To develop a list of look-alike drug names with tall man letters, that will facilitate and standardize the implementation of this technique in safety practices designed to reduce errors caused by look-alike names.
Material and methodsTwo structured surveys were carried out. The first survey included 46 pairs, groups, or individual look-alike drug names with tall man letters from the lists established by the FDA, ISMP and CAPCA/ISMP-Canada, and 32 selected from ISMP-Spain and the COF Council database. The second survey included 27 proposals made by those respondents who completed the first survey and 11 from the ISMP updated list. Participants were asked about the usefulness and current implementation of the technique. Ninety pharmacists from different hospitals participated in the first survey and 89 in the second.
ResultsThe list of look-alike drug names with tall man letters which has been developed includes 107 names structured into 44 pairs or groups. Of the respondents, 93.3% felt that this technique should be implemented for identifying medications, not only on pharmaceutical industry labels (91.1%) but also in other places where drug names appear, including computerized prescription screens (90%), pharmacy system screens (82.2%), automated dispensing cabinet screens (81.1%), labels for pharmacy preparations and shelves, etc. Only 9 hospitals (10%) were using this technique.
ConclusionsThe availability of this list of look-alike drug names for which tall man lettering is recommended may encourage the use of this technique for differentiating names in Spain where it is currently not greatly used.
Elaborar una relación de nombres de medicamentos similares con letras mayúsculas resaltadas, que facilite y estandarice la implantación de esta técnica en prácticas dirigidas a reducir errores por similitud de nombres.
Material y métodosSe realizaron dos encuestas estructuradas. La primera incluyó 46 pares, grupos o nombres de medicamentos similares con letras mayúsculas, procedentes de las listas establecidas por la FDA, ISMP y CAPCA/ISMP-Canadá, y 32 seleccionados de la base de datos del ISMP-España y Consejo de COF. La segunda incluyó 27 pares, grupos o nombres propuestos por los encuestados y 11 procedentes de la actualización del ISMP. Se formularon preguntas sobre la utilidad de la técnica y su implantación en los hospitales. Participaron en la primera encuesta 90 farmacéuticos de diferentes hospitales y 89 en la segunda.
ResultadosLa relación de nombres de medicamentos similares con letras mayúsculas resaltadas elaborada recoge 107 nombres agrupados en 44 pares o grupos. Un 93,3% de los encuestados opinó que esta técnica debería implantarse para denominar a los medicamentos, tanto en el etiquetado de la industria farmacéutica (91,1%) como en otros lugares donde aparecen los nombres, como en las pantallas de prescripción informatizada (90%), de farmacia (82,2%) o de los sistemas automatizados de dispensación (81,1%), en etiquetado de preparaciones y estantes, etc. Solo 9 (10%) de los hospitales utilizaban esta técnica.
ConclusionesLa disponibilidad de esta relación de nombres similares en los que se recomienda utilizar letras mayúsculas resaltadas podría facilitar su aplicación en prácticas de diferenciación de nombres, actualmente reducida en nuestro país.
Confusing the names of medications is a frequent cause of medication errors.1,2 These errors basically occur because of similarities between brand names (e.g. Sumial® and Luminal®) or generic names (e.g. dopamine and dobutamine), although they may also occur because of confusing brand names with generic names (e.g. Rohipnol® and ropinirole). Considering the very large number of medications on the market, it is not surprising that there are drugs with similar names. In Spain, the Institute for Safe Medication Practices (ISMP-Spain) works with the General Council of Official Associations of Pharmacists (COF) to maintain a database of names of medications that are likely to cause confusion, and this database currently contains more than 350 pairs of names.3,4 The United States and other countries, as well, publish their own lists of medications with similar names.5
Numerous organisms and institutions have published recommendations to help prevent medication errors from this cause.6–9 These recommendations focus mainly on two lines of action: establishing measures to avoid registration of new names that are similar to already existing names (pre-marketing strategies), and implementing specific practices to minimize errors caused by confusion among already existing names (post-marketing strategies). Among the latter, one technique that has been proposed for reducing this type of error consists of changing the appearance of the look-alike names of medications, in places where they are visually noticeable (computer screens, labels, etc.), emphasizing the differences by using capital (“Tall Man”) letters and/or other typographical elements in the parts of the words where the spellings differ.1 Some studies have shown that this technique may be effective for reducing dispensing errors when used on the labels of the medications,10 and for minimizing selection errors for names of medications in computerized prescription and dispensing systems.11,12
The use of tall man letters for preventing medication errors due to look-alike names has been encouraged by different organizations.8,13–16 It should be noted that the Institute for Safe Medication Practices (ISMP) and other experts11,17 recommend establishing a standardized reference list for using the technique and restricting the list to a limited number of drug names, in order to avoid diluting its effectiveness, which might happen if it were applied too broadly and in too many different ways.
