The administration of vitamin K immediately after birth has shown a significant decrease in the incidence of newborn bleeding, but there is not enough evidence to determine the most appropriate method of administration. The objective of this review is to determine the effectiveness of orally administered vitamin K compared to the intramuscular route in the prevention of haemorrhagic disease of newborn (HDN).
MethodsWe conducted a systematic review of the main databases (Medline, Embase and Cochrane, among others) without limitation by date, language or type of study. Selected studies evaluated the efficacy and safety of vitamin K. Excluded were studies in pregnant women in preterm infants or patients with pathology. The validity of these studies was assessed by CASPe tools for systematic reviews and clinical trials.
ResultsOnly two studies evaluated clinical aspects. They showed a reduction in the incidence of bleeding in the newborn after intramuscular prophylaxis with vitamin K. With regard to the oral route, different studies examined the effectiveness of vitamin K by determining biochemical parameters (factor X, prothrombin time and index, vitamin K1 in plasma and prothrombin antigen, among others) with inconclusive results regarding the route of administration and the number of doses.
ConclusionsThere is sufficient evidence to support the effectiveness of a single intramuscular dose of vitamin K to prevent the classic form of HDN. With regard to late HDN and oral route, the results are inconclusive because the studies used biochemical indicators of effectiveness, which cannot be correlated with the actual coagulation status of the newborn due to lack of scientific evidence.
La administración de vitamina K inmediatamente después del nacimiento ha demostrado un descenso significativo de la incidencia de hemorragias neonatales, pero no existe evidencia suficiente que determine la forma de administración más adecuada. El objetivo de esta revisión es determinar la eficacia de la vitamina K administrada por vía oral frente a la vía intramuscular en la prevención de la enfermedad hemorrágica del recién nacido.
MétodosSe realizó una revisión sistematizada de las principales bases de datos (Medline, Embase y Cochrane), entre otras sin limitación por fecha, idioma ni tipo de estudio. Los estudios seleccionados evaluaban la eficacia de la vitamina K. Se excluyeron aquellos estudios realizados en embarazadas, niños pretérmino o en pacientes afectos de alguna enfermedad. La validez de estos estudios fue evaluada mediante herramientas CASPe para revisiones sistemáticas y ensayos clínicos.
ResultadosLos estudios incluidos fueron cuatro ensayos clínicos y una revisión sistemática. Únicamente dos estudios evaluaron aspectos clínicos en los cuales se observó un descenso en la incidencia de hemorragias en el recién nacido tras la profilaxis con vitamina K por vía intramuscular. Con respecto a la vía oral, diferentes estudios analizaron la eficacia de la vitamina K mediante la determinación de parámetros bioquímicos (factor X, índice y tiempo de protrombina, vitamina K1 en plasma y antígeno de protrombina, entre otros) con resultados poco concluyentes en cuanto a la vía de administración y al número de dosis.
ConclusionesExiste evidencia suficiente que apoye la eficacia de una dosis única de vitamina K vía intramuscular para prevenir la forma clásica de la enfermedad hemorrágica del recién nacido. Para la forma tardía y la vía oral los resultados no son concluyentes dado que los estudios existentes utilizan indicadores bioquímicos de eficacia, los cuales no pueden correlacionarse con el estado de coagulación real del recién nacido por falta de evidencia científica.
Haemorrhagic disease of the newborn (HDN) is an acquired coagulation disorder secondary to a deficiency in the coagulation factors that are dependant on vitamin K (factors II, VII, IX and X). Diagnosis is based on bleeding in a newborn infant with a prolonged prothrombin time and normal platelet count and fibrinogen plasma levels. It is then confirmed by immediate correction of the prothrombin time and/or cessation of the bleeding after administering vitamin K.1,2 This condition is divided into three categories, depending on the age of the newborn infant when it develops1,3:
- 1.
Early HDN: occurs within 24h of birth, and cannot be prevented by postnatal vitamin K prophylaxis.
- 2.
Classic HDN: bleeding occurs during the infant's first week. Common bleeding sites are gastrointestinal, cutaneous, nasal and following a circumcision. This is the most common form.
- 3.
Late HDN: occurring from week two to the first three months. The most common bleeding sites are intracranial, being associated with a higher rate of mortality and disability, and are followed by cutaneous and gastrointestinal bleeding.
