFN, tests should include a complete blood count and TSH (A1).
In patients with HBV-related cirrhosis, the cumulative incidence of liver decompensation at five years is around 20%.12 The five-year survival rate for patients with decompensated cirrhosis is 15%-35%.96 These patients should be treated without delay with a nucleos(t)ide analogue with a high genetic barrier to resistance, regardless of their HBV DNA levels.12,34,97 At the same time, if they are potentially eligible candidates, they should be referred for assessment for liver transplantation.
The aim of antiviral therapy in these patients is twofold. On the one hand, it is intended to improve liver function and possibly avoid liver transplantation. On the other hand, it is meant to minimise the risk of HBV infection recurrence after liver transplantation, as this is related to viraemia levels at the time of transplantation. Antiviral therapy alters the natural history of decompensated cirrhosis, improves liver function and increases short- and long-term survival, especially when started early.98,99 Liver transplant-free survival rates are over 80% in patients treated with analogues,64,100 and up to a third of these patients can be removed from the transplant waiting list.98 This clinical improvement can take several months to become apparent, and patients may die of liver failure or its consequences in the meantime. The majority of deaths occur in the first six months after starting treatment, regardless of the extent to which virus replication is suppressed.99 Patients with high Model For End-Stage Liver Disease (MELD) or Child-Pugh scores have an increased risk of death. Hence the importance of prompt referral for assessment for liver transplantation, without waiting for viraemia levels to become undetectable.
PEG-IFN is contraindicated in this patient group. ETV and TDF are the first-line analogues in patients with decompensated cirrhosis. In choosing the analogue, any history of resistance to antivirals and any risk factors for kidney disease or bone disease should be taken into account. In patients with prior resistance to LAM, TDF is the drug of choice. In patients being treated with TDF who have underlying kidney or bone disease or are at risk of either, the possibility of switching to ETV or TAF should be considered,34 depending on prior resistance to LAM. The dose of ETV in decompensated cirrhosis is 1 mg per day (compared to 0.5 mg per day in compensated cirrhosis) because that was the dose used in the registry study for this population.101 However, the dose of 0.5 mg per day has shown similar efficacy.102
Analogue safety is very important in patients with decompensated cirrhosis, particularly those with very advanced liver disease. These patients should be closely monitored to detect potentially serious complications early. One such complication is lactic acidosis, which may occur with any analogue, but fortunately is rare. The risk thereof may be higher in patients with decompensated cirrhosis, especially those with MELD scores ≥ 20.1 Kidney failure is also common in these patients, requiring adjustment of their analogue dose and frequent monitoring of their kidney function. There are no data as yet on the safety and efficacy of TAF in patients with decompensated liver disease.97 These patients may be at increased risk for severe hepatic or renal adverse reactions. Therefore, close monitoring of liver biochemistry and kidney function tests is recommended if TAF is to be used.
Recommendations:
- •
Patients with decompensated cirrhosis should be treated without delay with an analogue with a high genetic barrier to resistance, regardless of their HBV DNA levels (A1). In choosing an analogue, history of resistance to other analogues and risk factors for kidney and bone disease should be taken into account.