In the USA, the FDA Office of Generics Drugs implemented this technique in 2001.13 Manufacturers of 34 generic medications were asked to voluntarily review the label design for these drugs and to use capital letters or also color to visually differentiate the names. The ISMP created an additional list that added 42 more pairs of medication names to the FDA list.17 New names have since been added,18 and healthcare institutions and practitioners have been encouraged to use this technique. Recently, ISMP-Canada carried out a project in conjunction with the Canadian Association of Provincial Cancer Agencies (CAPCA) to establish a list of oncology look-alike medication names for tall man letters.19
In our country we do not have a standardized reference list for use by practitioners and institutions. Lists from other countries do not line up perfectly with our healthcare situation, mainly because of differences in brand names and in our medication use profile. The main objective of this study has been to create a list of medications with look-alike names with tall man lettering that would facilitate the implementation of this technique in practices designed to reduce errors due to confusion among names and that would include the names that are deemed to present the greatest risk in our country. In addition, the study looked at opinions about the usefulness of this technique and its current implementation in hospitals.
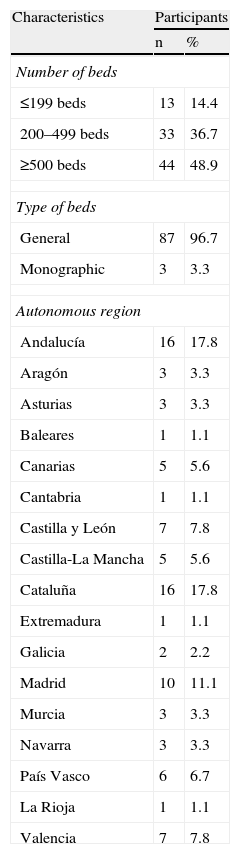
Material and methodsThe study was coordinated by ISMP-Spain which put together a group of experts on medication safety and management from 13 Spanish hospitals. It was based on the completion of two structured surveys by a representative sample of pharmacists from all autonomous regions and all sizes of hospital. Each member of the group of experts took charge of contacting and tutoring several participants from other hospitals, in order to create cascade dissemination for the study. Table 1 shows the distribution of the hospitals where the 90 pharmacists who completed the surveys worked by number of beds and by autonomous region.
Characteristics of the Hospitals Where the Study Participants Work (n=90).
| Characteristics | Participants | |
| n | % | |
| Number of beds | ||
| ≤199 beds | 13 | 14.4 |
| 200–499 beds | 33 | 36.7 |
| ≥500 beds | 44 | 48.9 |
| Type of beds | ||
| General | 87 | 96.7 |
| Monographic | 3 | 3.3 |
| Autonomous region | ||
| Andalucía | 16 | 17.8 |
| Aragón | 3 | 3.3 |
| Asturias | 3 | 3.3 |
| Baleares | 1 | 1.1 |
| Canarias | 5 | 5.6 |
| Cantabria | 1 | 1.1 |
| Castilla y León | 7 | 7.8 |
| Castilla-La Mancha | 5 | 5.6 |
| Cataluña | 16 | 17.8 |
| Extremadura | 1 | 1.1 |
| Galicia | 2 | 2.2 |
| Madrid | 10 | 11.1 |
| Murcia | 3 | 3.3 |
| Navarra | 3 | 3.3 |