The estimated incidence of classic HDN in Europe, when prophylaxis is not used, is 5 or 6 newborns per 100000 live births,3,4 while late HDN is between 5 and 7 cases per 100000 live births.1,5 Despite its low incidence, severity and mortality of affected cases are high. This incidence is higher in developing countries, where the warm climate and exclusive maternal nutrition are risk factors, given the low vitamin K content. In countries like Mexico, late HDN has presented a mortality of between 11% and 14%, and severe after effects in more than 40% of cases.6 As such, this disease is an important cause of morbidity and mortality in newborns, especially late HDN, where the risk of intracranial haemorrhage reaches 60%.7
Although vitamin K is firmly established as an effective prophylaxis for HDN by observational studies, clinical trials and meta-analysis (8), the way it should be administrated is still under debate. Since the American Academy of Pediatrics systematically recommended intramuscular vitamin K in 1961, different prevention policies have been developed, but which in many cases depend on the doctor's judgement.3,8,9 Even so, at present no consensus has been made on a standard procedure concerning the pharmacological product that should be used, the route of administration or the dosage (quantity, number and frequency of doses). One of the reasons for such heterogeneity concerning prophylaxis for HDN is because there are several forms of vitamin K3,10,11:
- 1.
Natural or liposoluble forms: vitamin K2 or menaquinone which is synthesised by the intestinal flora and vitamin K1 or phylloquinone, naturally present in food.
- 2.
Water-soluble or synthetic forms:
Menadione (vitamin K3), the synthetic form of menaquinone was the first to be used in the 1950s. Six years later, it was related to haemolysis and jaundice in newborns as it was used in high doses. It was therefore withdrawn, although it is still used in some third world countries. Marketed in vials for intramuscular use.
Phytomenadione, the synthetic form of phylloquinone, and currently present in two types of marketed preparations: one dissolved in cremophor EL, widely used in Europe and North America and marketed in vials for intramuscular use. And another presented as a micellar mixture with greater oral absorption. It is available in vials that can be administered orally and intramuscularly.
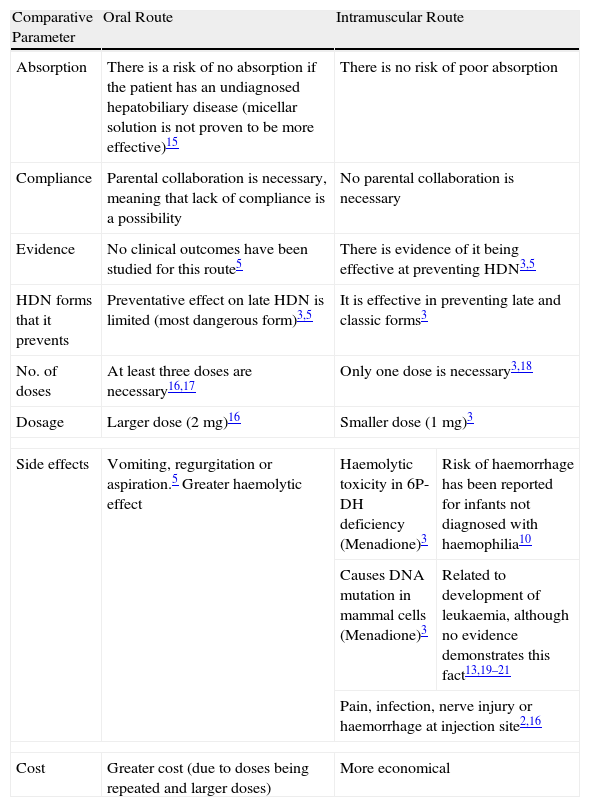
When intramuscular prophylactic vitamin K had shown to be effective at preventing classic HDN,3,5,12 1mg of intramuscular vitamin K was recommended at birth in most western countries. Later publications suggested that prophylactic vitamin K could be associated with the risk of developing childhood cancer.13 As such, some countries opted for oral prophylaxis regimens with repeated doses. However, the lack of consistent scientific evidence on the efficacy5 of oral vitamin K, and given that the incidence of HDN secondary to using this route of administration increased,5,6,14 another change was made to the regimens established, meaning that intramuscular vitamin K was recommended once more. At present, the route of administration remains a matter of controversy, holding with it a significant amount of uncertainty as to which prophylaxis is the most adequate for this disease. Table 1 shows the advantages and disadvantages of the two routes of administration.