- •
Patients with decompensated cirrhosis on analogue therapy should be closely monitored for treatment-related adverse effects such as kidney failure and lactic acidosis (A1).
- •
Patients with decompensated cirrhosis should be referred for assessment for liver transplantation if they are potential candidates, without waiting for HBV DNA levels to become undetectable (A1).
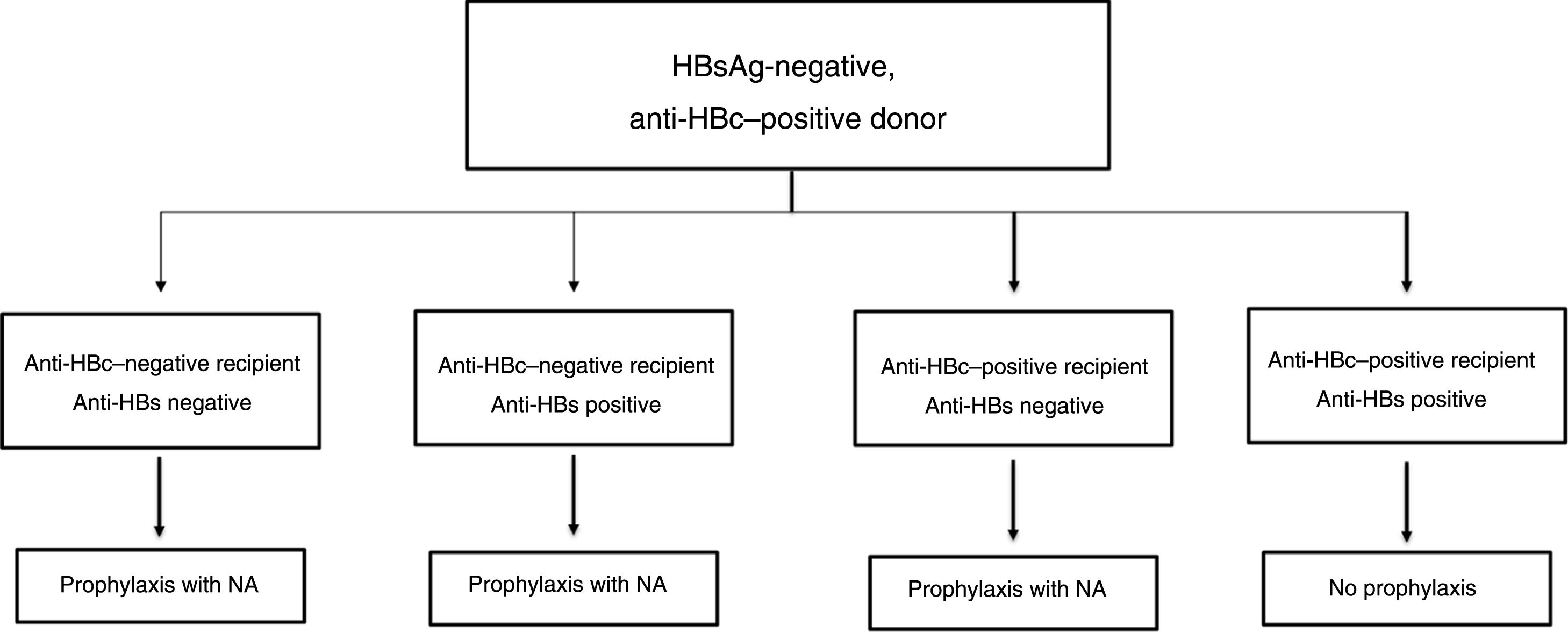
In the past, hepatitis B was considered a contraindication to liver transplantation as, in the absence of effective prophylaxis and treatment strategies, most patients underwent viraemic transplantation and graft reinfection was not only universal, but also accompanied in many cases by rapidly progressive and fatal hepatitis.103 The appearance first of hepatitis B immune globulin (HBIG), then of analogues, enabled development of effective prophylaxis and treatment strategies which have revolutionised liver transplantation in patients with HBV-related liver disease, to the extent that outcomes are now comparable to those achieved in other aetiologies.104,105
Treatment on waiting listIn patients on the liver transplant waiting list, the goal of treatment with analogues with a high genetic barrier to resistance (ETV or TDF/TAF) is to achieve undetectable HBV DNA levels as soon as possible and to maintain them until transplantation, in order to reduce the risk of post-transplant hepatitis B recurrence.103
Although it is desirable for viraemia to be undetectable at the time of liver transplantation, detectable HBV DNA levels should not delay the addition of the patient to the waiting list or be considered a contraindication thereto.106,107 This is particularly important in patients with acute-on-chronic HBV-related liver failure, who often have relatively high viraemia levels.108
Prophylaxis of hepatitis B recurrence after liver transplantationDe novo patientsA combination of an analogue and HBIG has become standard prophylaxis, and is capable of reducing the recurrence rate of HBV infection after liver transplantation to <5%.96,109 The analogue should be potent and have a high genetic barrier to resistance (ETV or TDF/TAF),110,111 and should be continued indefinitely after liver transplantation. In general, whichever analogue is started before transplantation is maintained, unless clinical circumstances favour switching to a different one (see section "Treatment of patients with decompensated cirrhosis").
There is more debate about the need for and duration of treatment with HBIG.112 Although most centres still use HBIG during the initial post-liver transplant period, the dose and duration are highly variable and the benefits of using HBIG in the long term remain uncertain. HBIG doses can be administered as needed or at set times; administration at set times is the preferred option in patients with poor adherence. In maintenance prophylaxis, the route of administration can be intravenous, intramuscular or subcutaneous, depending on the dose and the protocol at each hospital.112,113
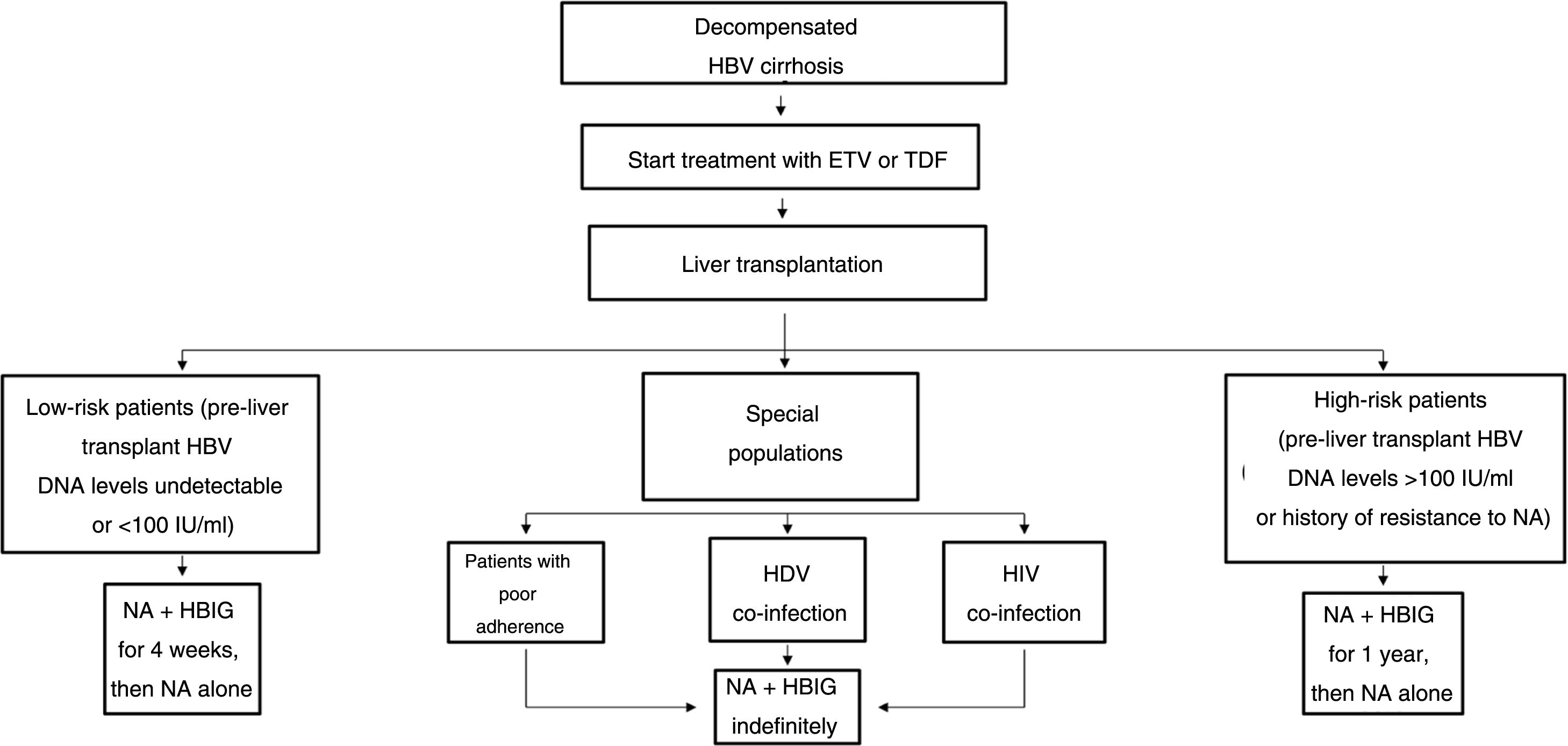
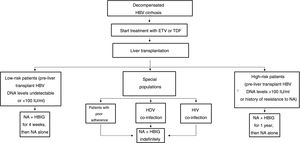
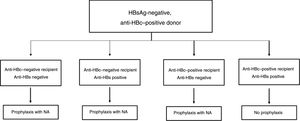
The drawbacks of HBIG, such as its high price, parenteral administration and side effects, have led to an ongoing search for strategies to optimise its use, such as using it at low doses, withdrawing it after a variable period of time following liver transplantation and even omitting it altogether from prophylaxis protocols. According to the data currently available, it seems that personalised HBIG regimens could be used depending on risk of post-liver transplant hepatitis B recurrence (Fig. 1).