| País Vasco | 6 | 6.7 |
| La Rioja | 1 | 1.1 |
| Valencia | 7 | 7.8 |
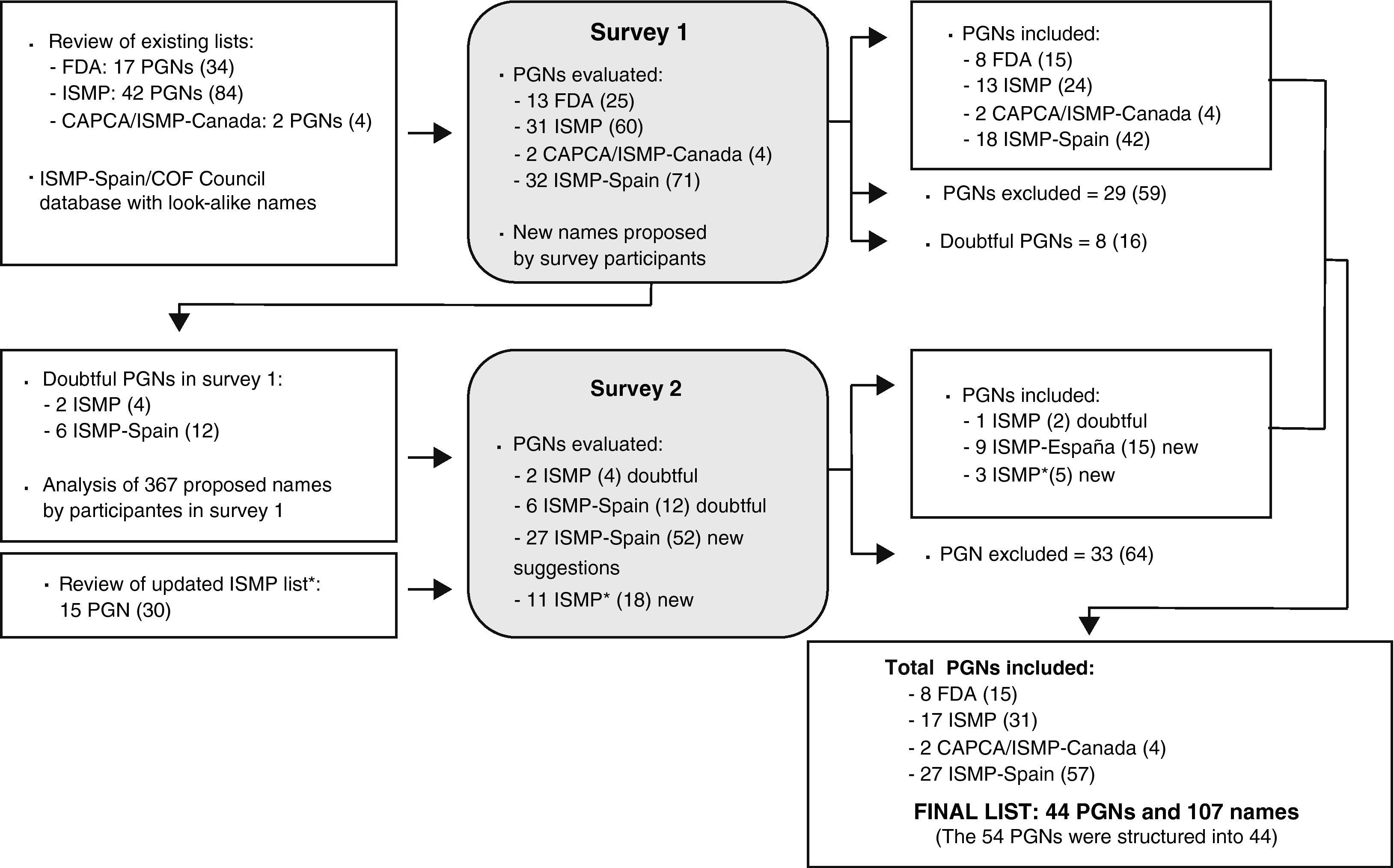
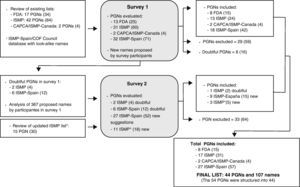
The first survey was created after reviewing the list of medication names with tall man letters available in the bibliography, and after analyzing the database of look-alike medication names maintained by ISMP-Spain and the Council of COF, which is elaborated based on medication error reports received that were due to confusion among names. Fig. 1 diagrams the process for selecting the names and developing the list of names with tall man letters. In order to facilitate understanding, the acronym PGN has been used to indicate the pairs of look-alike names with tall man letters (e.g. DOBUTamine-DOPamine), groups of names (e.g. cefAZOLin-cefTRIAXone-cefTAZIdime) or even individual names evaluated with respect to other pairs or groups (e.g. cefOXitin−compared to other cephalosporin).
Procedure followed for developing the list of look-alike drug names with tall man letters. The number of individual drug names is included in parenthesis in bold letters. PGN: pairs, groups, or individual drug names. FDA: United States Food and Drug Administration.13 ISMP: Institute for Safe Medication Practices.17 ISMP*: Institute for Safe Medication Practices.18 CAPCA/ISMP-Canada: Canadian Association of Provincial Cancer Agencies/Institute for Safe Medication Practices-Canada.19
Four blocks of PGNs were used for this first survey:
- –
13 PGNs from the FDA-approved list of 34 generic names (17 PGNs) established in 2001.13 Nine names were eliminated because they are not marketed in Spain.
- –
31 PGNs from the ISMP list with additional 42 PGNs.17 Of the on that list, 11 names were eliminated because they are not marketed in Spain. A double option was offered for one PGN.
- –
2 PGNs from the list of generic names for oncology medications created by ISMP-Canada and CAPCA.19 All names already included on the lists above were eliminated.
- –
A list of 32 PGNs of pairs or groups of look-alike names not included on the lists above and which had caused errors in Spain. As previously stated, look-alike names were selected from the database of the ISMP-Spain and the Council of COF.3,4
For the new names the MID rule11 was used whenever possible to select the letters or sections of names to capitalize. In the drug names from previous lists we kept the same capital letters, except in certain cases involving new names added to a pair or group already included.
The survey respondents were asked to evaluate each PGN using a Likert scale of 5 categories for their answers, scoring from 1 to 5, corresponding to these opinions respectively: strongly disagree, disagree, indifferent, agree, strongly agree.