Characteristics Associated With Route of Administration.
| Comparative Parameter | Oral Route | Intramuscular Route | |
| Absorption | There is a risk of no absorption if the patient has an undiagnosed hepatobiliary disease (micellar solution is not proven to be more effective)15 | There is no risk of poor absorption | |
| Compliance | Parental collaboration is necessary, meaning that lack of compliance is a possibility | No parental collaboration is necessary | |
| Evidence | No clinical outcomes have been studied for this route5 | There is evidence of it being effective at preventing HDN3,5 | |
| HDN forms that it prevents | Preventative effect on late HDN is limited (most dangerous form)3,5 | It is effective in preventing late and classic forms3 | |
| No. of doses | At least three doses are necessary16,17 | Only one dose is necessary3,18 | |
| Dosage | Larger dose (2mg)16 | Smaller dose (1mg)3 | |
| Side effects | Vomiting, regurgitation or aspiration.5 Greater haemolytic effect | Haemolytic toxicity in 6P-DH deficiency (Menadione)3 | Risk of haemorrhage has been reported for infants not diagnosed with haemophilia10 |
| Causes DNA mutation in mammal cells (Menadione)3 | Related to development of leukaemia, although no evidence demonstrates this fact13,19–21 | ||
| Pain, infection, nerve injury or haemorrhage at injection site2,16 | |||
| Cost | Greater cost (due to doses being repeated and larger doses) | More economical | |
Given the magnitude of the issue and the lack of consensus, this paper aims to collect updated scientific evidence to determine the efficacy and effectiveness of both oral and intramuscular vitamin K for preventing HDN.
MethodsWe performed a systematic review of the scientific literature to find the main studies on using vitamin K to prevent HDN. To do so we used the following sources: Medline, Embase, Cochrane Library, Tripdatabase, Centre for Reviews and Dissemination, E-Guidelines, clinicaltrials.gov, Current Controlled Trials, Nice, Guiasalud, Scottish Intercollegiate Guidelines Network (SIGN), Health Technology Assessment International (HTAI), Clinical evidence, Galenicom, Scopus, Web of Science, and Current context until 30 April 2008. The search had no date or language restrictions; however, we narrowed the search to systematic reviews, clinical trials and meta-analyses. We also consulted websites from the Asociación Española de Pediatria: http://www.aeped.es/ and the American Academy of Pediatrics: http://www.aap.org/.
Search strategies used on Medline, Embase, Tripdatabase, Cochrane, HTA database, Database of Abstracts of Reviews of Effects (DARE), National Health Service Economic Evaluation Database (NHS EED), among others, are shown in Annex 1. In the other databases, we performed free-text searches, with more open options. We also performed manual searches from the articles included in the bibliography list.
We included all articles that evaluated the efficacy of the different doses and routes of administration of prophylactic vitamin K for HDN in healthy infants born at term. We excluded any articles that studied the use of vitamin K during the pregnancy, in preterm infants or patients with any type of illness. We critically reviewed the articles and performed a qualitative synthesis with the main characteristics of each of the articles, therefore assessing the design and methodology used for each of the studies.
Given that results are measured in so many different ways in the literature, we decided to classify them considering the comparison method used and the result type. We created three groups for comparison methods, as shown in Annex 2:
Primary results: those that measured clinical outcomes such as spontaneous bleeding or bleeding following a circumcision.
Other results (OR): referring to biochemical parameters such a vitamin K1 plasma levels, the presence of Protein induced by Vitamin K Absence (PIVKA-II) levels, activity of coagulation factors II, VII and X, prothrombin time (PT), prothrombin index (PI), activated partial thromboplastin time (APTT), prothrombin antigen, factor II-X and factor II/X-II coefficient.
The quality of the articles was assessed independently by two reviewers using the tool Spanish Critical Appraisal Skills Programme (CASPe)22 for systematic reviews and clinical trials. The two first series of questions were used, each having a maximum of three points. The results are presented in Table 2, showing the points obtained by both series of questions (“screening” and “detailed”).