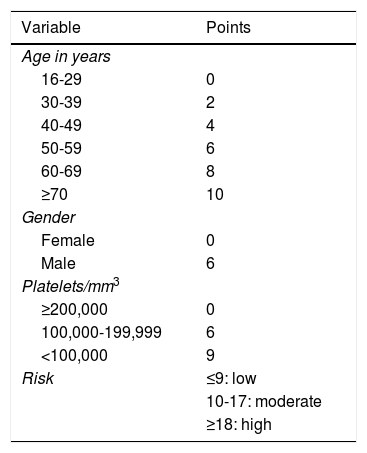
The main risk factor for recurrence of HBV infection after liver transplantation is viraemia >100 IU/ml at the time of transplantation. A history of resistance to analogues is also a risk factor for post-transplant recurrence of HBV infection.114 In high-risk patients, the recommended prophylaxis strategy is a combination of indefinite administration of ETV or TDF/TAF and long-term administration of HBIG. The optimal duration of HBIG use has not been firmly established, but it is recommended that it be administered for at least one year after achieving negative results for HBV DNA following liver transplantation. Maintaining the following pre-dose anti-HBs titres is recommended: >500 IU/ml up to 3 months, >100-250 IU/ml up to 6 months and >50-100 IU/ml from 6 months onwards.
Low-risk patientsPatients considered low risk are those with undetectable or low viraemia (<100 IU/ml) at the time of liver transplantation. The recommended prophylaxis strategy in these patients is a combination of indefinite administration of ETV or TDF/TAF and short-term administration of HBIG. Once again, the optimal duration of HBIG use has not been firmly established. Although excellent outcomes have been reported with HBIG treatment periods as short as 5-7 days,115,116 a period of one month seems both sufficient and reasonable in patients with optimal antiviral treatment.117 In patients with undetectable viraemia prior to liver transplantation, the use of a prophylaxis regimen based solely on administration of ETV or TDF/TAF, with no HBIG, may be a valid alternative, especially at centres where the cost of prophylaxis is an important consideration.107,118
Special populationsPatients with HDV or HIV co-infection and patients with questionable adherence to treatment with analogues do not really represent high-risk groups per se, but they do have special characteristics that make them candidates for indefinite administration of HBIG. HDV co-infection is not associated with an increased risk of hepatitis B recurrence, but recurrence of hepatitis D is itself risky, as there are no drugs to treat it effectively. A recent analysis assessed HBIG withdrawal at variable times after liver transplantation in 81 patients who underwent liver transplantation due to hepatitis D in different studies, and found only one case of HDV recurrence.119 Despite these data, prophylaxis with analogues alone is not currently recommended outside the context of clinical trials. Patients with HIV co-infection receiving analogues often have post-transplant intermittent low-grade viraemia,120, which supports the use of HBIG to minimise the risk of prophylaxis failure. Patients with questionable adherence to analogues are candidates for indefinite combination prophylaxis with an analogue + HBIG.117
Pre-liver transplant HCC is not a risk factor per se for post-transplant hepatitis B recurrence, but recurrence is more common in more advanced HCC (beyond Milan criteria) than in HCC within Milan criteria.121 Recurrence of HCC, however, is clearly associated with post-liver transplant hepatitis B recurrence, but in these cases combined prophylaxis is not very effective for prevention.114,122 Therefore, prophylaxis in patients with HCC should be chosen based on virological risk, as in patients without HCC.
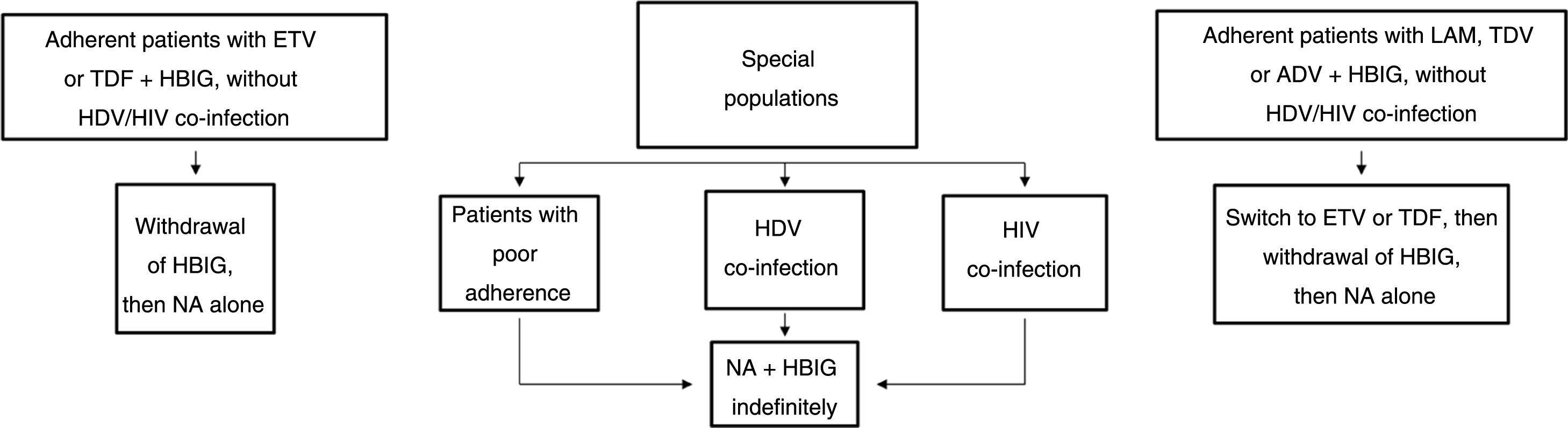
Long-term prophylaxisLong-term HBIG withdrawal in stable patientsLong-term HBIG withdrawal after a variable period of prophylaxis in combination with an analogue following liver transplantation is one of the strategies used to reduce the costs and drawbacks of HBIG in stable patients.114 For patients treated with analogues with a high barrier to resistance, the information gathered from the different studies can be summarised as follows: the time since liver transplantation is at least six months; the recurrence rate (HBsAg positivity) ranges from 0% to 8.7%;123–125 very few patients with recurrence after HBIG withdrawal had detectable HBV DNA levels with or without elevated liver enzymes; and, lastly, this situation typically arose in patients with poor adherence to analogues.117
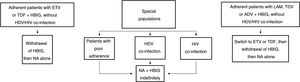
Based on the above, before HBIG is withdrawn in stable patients (with negative HBsAg and HBV DNA) the following factors should be taken into account: adherence, presence of HDV or HIV co-infection and type of analogue used while the patient was on combination prophylaxis (low or high barrier to resistance) (Fig. 2). If the patient was receiving an analogue with a low barrier to resistance, switching to ETV or TDF/TAF is recommended prior to withdrawing HBIG. Maintaining combination prophylaxis indefinitely is also recommended in patients with HDV or HIV co-infection and in patients with adherence problems.