The following sections were added to the first survey:
- –
An open question allowing survey respondents to propose new look-alike medication names for tall man letters. They could specify whether the suggestion for inclusion was based on a particular medication error that had already occurred or only on a personal opinion.
- –
A question about the types of drug names (generic, brand, or both) that were used in the participants’ hospitals for identifying medications in different processes of the medication use system.
- –
Four questions designed to gather information about the opinions of the practitioners taking the survey as to the usefulness of the tall man lettering technique and the practices in which the technique was likely thought to be most effective.
- –
Two questions regarding the current degree of implantation of the tall man letters technique in Spanish hospitals.
The first survey was sent out, along with the objective and methodology for the project, in September, 2010, and a time limit of one and a half months was set up of for dissemination and completion. Afterwards, survey answers were analyzed and the second survey was prepared.
The second survey was sent out in February, 2011 and was answered by 89 of the 90 former participants. It included the following blocks of PGNs for evaluation:
- –
8 PGNs that were considered doubtful after analyzing the results of the first survey.
- –
27 PGNs that included 52 new names from the 367 proposed by participants in the first round. Considering the recommendation of the ISMP and other experts to limit the number of names in order to avoid loss of effectiveness of the technique, these PGNs were evaluated and only the ones presenting the greatest risk of causing serious incidents in case of confusion were selected. In making the choices, the probability of confusion was evaluated in function of several risk factors (strength, route of administration, dosage form, frequency of administration, indications, storage conditions, patient and prescriber population) and the severity of the consequences of errors should they occur.4,20
- –
11 PGNs from the list of look-alike drug names with tall man letters updated by the American ISMP in November, 2010.18 Of the 30 new names added to that list, 12 were eliminated because they are not marketed in Spain or because they were included in the first survey at the prior suggestion of ISMP-Spain (e.g. valACYclovir-valGANCiclovir).
Finally, a question was included to allow survey takers to express their opinions as to the typographical resources that they considered most appropriate with tall man letters in the names when applying the technique to computer application screens, or on labels, documents, and other uses where a broader set of resources is available.
Statistical AnalysisA statistical analysis was carried out on both surveys and consisted of calculating the principal measures of central tendency, dispersion, and position. In this way a complete vision of the set of points given by the respondents for each PNG was obtained, as well as the percentage of answers with low scores (given a 1 or a 2), medium scores (given a 3) or high scores (given a 4 or a 5) on the Likert scale that was used. The criteria, applied to establish agreement by respondents in the selection of a PGN, were to have an average score≥4 points and a percentile 25≥4, since this indicated that only 25% of the survey takers had no opinion or disagreed with including the corresponding PGN. A result was considered doubtful and, therefore, consensus could not be affirmed, when the average was ≥4 and the percentile 25 of 3.
Data analysis was carried out using the IBM SPSS Statistics 19 program.
ResultsTable 2 shows the list of look-alike drug names with tall man letters developed with the PGNs that survey respondents agreed upon. Of a total of 116 PGNs evaluated between the two surveys, 54 (46.6%) were included on the final list by consensus. From the first survey, 52.6% of the PGNs were included and 8 (10.3%) were considered doubtful. From the second survey the percentage of PGNs included was smaller (28.3%) with agreement reached on 1 of the 8 doubtful PGNs and on 10 of the 37 newly evaluated PGNs. There were no doubtful PGNs. Some PGNs were regrouped on the final list in order to facilitate application, given that the surveys had been structured according to the source and some of these PGNs were drugs belonging to the same therapeutic groups. Thus, the final list contains a total of 107 drug names grouped into 44 PGNs, instead of 54. Of these 44 PGNs, 28 (63.6%) contain generic names (9 of which contain one or more cytostatic drug), 15 brand names, and 1 contains a generic name and a brand name.
List of Look-alike Drug Names With Recommended Tall Man Letters.