CASPe Assessment for Clinical Trials.
| Reference | CASPe (Maximum 3/3a) | Observations/Blindness |
| Jafarzadeh et al.27 | 3/2 | No clinician or researcher blindness |
| Chawla et al.28 | 3/3 | Patient and clinician blindness, but nothing to indicate that researchers were blinded |
| Clarke et al.29 | 3/2 | Blindness not indicated |
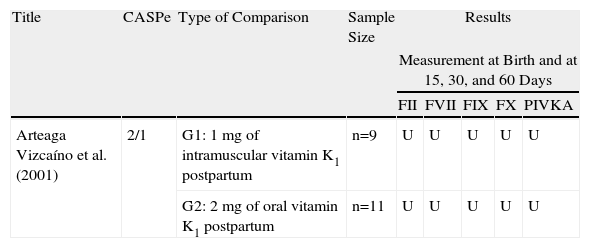
| Arteaga Vizcaíno et al.26 | 2/1 | Blindness not clear, no randomisation, no comparison between groups, which are not homogeneous |
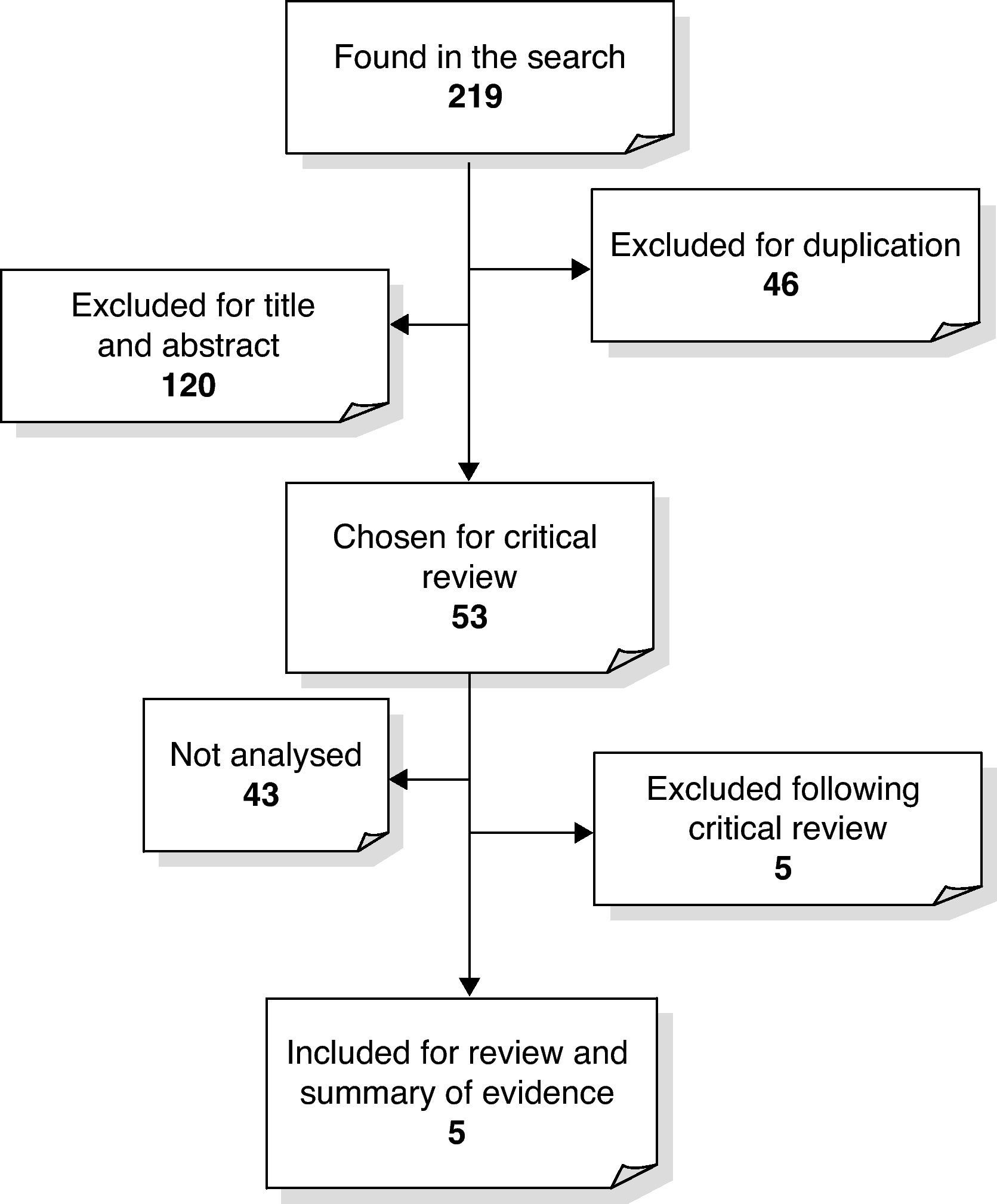
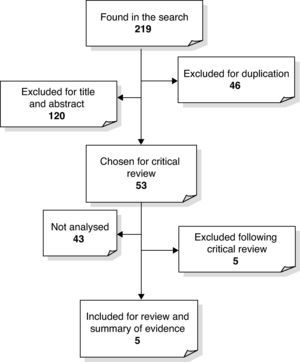
We obtained a total of 218 references corresponding to clinical trials, systematic and narrative reviews, complete reports, original articles and recommendations which assessed the role of prophylactic vitamin K for HDN. As is shown in Fig. 1, 46 of the references were duplicated and 120 were excluded because the title and abstract did not include any information related to the subject matter that we were interested in. Then, given that a Cochrane Review had been updated in 2000 and a HTA report in 1998, of the 53 remaining articles chosen for critical review, we only included clinical trials, reports from assessment agencies and systematic reviews published after 2000 which did not appear in any of the previously mentioned studies. We only included the abstract of the technology assessment report, which concluded that there was enough evidence to support the use of vitamin K in HDN prevention,23 because it was published before the Cochrane Review. Two studies24,25 were excluded because they did not discuss the objective that we were interested in. We were unable to recover the original and complete format of two clinical trials and a technology assessment report, meaning that we eventually had five articles to analyse: four clinical trials and one systematic review.
After having applied the CASPe quality assessment scale, we observed that the selected articles had a good methodological quality, except for one,26 which obtained lower results. The treatment groups had been randomly selected for three of the studies, and the allocation method was not explained for one of them. We are not certain as to what blindness was used for two of the trials, and there were no significant follow-up losses for any of them. We found that the systematic review was high quality. The quality assessment results are shown in Tables 2 and 3.