VaccinationActive immunisation with HBV vaccine after liver transplantation is in theory an appealing alternative to indefinite administration of prophylaxis in general and HBIG in particular. However, studies which have assessed the efficacy of post-liver transplant vaccination have been very mixed in terms of characteristics and number of patients enrolled, donor type (cadaveric versus living), vaccine type, use of adjuvants, vaccination protocols and definition of response. In patients having undergone transplantation for chronic liver disease, outcomes are generally unsatisfactory, with overall response rates of less than 30%-40%.126 However, these rates are much higher (75%-100%) in patients having undergone transplantation for acute liver failure.126 This strategy is not currently recommended in clinical practice with the aim of discontinuing antiviral prophylaxis and should only be considered in the context of clinical trials, though it may be considered in transplants due to acute liver failure.
Complete withdrawal of prophylaxisThe notion that prophylaxis for hepatitis B recurrence should be maintained indefinitely following liver transplantation is based on indirect findings, primarily frequent determinations of serum HBV DNA levels (titres generally being low or transient) or peripheral blood or liver mononuclear cells in patients without clinical or serological evidence of post-transplant hepatitis B recurrence.127,128 This notion was called into question in a prospective study in which the efficacy and safety of planned complete withdrawal of prophylaxis was analysed in 30 heavily screened patients with a theoretically low risk of recurrence of hepatitis B following liver transplantation, since in all cases serum HBV DNA levels were undetectable at the time of transplantation. The recurrence rate for hepatitis B (HBsAg positivity with or without detectable viraemia) was 20% (6/30), and only three patients needed to restart prophylaxis.129,130 In another recent study, retrospective analysis of complete withdrawal of prophylaxis (HBIG + analogue) in a group of ten patients who had voluntarily stopped taking prophylaxis due to adherence problems also found positive outcomes.131 Despite the above results, complete withdrawal of prophylaxis cannot currently be recommended in practice and should only be attempted in the context of clinical trials.
Recommendations:
- •
All patients on the liver transplant waiting list due to HBV-related liver disease should be treated with an analogue with a high genetic barrier to resistance (A1).
- •
Prophylaxis for post-liver transplant hepatitis B recurrence should be based on indefinite administration of ETV or TDF/TAF (A1).
- •
With respect to HBIG, an personalised approach to its use is recommended based on risk of post-liver transplant HBV infection recurrence (A1).
The prevalence of anti-HBc–positive donors varies by geographic area: 2%-3% in the US, around 10% in Europe and over 50% in Asia.132 In Spain, the prevalence is around 10%,133 but increases with donor age up to 30% in donors over the age of 60.134 The use of these grafts is associated with a risk of hepatitis B transmission even in the absence of detectable viraemia in the donor, due to the presence of occult infection in a significant proportion.135,136 In the absence of prophylaxis, the risk of hepatitis B transmission depends mainly on the anti-HBc/anti-HBs status of the recipient at the time of liver transplantation.137–139 The highest risk is observed in naive recipients (anti-HBc–negative and anti-HBs–negative) with a prevalence of de novo hepatitis B of 47.8% to 65.5%. Recipients with isolated positive anti-HBc serology have a risk ranging from 13% to 17.9%. Surprisingly, patients who are anti-HBs–positive by vaccination (with isolated positive anti-HBs serology) are not protected, with a risk of de novo HBV infection ranging from 9.7% to 23.3%. However, it should be noted that details as seemingly important as anti-HBs levels at the time of liver transplantation, changes over time in post-transplant levels and the presence of vaccine-related pre-S mutants are not generally available in published studies, but might shed light on the development of de novo hepatitis B in these patients.140 Lastly, recipients who are positive for anti-HBc and anti-HBs have the lowest risk (1.4%-5.5%).
Overall, prophylaxis significantly reduces the risk of de novo hepatitis B in all patient groups, except those positive for anti-HBc and anti-HBs, due to the low incidence of de novo hepatitis B with no prophylaxis in this group.137Fig. 3 shows an algorithm for the use of liver grafts from HBsAg-negative and anti-HBc–positive donors. Analogues are the drugs of choice for prophylaxis and should be administered indefinitely. Until now, LAM has been considered the drug of choice for prophylaxis due to its cost-effectiveness. With the reduction in the cost of ETV and TDF and the risk of resistance to LAM over time,141,142 these drugs are now a better alternative than LAM.143 Recent studies have analysed active immunisation before and after liver transplantation as a strategy to prevent de novo HBV infection and eliminate the need to administer an analogue.144,145 Unfortunately, the small size of the series and the lack of uniformity of the regimens have yielded contradictory results. This strategy requires frequent monitoring of anti-HBs levels and could be more effective in living donor liver transplantation, in which the vaccine could be administered to non-immunised recipients before the transplant procedure.145
Recommendations:
- •
Liver grafts from HBsAg-negative anti-HBc–positive donors should preferably be used in patients undergoing transplantation due to hepatitis B, as they will already be receiving effective prophylaxis against hepatitis B recurrence. As a second choice, they may be used in recipients who are positive for anti-HBc or for anti-HBs. As a third and last choice, they may be used in naive recipients (B1).