| Drug name pairs or groups with tall man letters | Source/s |
| ADRENalina-ATROPina | ISMP-Spain |
| ADVAgraf-PROgraf | ISMP-Spain |
| ARICept-AZILect | ISMP-Spain |
| azaCITIDina-azaTIOprina | ISMP |
| BUpivacaína-MEpivacaína-ROpivacaína | ISMP-Spain |
| cefAZOLina-cefTRIAXona-cefTAZIdima-cefOXitina/cefOTAXima | ISMP/ISMP-Spain |
| CISplatino-CARBOplatino | ISMP |
| cloTIApina-cloZApina | ISMP-Spain |
| cloTRIMazol-cloMETIazol | ISMP-Spain |
| DACTINomicina-DAPTOmicina | ISMP |
| DASAtinib-LAPAtinib/SUNItinib-SORAfenib | ISMP-Spain/CAPCA/ISMP-Canada |
| DAUNOrubicina-DOXOrubicina/IDArubicina/EPIrubicina | FDA/ISMP/ISMP-Spain |
| DOBUTamina-DOPamina | FDA |
| DOCEtaxel-PACLitaxel | CAPCA/ISMP-Canada |
| DOLquine-DEPAkine | ISMP-Spain |
| EbiXA-EviSTA | ISMP-Spain |
| eFEDrina-EPINEFrina/FENILEFrina | ISMP/ISMP-Spain |
| EmCONcor-EnTOcord | ISMP-Spain |
| FLUoxetina-DULoxetina-PARoxetina | ISMP |
| FOSamax-TOPamax-IOPimax | ISMP-Spain |
| gliPIZida-gliBENCLAMida/gliCLAZida-gliMEPIRida | FDA/ISMP-Spain |
| hidrALAZINA-hidrOXIzina | FDA |
| HumaLOG-HumuLINA | ISMP |
| ISOtretinoína-tretinoína | ISMP |
| loRAtadina-loVAStatina | ISMP-Spain |
| metAMIzol-metroNIDAzol | ISMP-Spain |
| mitoXANTRONA/mitoMICINA | FDA/ISMP-Spain |
| MOVIcol-MONurol-MAGNurol | ISMP-Spain |
| niCARdipino-niFEdipino/niMODipino | FDA/ISMP |
| NovoRAPID-NovoMIX | ISMP-Spain |
| OXcarbazepina-carBAMazepina | ISMP |
| predniSONA-prednisoLONA | FDA |
| ProZAC-ProSCAR | ISMP-Spain |
| quiNINA-quiNIDina | ISMP |
| riTUXimab-inFLIXimab | ISMP |
| RoHIPnol-roPINIrol | ISMP-Spain |
| SandIMMUN-SandoSTATIN | ISMP |
| SinOGan-SinEQUan | ISMP-Spain |
| sulfaDIAzina-sulfaSALAzina | ISMP |
| SUmial-LUminal | ISMP-Spain |
| TiAPRIzal-TriLEPtal-TryPTIzol | ISMP-Spain |
| UrSOCHol-UroTRol-UroLOSIN | ISMP-Spain |
| valACiclovir-valGANCiclovir | ISMP-Spain |
| vinBLAStina-vinCRIStina/vinFLUNina-vinORELBina | FDA/ISMP-Spain |
Notes: This list has the international non-proprietary names in Spanish, which correspond to the Spanish Official Name.
Brand names always start with an uppercase letter. Some brand names incorporate tall man letters in initial characters and may not be readily recognized as brand names.
FDA: United States Food and Drug Administration.13 ISMP: Institute for Safe Medication Practices.17,18 CAPCA/ISMP-Canada: Canadian Association of Provincial Cancer Agencies/Institute for Safe Medication Practices-Canada.19
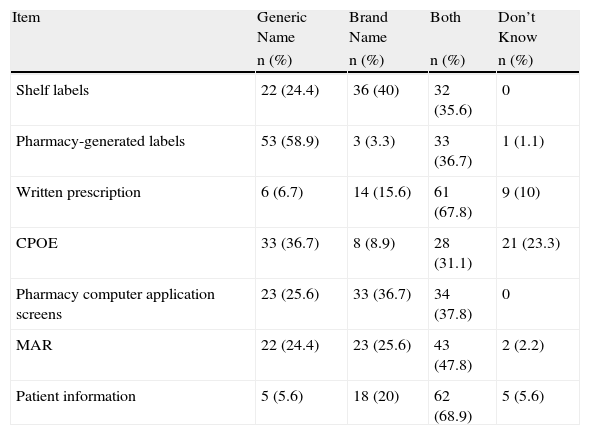
The types of names used in the hospitals in which the survey respondents worked are shown in Table 3. Generic names were most used for labeling pharmacy preparations (58.9%) and in computer prescription order entry (36.7%), while both names were used on manual prescription (67.8%) and on the information given to patients (68.9%). Brand names were most used for storing medications (40%).
Types of Drug Names Used Throughout the Medication Use Process in the Hospitals Where Respondents Work (n=90).