CASPe Assessment for Reviews.
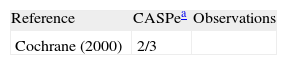
| Reference | CASPea | Observations |
| Cochrane (2000) | 2/3 |
Primary results: we found two studies (Sutherland1 and Vietti1) from the Cochrane systematic review, whose results showed a significant difference in favour of the use of prophylactic vitamin K for HDN. They assessed clinical aspects such as bleeding during the first week of life, or bleeding following a circumcision during the first three days of life with relative risks (RR) of 0.73 (CI 95%: 0.56–0.96) and 0.18 (CI 95%: 0.08–0.42), respectively.
Other results: later studies used specific biochemical markers to assess efficacy. Therefore, the presence of PIVKA-1126 was used (obtaining a significant reduction between days 15, 30, and 60, between both study groups (P<.001), as well as prothrombin time),27 with an average in the intramuscular group being 16.77±4s compared with 16.39±2.98s in the oral group. We have yet to find more studies that analyse this comparison.
Comparison 2: Oral Versus Placebo or No TreatmentPrimary results: we did not find any study that evaluated the clinical aspects.
Other results: five trials studied the efficacy measuring biochemical parameters. Three of them determined the presence of PIVKA-II during the first three days of life,30–32 meaning that as a collective they resulted in being favourable for vitamin K-treated group (RR: 0.40 [0.26–0.61]). The study conducted by Ulusahin in 199631 monitored infants during the first month, and did not show any significant differences between the two groups. Another trial33 analysed the factor X-II, not showing any significant differences. However, it also determined the factor II/ factor X coefficient, and the prothrombin time, which on this occasion provided a favourable result when using vitamin K orally (difference between means: 0.37 [0.06–0.68]). A study that evaluated the prothrombin index34 also showed results in favour of the use of vitamin K orally. We have yet to find more studies that analyse this comparison.
Efficacy of Intramuscular Versus Oral HDN ProphylaxisComparison 3: Oral Dose Versus Intramuscular DosePrimary results: none of the trials specifically assessed the onset of clinical symptoms for either of the two cases.