- •
The need for prophylaxis depends on the anti-HBc/anti-HBs serological profile of the recipient at the time of liver transplantation and is based on indefinite administration of an analogue (A1).
- •
Although LAM has been shown to be highly effective, ETV and TDF are preferable due to their high genetic barrier to resistance (B1).
Chronic HBV infection is the most common cause of HCC worldwide.146 Most of the knowledge about the risk of HCC in HBV infection comes from studies conducted in Southeast Asia and sub-Saharan Africa, where HBV infection is endemic and acquired in the perinatal period. However, the incidence of HCC in patients with hepatitis B is estimated at 0.4%-0.6% per year.147 HCC usually occurs in patients with liver cirrhosis associated with B virus infection, meaning that these patients are in advanced stages of the disease (70%-90%), but it is important to mention the carcinogenic potential of the virus (whose genome is able to integrate into human DNA), particularly one of the proteins it encodes (protein X). In non-cirrhotic patients the risk is lower, but not negligible, and especially affects men over the age of 40 with a family history of HCC or long-lasting infection (vertical transmission), as well as patients from areas of high endemicity (Africa or Asia) with co-infection by other viruses (HCV/HIV) and genotype C infection.1,12,19,146,148,149
As with other viral infections, viral suppression by nucleos(t)ide analogues is able to modify the risk of HCC among patients with hepatitis B. Treatment with analogues has been associated with a reduced, but not eliminated, risk of HCC.41,150–153 A study in Caucasian patients showed that the risk of HCC in patients with cirrhosis and patients over the age of 50 persisted after five years of antiviral treatment.154
Indications for screeningThe American Association for the Study of Liver Diseases recommends screening based on HCC incidence rate thresholds ≥0.2% per year among those without cirrhosis and from 0.2% to 1.5% per year among patients with underlying cirrhosis.155,156 Family history of HCC, age and race are considered additional criteria for stratifying HCC risk in patients with hepatitis B. HCC surveillance is recommended for all patients with cirrhosis and for patients without cirrhosis but with a family history of HCC or African or Asian origin who are over the age of 40 if male or over the age of 50 if female.
All at-risk patients should undergo strict screening with abdominal ultrasound every six months to enable early detection of cancer. Scoring systems have also been developed in the last few years to predict the risk of HCC in patients with hepatitis B. However, while these scoring systems can achieve high negative predictive values (exceeding 95%) for the development of HCC over a period of three to ten years in untreated patients, they may not adequately predict the risk of HCC in patients on antiviral therapy. In addition, most of them were validated in and Asian population (genotypes B/C), which differs from the population in the Mediterranean region (Caucasian race and genotypes A/D).12 PAGE-B is a recently developed score validated in Caucasian patients treated with nucleos(t)ide analogues which has a good predictive capacity for the development of HCC in the first five years of treatment with ETV or TDF. This score can be easily applied in clinical practice as it is based on widely available parameters (platelets, age and sex)157(Table 6). Cumulative probabilities of developing HCC at five years in low, moderate and high-risk patients were 0%, 3%, and 17%, respectively.
PAGE-B model for predicting the risk of HCC at 5 years in Caucasian patients treated with ETV or TDF.
| Variable | Points |
|---|---|
| Age in years | |
| 16-29 | 0 |
| 30-39 | 2 |
| 40-49 | 4 |
| 50-59 | 6 |
| 60-69 | 8 |
| ≥70 | 10 |
| Gender | |
| Female | 0 |
| Male | 6 |
| Platelets/mm3 | |
| ≥200,000 | 0 |
| 100,000-199,999 | 6 |
| <100,000 | 9 |
| Risk | ≤9: low |
| 10-17: moderate | |
| ≥18: high | |
All patients with HCC should receive antiviral therapy with nucleos(t)ide analogues, regardless of viral load, as said therapy has been shown to reduce the risk of HCC recurrence and improve the prognosis of the liver disease.35 In a meta-analysis that included 8,060 cases, the recurrence risk ratio (RR) at one year and three years was clearly lower in patients on antiviral therapy (RR 0.41; 95% CI: 0.28-0.61 at one year; RR: 0.63 ; 95% CI: 0.43-0.94 at three years).36
Recommendations:
- •
Patients with cirrhosis due to HBV should have ultrasound screening for HCC every six months (A1).
- •
In patients who do not have cirrhosis but do have a family history of HCC or are from an African or Asian country and are over 40 years of age (if male) or over 50 years of age (if female), especially in case of vertical transmission or genotype C infection, ultrasound screening for HCC every six months is recommended (B1).
- •
In patients treated with ETV or TDF, it is recommended that the PAGE-B score be calculated at the start of treatment. High-risk patients (score ≥18 points) should be included in a programme of screening for HCC every six months; screening may be considered for moderate-risk patients (10-17 points) (B2).