| Item | Generic Name | Brand Name | Both | Don’t Know |
| n (%) | n (%) | n (%) | n (%) | |
| Shelf labels | 22 (24.4) | 36 (40) | 32 (35.6) | 0 |
| Pharmacy-generated labels | 53 (58.9) | 3 (3.3) | 33 (36.7) | 1 (1.1) |
| Written prescription | 6 (6.7) | 14 (15.6) | 61 (67.8) | 9 (10) |
| CPOE | 33 (36.7) | 8 (8.9) | 28 (31.1) | 21 (23.3) |
| Pharmacy computer application screens | 23 (25.6) | 33 (36.7) | 34 (37.8) | 0 |
| MAR | 22 (24.4) | 23 (25.6) | 43 (47.8) | 2 (2.2) |
| Patient information | 5 (5.6) | 18 (20) | 62 (68.9) | 5 (5.6) |
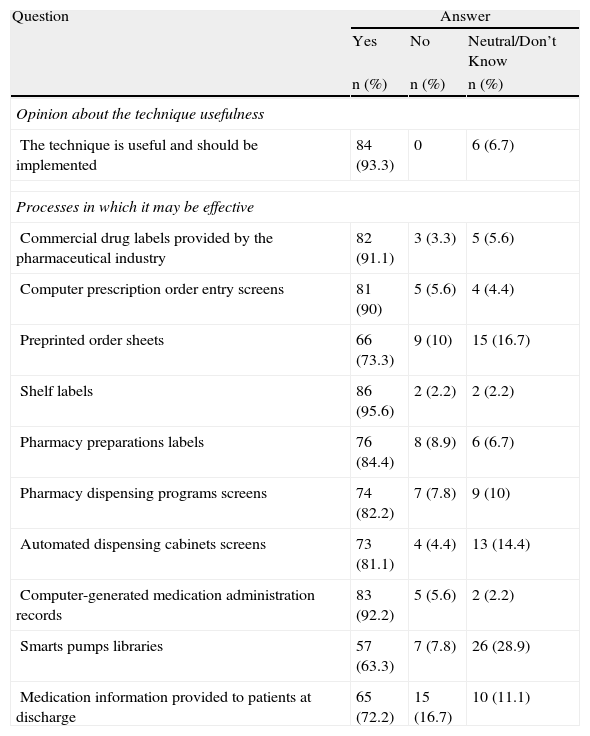
The majority of respondents (93.3%) believe that the use of tall man letters is a useful technique that should be implemented (Table 4). Going along with the type of names used in hospitals, a high percentage (72.2%) of participants stated that this technique should be implemented for generic and brand names, 20% responded that preference should be given to generic names, and only 5.6% felt that it should be used exclusively for generic names.
Respondent Opinion About Tall Man Lettering Usefulness (n=90).
| Question | Answer | ||
| Yes | No | Neutral/Don’t Know | |
| n (%) | n (%) | n (%) | |
| Opinion about the technique usefulness | |||
| The technique is useful and should be implemented | 84 (93.3) | 0 | 6 (6.7) |
| Processes in which it may be effective | |||
| Commercial drug labels provided by the pharmaceutical industry | 82 (91.1) | 3 (3.3) | 5 (5.6) |
| Computer prescription order entry screens | 81 (90) | 5 (5.6) | 4 (4.4) |
| Preprinted order sheets | 66 (73.3) | 9 (10) | 15 (16.7) |
| Shelf labels | 86 (95.6) | 2 (2.2) | 2 (2.2) |
| Pharmacy preparations labels | 76 (84.4) | 8 (8.9) | 6 (6.7) |
| Pharmacy dispensing programs screens | 74 (82.2) | 7 (7.8) | 9 (10) |
| Automated dispensing cabinets screens | 73 (81.1) | 4 (4.4) | 13 (14.4) |
| Computer-generated medication administration records | 83 (92.2) | 5 (5.6) | 2 (2.2) |
| Smarts pumps libraries | 57 (63.3) | 7 (7.8) | 26 (28.9) |
| Medication information provided to patients at discharge | 65 (72.2) | 15 (16.7) | 10 (11.1) |
Most of the professionals consulted (91.1%) thought that this technique could help to minimize errors made in selecting medications if it were used not only on drug labels provided by the pharmaceutical industry, but also in other procedures or elements used in hospitals, including computerized prescription order entry (90%), pharmacy systems (82.2%) and automated dispensing cabinet screens (81.1%), labeling for drawers and shelves (95.6%) and preparations (84.4%), etc. Nineteen survey respondents indicated various procedures for which they thought this technique could be well used, including in the formulary, reports, prescriptions at discharge, etc. Nine participants agreed on the usefulness of the technique for unit dose repackaging.
Only 9 of the 90 participants currently used tall man letters to differentiate among names of look-alike drugs in some practices. Specifically, 4 hospitals used the technique on labels for drawers and shelves, 3 on computer prescription screens, and 2 hospitals on labels for pharmacy preparations, on the screens for the dispensing system, on automated dispensing cabinet screens, and on the computer-generated medication administration records.