Other results: no significant differences were found between the two routes of administration, when comparing the presence of PIVKA-II,30,31,35,36 PIVKA-II protein levels, the prothrombin antigen or combined activity of coagulation factors I, VII, and X37 as a variable of results. Trials with markers such as the prothrombin index during the first week,34 prothrombin time during the first week27,35 and vitamin K1 in plasma during the first week38 did not show any significant differences either. However, a clinical trial that studied the vitamin K1 plasma levels at two weeks36 did show a significant difference, finding higher plasma levels in the intramuscular group (difference between means: −0.79ng/ml [−1.02−{−0.56}]). The results from this study combined with those obtained by Maurage,37 both lasting one month, showed a significant difference between the vitamin K1 plasma levels for the intramuscular group (difference between means: −0.23ng/ml [−0.30−{−0.16}]). A more recent study26 showed that there was a lack of significant differences between the two administration methods when measuring the prothrombin time during the first 6h of life.
Comparison 4: Three Oral Doses Versus One Intramuscular DosePrimary results: none of the studies measured clinical results.
Other results: there was a significant statistical difference between the vitamin K in plasma at two weeks and at three months39 in favour of oral administration (difference between means: 0.80ng/ml [0.34–1.27]; 0.30ng/ml [0.10–0.50], respectively). However, after one month, this measurement did not show any significant differences as occurred when the international normalised ratio (INR) was used as an indicator of treatment efficacy.39 Later studies coincide with this last finding: they determined the presence of PIVKA-II and the combined activity of coagulation factors II, VII and X during the first two months of life26 and the prothrombin time from the third to the twentieth day of life.27 The clinical trials after 2000, their main results, and the CASPe in accordance with order of publication are detailed in Tables 4 and 5.
Clinical Trial Outcomes (I).
| Title | CASPe | Type of Comparison | Sample Size | Results | ||||
| Measurement at Birth and at 15, 30, and 60 Days | ||||||||
| FII | FVII | FIX | FX | PIVKA | ||||
| Arteaga Vizcaíno et al. (2001) | 2/1 | G1: 1mg of intramuscular vitamin K1 postpartum | n=9 | U | U | U | U | U |
| G2: 2mg of oral vitamin K1 postpartum | n=11 | U | U | U | U | U | ||
U: unavailable. Only changes to certain levels for each parameter represented by graphs and accompanied by their respective P values.
Clinical Trial Outcomes (II).
| Title | CASPe | Type of Comparison | Sample Size | Results | |
| Measurement at 12h of Life | |||||
| PT (P=.38) | APTT (P=.69) | ||||
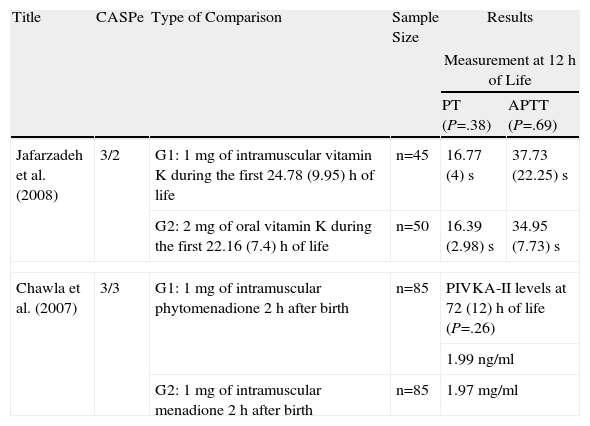
| Jafarzadeh et al. (2008) | 3/2 | G1: 1mg of intramuscular vitamin K during the first 24.78 (9.95)h of life | n=45 | 16.77 (4)s | 37.73 (22.25)s |
| G2: 2mg of oral vitamin K during the first 22.16 (7.4)h of life | n=50 | 16.39 (2.98)s | 34.95 (7.73)s | ||
| Chawla et al. (2007) | 3/3 | G1: 1mg of intramuscular phytomenadione 2h after birth | n=85 | PIVKA-II levels at 72 (12)h of life (P=.26) | |
| 1.99ng/ml | |||||
| G2: 1mg of intramuscular menadione 2h after birth | n=85 | 1.97mg/ml | |||
Following the review, we are able to distinguish two types of clinical trials: those based on clinical outcomes and those that assessed biochemical outcomes. Annex 2 shows the results from the clinical trials and the systematic review assessed in accordance with the comparison type and results’ indicators. Only the trials based on clinical outcomes proved that intramuscular vitamin K was effective for classic HDN prophylaxis, and we did not find any similar trial that assessed oral administration. The actual relationship that could exist between the various biochemical parameters used to measure the results of the different trials and the newborn's coagulation status, or even its clinical condition is still unknown. As such, given the trials’ results based on these parameters, their clinical relevance cannot be deduced. There are only two studies that assess clinical outcomes. One probable cause for the scarcity of this type of results’ variables in trials is the low incidence of HDN, which would therefore mean that an excessively large sample would be needed to obtain statistically significant results. Meanwhile, prolonged monitoring is needed to be able to detect late HDN, which is difficult to achieve.