Co-infection with HBV and HIV is a common health problem. Among the approximately 37 million people infected by HIV in the world, it is estimated that 5%-20% have HBV co-infection. HIV infection has a significant impact on the natural history of chronic hepatitis B. Co-infected patients have higher HBV DNA levels and are more likely to have HBeAg and an increased risk of morbidity and mortality due to chronic liver disease and HCC, compared to HIV-negative patients and to HIV-positive patients without hepatitis B.158–160 HIV-positive patients without serological evidence of HBV infection should be offered HBV vaccination, and antibody response should be monitored and rechecked annually in immunocompromised patients to assess the need for booster doses.161
The indications for treatment of hepatitis B in co-infected patients are the same as in patients without HIV. Antiretroviral therapy guidelines recommend starting treatment in all patients with HIV-1 infection with or without symptoms regardless of their CD4+ lymphocyte count. Therefore, treatment for both infections should be prescribed simultaneously using a combination of HIV reverse transcriptase inhibitors active against HBV (TDF or TAF with LAM or emtricitabine).12,162–164 These combinations provide very good rates of antiviral efficacy without development of resistance.165 TAF reduces the incidence of adverse effects of TDF on renal tubular function and bone density without affecting suppression of virus replication in both viruses, and switching from TDF to TAF does not alter the virological control of the two infections. It was recently reported that residual B viraemia not associated with the appearance of resistant mutants is detected in up to 10% of patients with HBV/HIV co-infection with good adherence to treatment.164 However, the mechanisms and clinical significance of the persistence of HBV DNA levels are not fully understood. Data with PEG-IFN are limited in co-infected and HBeAg-positive patients, but recent studies failed to show increased HBeAg loss or seroconversion after addition of PEG-IFN to treatment with LAM or emtricitabine and TDF.166
Patients co-infected with hepatitis B and CHepatitis B and C viruses share several transmission mechanisms, and hepatitis C virus co-infection has been reported in 7%-15% of patients with chronic hepatitis B.167 Chronic infection by both viruses accelerates the progression of liver disease and increases the risk of developing HCC.168 HCV infection often inhibits replication of HBV, but the presence of HBV neither compromised treatment efficacy in the past with PEG-IFN and ribavirin nor compromises it now with direct antivirals. Rates of sustained viral response are similar (more than 95%) in patients with HBV/HCV co-infection and patients with HCV infection alone.168 There is also no evidence that the antiviral efficacy of nucleos(t)ide analogues for hepatitis B is affected by HCV co-infection. However, there is a risk of reactivation of HBV replication during or after treatment with direct antivirals. Consequently, HBV DNA levels should be monitored in all HBsAg-positive patients being treated for hepatitis C, and treatment with ETV or TDF/TAF should be prescribed where indicated.169 If there is no clear indication for hepatitis B treatment, prophylaxis with any analogue taken during treatment for hepatitis C and continued for 12 weeks after completion thereof may be considered. The risk of reactivation is practically non-existent in HBsAg-negative and anti-HBc–positive patients, but HBsAg or HBV DNA levels should be monitored for elevated transaminases.
Patients co-infected with hepatitis B and DHDV only infects HBsAg-positive patients, so all patients with hepatitis B need to be screened for HDV infection. Although the incidence of HDV has decreased greatly in recent years, in some geographic areas, such as the Mediterranean basin and Eastern European countries, between 5% and 10% of HBsAg-positive patients have HDV co-infection.170 Hepatitis D is characterised by more severe liver disease and a higher incidence of cirrhosis than seen in patients with chronic hepatitis B.
IFN is the only drug recommended for the treatment of chronic hepatitis D. In some historical series, the use of high doses of standard IFN-〈 00A0;(9-10 MU 3 times weekly) achieved response rates of around 15%, with histological improvement in the patients who responded.171,172 More recently, treatment with PEG-IFN for one year achieved a response (HDV RNA undetectable 24 weeks after completion of treatment) in 28% of patients.173 Other studies have reported virological response at week 24 post-treatment in 25%-37% of patients, but late relapses have been reported one year after completion of treatment. The response rate is not improved by either prolongation of PEG-IFN therapy or addition of nucleos(t)ide analogues.173,174 In the absence of cirrhosis, nucleos(t)ide analogues should be administered only if there is significant HBV replication (HBV DNA levels repeatedly more than 2,000 IU/ml). The therapeutic benefit and the risk of developing clinical events are closely related to achieving negative results for HDV RNA.175,176
It is essential to develop new drugs for chronic hepatitis D. Prenylation inhibitors (lonafarnib) and entry inhibitors (Myrcludex B) are currently in different stages of clinical development.172
Patients with chronic kidney diseasePatients with chronic kidney disease on haemodialysis are a risk group for HBV infection. Although their response to the hepatitis B vaccine is lower than that of the general population, the prevalence of chronic HBV infection in these patients has been drastically reduced in recent years. However, as it can still cause morbidity, screening is mandatory.12 The vaccine is indicated in all seronegative patients and, as the immunisation rate is lower than that of the general population, booster doses are required.177 Patients on haemodialysis can be treated with ETV or TAF according to the same criteria used in immunocompetent patients without chronic kidney disease; if ETV is used, then the dose should be adjusted (Table 3). As stated earlier, the drugs with the best renal safety profile and a high genetic barrier must be used in these patients to prevent resistance.178 ETV is the analogue of choice in naive patients; TAF is reserved for those with resistance to LAM. Antiviral therapy with nucleos(t)ide analogues has also been used in patients with HBV-associated glomerulonephritis, with two-thirds of them achieving clinical remission and improvement in proteinuria.179 HBV-infected kidney transplant recipients should also similarly receive treatment or prophylaxis with analogues (ETV in naive patients and TAF in naive or pretreated patients, although efficacy and safety data are not available in this subgroup). Both LAM (risk of resistance) and IFN (risk of promoting rejection of the transplanted kidney) should be avoided.180
Acute hepatitisMost cases (95%) of acute hepatitis B in immunocompetent adults resolve spontaneously, in which case there is no indication for treatment. However, in cases of severe acute hepatitis (coagulation disorders or signs of acute liver failure), antiviral treatment should be prescribed. ETV and TDF (and most likely also TAF, although there are no data with this drug in patients with severe acute hepatitis) are the best options due to their potency and high genetic barrier, especially if liver transplantation is being considered.181,182 IFN therapy is not recommended in patients with severe acute hepatitis.
PregnancyThe clinical approach to hepatitis B during pregnancy poses a number of challenges, including treatment of the disease in the pregnant woman and prevention of transmission to the newborn, whose risk of developing chronic hepatitis is as high as 90%. IFN (standard or pegylated) is contraindicated in pregnancy, and nucleos(t)ide analogues are not registered for such use; hence the indication for treatment should be carefully assessed.183 Although LAM, ETV and ADV lack consistent safety data in humans, TBV and TDF have shown no evidence of toxicity to the fetus.12 Of the two, TDF is the better option due to its high genetic barrier and non-existent risk of developing resistance. There are no safety data for TAF in pregnant patients and its use cannot be recommended.