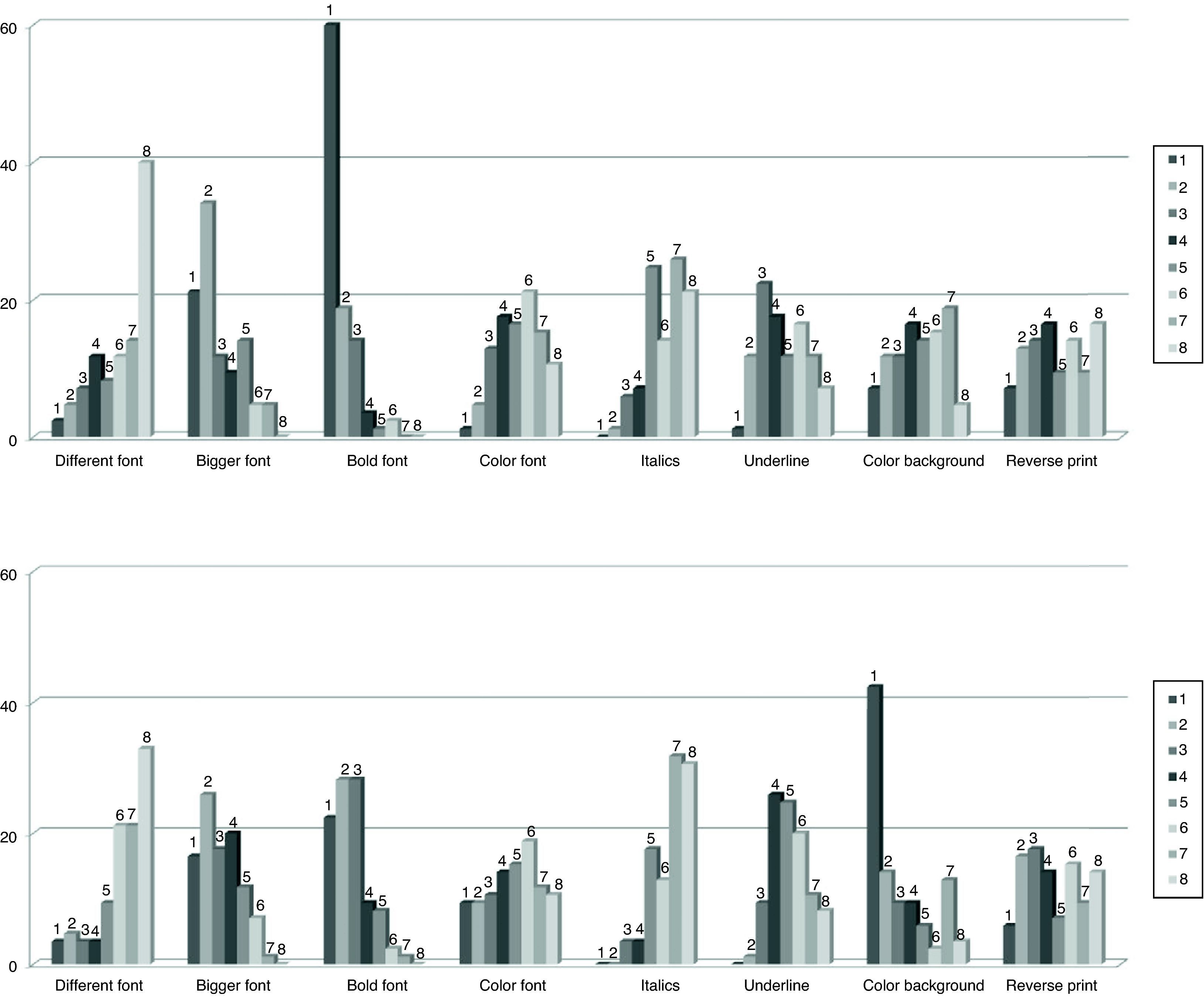
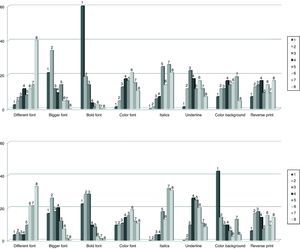
Fig. 2 shows the frequency distribution of opinion of the respondents as to possible typographical resources that could be used to highlight the tall man letters on the look-alike medication names. As far as applications for computerized systems, the resource chosen as first option with greatest frequency was the use of bold lettering (60%), and second was increasing font size (21.2%). In the case of highlighting these letters on the labels of medications or on documents where other resources are possible, especially for the use of color, the majority of participants preferred as a first option, the use of a background color (42.4%), second, use of bold letters (22.4%), bigger font size (16.5%) and different colors (9.4%). Some respondents indicated that the combination of bold letter with larger font size would be the optimum first choice, and second would be the use of background color and larger font size.
Frequency distribution of the opinion of respondents (n=89) on the typographical resources they consider most appropriate for highlighting the distinctive letters in the drug names on computer application screens (above) or on labels and documents (below). Respondents expressed their preferences ordering from 1 to 8 the 8 different options presented.