To date, various HDN prophylaxis programmes have been developed globally. In an attempt to reduce the target population, it has been suggested that a group of milk-fed infants with risk factors should be selected, for example, newborn breast-fed infants. However, this measure is not feasible, given that there are no criteria determining high risk and that new cases of HDN are developing following the application of this practice.
Different types of vitamin K (K1, K2, K3, and K1 included in a mixed micellar solution) have been used in the studies assessed. However, they have not been included in the classification given that it would be too complicated to then try and interpret them. However, we have not found any studies that show evidence on the type of vitamin and its efficacy to prevent HDN. Phytomenadione is the preparation used in most countries (both orally and intramuscularly), although other countries such as Japan and India use other alternatives.
No randomised clinical trial has studied the efficacy of oral or intramuscular vitamin K for late HDN. The review consulted1 recommends using intramuscular vitamin K versus the oral form to prevent practically all cases of HDN. Undiagnosed hepatobiliary disease in the newborn is one of the main reasons that oral prophylaxis fails for late HDN. It was initially considered that the K1 form in the micellar mixtures was of particular importance, given its ability to be better absorbed, although it has not been demonstrated. Recent studies did not show any significant results by comparing this vitamin form with prophylactic regimens consisting of three classic oral vitamin K doses.29
A single oral dose of vitamin K at birth seems to be less effective than an intramuscular dose. As such, some publications support prophylactic regimens consisting of repeated oral vitamin K doses. Regularity of the doses is a matter of debate, as there are various possibilities, from weekly to daily regimens. However, there is no evidence concerning which is more effective.
Given the lack of evidence for several aspects concerning prophylactic vitamin K for HDN, certain factors, in addition to efficacy, must be taken into consideration when deciding which prophylaxis method should be used:
Considering if parents, carers or healthcare professionals need to participate in the continued treatment regimens, and whether exhaustive monitoring is necessary.
Intramuscular administration is invasive and could be therefore rejected by the newborn's family.
Oral administration needs more active ingredients (double that of intramuscular) and has to be administered more than once, which should be taken into account when considering costs. Furthermore, there is currently no oral vitamin K form marketed, as it is usually available in parenteral form and there are no others at a lower cost.
Newborn diet (natural or processed milk), as well as ethical and geographical differences could affect vitamin K metabolism.
This review has some limitations, such as a possible publication bias, given that not all studies conducted reach publication or even become indexed in the databases from which we extracted the studies. Furthermore, having used a systematic revision (i.e. it included all the information on the study subject until it was conducted) could have partly biased the studies that analysed the use of prophylactic vitamin K for HDN. Furthermore, there are very few recent studies analysing this subject and only 20% of the bibliography references had been published in the last 5 years, which emphasises that the information available has not been updated.
To conclude, there is enough evidence supporting that a single dose of intramuscular vitamin K is sufficient to prevent classic HDN. The biochemical indices of the coagulation status during the first week of life has improved with regards laboratory results for oral and intramuscular administrations, without there being significant differences between them. However, there are no studies that analyse the vitamin K's prophylactic role for late HDN. Furthermore, when comparing a single intramuscular vitamin K dose with a single oral dose, it was observed that after two weeks, the intramuscular group's vitamin K1 plasma levels increased, which cannot be related to the infant's coagulation status due to a lack of scientific evidence. When comparing a single intramuscular vitamin K dose with three oral vitamin K doses, the vitamin K plasma levels at two weeks and at two months following administration are higher in the oral group. However, there is no evidence that this translates to differences in the newborn's coagulation status.
Conflict of InterestThe authors affirm that they have no conflicts of interest.
Please cite this article as: Martín-López JE, et al. La vitamina K como profilaxis para la enfermedad hemorrágica del recién nacido. Farm Hosp. 2011;35(3):148–155.