Treatment decision-making must weigh the severity of the disease and the risk of perinatal transmission.1,183 If a woman in whom treatment is indicated wants to become pregnant in the near future, in most cases antiviral therapy with nucleos(t)ide analogues can be delayed until after the birth. However, if the patient has predictive factors for response to PEG-IFN, then this treatment may be indicated. As the duration of PEG-IFN therapy is limited to 48 weeks, the patient could plan to become pregnant afterwards. Should a patient on analogue therapy become pregnant, the medication should not be stopped. Nevertheless, the antiviral of choice under these circumstances is TDF, so if the patient is taking ETV or LAM, she should be switched to TDF.12,34
Combined immunoprophylaxis with a vaccine and specific gamma globulin is indicated in all newborns with an HBsAg-positive mother. This strategy reduces the risk of transmission very significantly (by more than 90%). However, patients with high viraemia levels (>200,000 IU/ml) should be prescribed an antiviral (again, TDF is the drug of choice) as of weeks 24-28 of pregnancy.1,12,34. The duration of antiviral treatment as prophylaxis for vertical transmission has not been established, and there is debate over whether it should be discontinued immediately after delivery or continued for up to three months afterwards. Breastfeeding is not contraindicated in women with hepatitis B or in those on treatment with TDF.
ChildrenAfter almost 30 years of universal vaccination of all newborns in Spain, the incidence of HBV infection in children has decreased dramatically. Most of those who develop chronic hepatitis are in the HBeAg-positive chronic infection phase, with high viraemia levels and minimal or no necroinflammatory activity in the liver (immune tolerant phase), and have mild disease which does not require treatment.184 The rate of spontaneous HBeAg seroconversion is very high in children. Therefore, those who show signs of disease activity must be closely monitored before treatment is started, and treatment is only indicated in children or adolescents with active or potentially severe liver disease (necroinflammation and moderate/advanced fibrosis). Standard IFN, LAM and ADV have been used in the past with outcomes in terms of efficacy, safety profile and risk of resistance similar to those seen in adult patients.184,185 Both ETV and TDF are equally effective in the paediatric population and, as in the adult population, are the drugs of choice if treatment is indicated.
Recommendations:
- •
The indications for treatment of hepatitis B in HIV-positive patients are the same as in non–HIV-positive patients. All co-infected patients should be prescribed treatment with combinations based on TDF/TAF with LAM/emtricitabine, regardless of their CD4+ lymphocyte count (A1).
- •
Patients on haemodialysis should be treated with nucleos(t)ide analogues with a high genetic barrier (ETV/TAF); if ETV is used, then the dose should be adjusted. In patients without resistance to LAM, ETV is the first choice and TAF is reserved for those pretreated with LAM (B1).
- •
PEG-IFN for 48 weeks is the recommended treatment for compensated chronic hepatitis D and achieves virological response in approximately 25% of patients. Oral antivirals for HBV are not effective for HDV, but should be used if there is active HBV replication (HBV DNA levels >2,000 IU/ml in patients without cirrhosis or any HBV DNA levels in patients with cirrhosis) (A1).
- •
In patients co-infected with hepatitis B and C viruses, the rate of response to treatment with direct antivirals is similar to that of patients infected with HCV alone. However, there is a risk of reactivation of HBV during or after treatment with direct antivirals. HBV DNA levels should be closely monitored in HBsAg-positive patients being treated for hepatitis C, treatment should be started with nucleos(t)ide analogues (ETV or TDF/TAF) where so indicated and, in the remaining patients, prophylaxis should be considered during hepatitis C treatment and for 12 weeks afterwards.
- •
In pregnant women, treatment decision-making must weigh the severity of the disease and the risk of perinatal transmission. In addition to active and passive immunoprophylaxis with a vaccine and specific gamma globulin in all newborns, in patients with high viraemia levels (>200,000 IU/ml) treatment with TDF is recommended as of weeks 24-28 of pregnancy. In women already being treated with analogues, the medication should not be discontinued, but they should be switched to TDF if they were receiving another antiviral (A1).
- •
The incidence of HBV infection in children has decreased significantly in recent years (A1). In the few cases in which treatment is indicated, ETV and TDF are the drugs of choice, as in the adult population (B1).
People with chronic infection (HBsAg-positive) or previous contact with HBV (HBsAg-negative and anti-HBc–positive) who are taking immunosuppressant therapy or biologic drugs are exposed to reactivation of the infection, the severity of which can range from an asymptomatic, transient elevation in transaminases to fulminant hepatitis. When HBV infects a susceptible person, the virus penetrates hepatocytes, and its DNA integrates into hepatocyte DNA and becomes cccDNA. cccDNA is fairly stable in infected cells and can persist for decades in a latent state, serving as a reservoir for HBV reactivation, even in patients whose HBV infection had apparently resolved (HBsAg-negative and anti-HBc–positive).186
Reactivation of HBV is defined as an increase in virus replication (usually by >1 log of HBV DNA) in individuals with detectable viral load or as HBV DNA positivity in people with undetectable viral load or resolved infection.187,188 In HBsAg-positive patients, reactivation is characterised by a rapid increase in HBV DNA levels, followed by elevation of transaminases. In HBsAg-negative patients, this process tends to be preceded by HBsAg positivity.189,190 The mechanism thought to be responsible for reactivation begins with an increase in HBV replication and expression of HBV antigens in hepatocytes during immunosuppression, followed by T lymphocyte-mediated destruction of infected hepatocytes during immune recovery.
Factors related to the host, virus and type of treatment are thought to be associated with an increased risk of HBV reactivation.187,188 Having detectable HBsAg or HBV DNA levels greatly increases the risk of reactivation. In HBsAg-negative, anti-HBc–positive patients, anti-HBs positivity seems to be a protective factor against possible reactivation. However, information is limited and this factor does not currently alter the indication for prophylactic treatment.191 Underlying disease (e.g. lymphoma) can also affect the risk of reactivation. In general, the more potent the cytotoxic or immunosuppressant therapy, the higher the risk of virus reactivation. The level of immunosuppression necessary to cause reactivation in HBsAg-positive patients is lower than in patients who are HBsAg-negative and anti-HBc–positive. However, the prevalence of individuals with resolved HBV infection is much higher, such that this situation clinically significant. Therapies that deplete B lymphocytes, such as rituximab, significantly increase the risk of reactivation both in HBsAg-positive patients and in patients who are HBsAg–negative and anti-HBc–positive. In the case of bone marrow transplantation, HBV reactivation is almost universal in HBsAg-positive patients and occurs in approximately 50% of those with resolved infection.18,192
The first step in preventing HBV reactivation is identifying patients at risk. HBV serological status should be assessed in all candidates for immunosuppressant therapy or chemotherapy by testing for HBsAg and anti-HBc. If serology is negative, vaccination status (anti-HBs) could be assessed and the regimen could be completed if necessary. However, in these patients it is often not possible to delay immunosuppressant therapy after the administration of the vaccine. HBsAg-positive patients should be referred to a specialist who will assess and diagnose their phase of HBV infection and thus determine whether or not they meet criteria for starting antiviral therapy. In patients who are HBsAg–negative and anti-HBc–positive, HBV DNA levels should be determined; if detectable, these patients should be treated in the same way as HBsAg-positive patients and referred to a specialist.
Taking into consideration the two main factors (the type of HBV infection and the type of immunosuppressant therapy), the risk of reactivation can be classified as low (<1%), moderate (1%-10%) or high (>10%)18,190,191 (Table 7). HBsAg-positive patients in whom treatment is not indicated should be given antiviral prophylaxis if they belong to high- or moderate-risk groups. The same criteria for starting prophylaxis should be applied to HBsAg-negative, anti-HBc–positive and HBV DNA-positive patients, while in HBV DNA-negative patients, prophylaxis would be indicated if these patients belong to the high-risk group. Patients with this profile who belong to moderate or low-moderate risk groups should be closely monitored (transaminase levels, HBV DNA levels and HBsAg levels at least every 3 months during and for 6 months after the end of immunosuppressant treatment); if such periodic monitoring is not possible for any reason, antiviral prophylaxis should be considered.
Risk of HBV reactivation by immunosuppressant treatment and HBV serological status.
| Type of immunosuppressant treatment | HBsAg positive | HBsAg negative and anti-HBc positive |
|---|---|---|
| B-cell–depleting therapies (e.g. rituximab, natalizumab or alemtuzumab) | High | High |
| Immunosuppression associated with bone marrow transplantation | High | High |
| Potent TNF〈 inhibitors (e.g. infliximab, adalimumab, certolizumab or golimumab) | Moderate/high | Low/moderate |
| Anthracycline derivatives (e.g. doxorubicin) | High | Low/moderate |
| Local treatment of HCC (TACE) | High | Low/moderate |
| Systemic chemotherapy or cytokine or integrin inhibitors (e.g. abatacept, ustekinumab, natalizumab or vedolizumab) | Moderate | Low/moderate |
| Cyclophilin inhibitors (e.g. ciclosporin) | Moderate | Low/moderate |
| Tyrosine kinase inhibitors (e.g. imatinib) | Moderate | Low/moderate |
| Proteasome inhibitors (e.g. bortezomib) | Moderate | Low/moderate |
| Histone deacetylase inhibitors (e.g. romidepsin) | Moderate | Low/moderate |
| Less potent TNF-〈 inhibitors (e.g. etanercept) | Moderate | Low |
| Prednisone (or equivalent) ≥10 mg/d ≥4 weeks | High | Moderate |
| Prednisone (or equivalent) <10 mg/d ≥4 weeks | Moderate | Low |
| Prednisone (or equivalent) <1 week | Low | Low |
| Antimetabolites (e.g. AZA/6-MP or methotrexate) | Low | Low |
| Intra-articular corticosteroid injections | Low | Low |
AZA: azathioprine; HCC: hepatocellular carcinoma; MP: mercaptopurine; TACE: transarterial chemoembolisation; TNF: tumour necrosis factor.
Various studies have shown that antiviral therapy with nucleos(t)ide analogues significantly reduces the risk of HBV reactivation.193 Moreover, preventive antiviral therapy is more effective than treatment of reactivation once it has occurred.194 LAM is a drug with demonstrated efficacy in this situation that has been the drug of choice due to its proven safety and low cost. However, due to the risk of resistance developing in HBsAg-positive patients and in patients expected to need long-term antiviral therapy, it may be preferable to use more potent analogues with a high genetic barrier such as ETV and TDF, which are now affordable alternatives.12,195 Although there is no experience with the use of TAF in this context, prescription of TAF would be indicated in patients with kidney disease or bone disease and a history of resistance to LAM.
The antiviral regimen should preferably be started at least two weeks before immunosuppressant treatment or chemotherapy, especially in patients with detectable HBV DNA levels. It should be maintained during treatment, should be continued for at least 12 months (18 months for regimens based on rituximab or other anti-CD20 antibodies) after cessation of immunosuppressant treatment and should be discontinued only if the underlying disease is in remission.12 Antiviral therapy should be maintained in HBsAg-positive patients with a baseline situation of chronic hepatitis B and indication for treatment. It is advisable to monitor liver function tests and HBV DNA levels every three to six months during prophylactic treatment and for at least 12 months after cessation thereof, as some HBV reactivations occur after discontinuation of prophylaxis.
Recommendations:
- •
All candidates for chemotherapy and immunosuppressant or biologic therapy should undergo serological screening for HBV (A1).
- •
Antiviral prophylaxis is recommended in HBsAg-positive patients at moderate or high risk of reactivation and in HBsAg-negative, anti-HBc–positive patients at high risk of reactivation. For HBsAg-negative, anti-HBc–positive patients at moderate or low-to-moderate risk, monitoring during and for six months after finishing immunosuppressant therapy is recommended (B1).
- •
The recommended drugs in antiviral prophylaxis are ETV, TDF and TAF (A2).
- •
Antiviral prophylaxis should preferably be started two weeks before immunosuppressant therapy, especially in cases with detectable HBV DNA levels, and should be continued for 12 months (18 months for regimens based on rituximab or other anti-CD20 antibodies) after completion thereof (B1).
All the authors have worked as consultants and speakers for Gilead Sciences and Bristol Meyers Squibb.
Please cite this article as: Rodríguez M, Buti M, Esteban R, Lens S, Prieto M, Suárez E, et al. Documento de consenso de la Asociación Española para el Estudio del Hígado sobre el tratamiento de la infección por el virus de la hepatitis B (2020). Gastroenterol Hepatol. 2020;43:559–587.