Medication errors caused by look-alike drug names are a concern for institutions dedicated to patient safety and to healthcare authorities, who have designed safety practices to help institutions and practitioners to reduce such errors. In the year 2007, the degree of implantation of these practices in our country's hospitals was generally low, and especially so for such specific practices as using auxiliary warnings, or putting tall man letters on labels and storage shelves.21
This study has shown that the current use of tall man letters in order to distinguish between look-alike drug names in Spanish hospitals is still at a minimum. This situation is in contrast to that of the USA, where, according to ISMP surveys, the technique is used in more than 50% of hospitals to differentiate names on pharmacy preparation labels and on storage shelves, as well as on the screens of computer pharmacy systems, computer prescription order entry, and on automated dispensing cabinet. The differences between the two countries are likely attributable to the emphasis placed on this technique in the USA by diverse organizations, particularly the Joint Commission, which urged its use back in 2005 when it incorporated the implantation of measures to prevent errors caused by similarity in naming as an objective of the National Patient Safety Goals, and in 2010 was moved to medication standards,14 and to the fact of having available specific lists with look-alike names for which the use of tall man letters is recommended.13,17–19
Lacking a reference list not only makes it more difficult to apply the technique, but may also lead to inconsistent and motley use of it. The lists available in other countries do not really adapt themselves easily to our situation, and so when practitioners at each hospital attempt to differentiate the names that cause errors in their institutions, each one opts for applying tall man letters to different names and/or different letters or parts of the names. For this reason, when this project was conceived, the idea was to create a list starting off from two fundamental points: first, the necessity of promoting an international standard, respecting and keeping all names that were previously established on available lists and which had actually caused errors in our country, and second, adjusting the list making it more appropriate for our situation, incorporating other problematic names, not present on already existing lists, perhaps because they were drugs not marketed in other countries or else marketed under different names. For example, methamizole is a medication that is not on the market in other countries, yet it is widely used in Spain, and has often been confused with metronidazole. Other examples are Sumial®-Luminal® or Prozac®-Proscar®.
The use of generic names using the international non-proprietary name assigned by the World Health Organization is a recommended safety practice6,22 and, in fact, the techniques for differentiating among names focus more on generic names. Therefore, the list we created contained a greater proportion of these names. Nevertheless, since in our current practices both types of names are used, as was shown in the answers provided by our respondents, we felt that both types of names should be included in the surveys and some of them made it onto the final list by consensus. A medication was included not only in its international non-proprietary name (epinephrine) but also in the brand name currently in use (Adrenalina®), maybe because this last name is also used to indicate this pharmaceutical substance in pharmacology books and it is the official name in the European Pharmacopeia and some countries.
The typographical resources that were preferred by the practitioners consulted were similar to those selected in surveys carried out by the American ISMP, although it is difficult to make comparisons, since in this study two options were offered according to where the letters would be applied. In any case, these were simply opinions expressed that have not been supported by studies demonstrating objective evidence of effectiveness. In this sense, in one study on the effectiveness of certain typographical resources for differentiating among look-alike drug names it was observed that, although participants had subjectively stated that the use of color was a more effective resource, this does not correlate with objective findings and the combination of color and tall man lettering did not improve the effect of the tall man letters alone.23
The list of look-alike drug names with tall man letters that has resulted from this study may turn out to be a useful tool that will promote the use of this technique. Still, there will be some limitations. In the first place, there will be limitations derived from the methodology followed in order to create the list, since two surveys were used and the effectiveness of the application in terms of error reduction has not been evaluated. This limitation is analogous to the limitations that affect all the rest of the available lists, since there is no scientific evidence as to whether errors due to confusion between pairs of names included on the lists have been reduced after its application. Also, the respondents were hospital pharmacists and the results would have been enriched by participation from other healthcare practitioners in different working environments. Nevertheless, the working group feels that this list is a first step and that what has been created is an open list that will need updating periodically. Finally, the limitations inherent in the technique must be born in mind, since there are very few studies available on letters or parts of names that should be highlighted and which type of typography would be the most efficient for doing so.
The main contribution of this study is to provide practitioners and institutions who would like to use this technique with an initial reference list. It should be noted that the use of tall man lettering makes it easier to distinguish among names, basically because it calls attention to the names that carry these letters, as revealed by studies that have been carried out.23 For this reason, it is important to inform practitioners appropriately of the objective of the technique at the time of its implantation on safety practices in the institution. The working group hopes that this list will contribute to making practitioners more aware of this problem and that it will encourage the implantation of safety practices designed to minimize errors caused by confusion between names in all the process involved in the medication use system, which will lead to improved patient safety.
Special ThanksThe working group would like to thank Fernando A. Navarro, medical translator, and Trinidad Sánchez Muñoz of the Center for Linguistic Research of the University of Salamanca for their collaboration in translating certain linguistic terms.
Conflict of InterestThe authors have no conflict of interest to declare.
ISMP-España, Hospital Universitario de Salamanca: María José Otero López, Rita Martín Mu¿noz, Ana María Moreno Gómez, Alfonso Domínguez-Gil Hurlé. Unidad de Investiga-ción CAIBER, Hospital Universitario de Salamanca: Mercedes Sánchez Barba. Hospital Universitario Miguel Servet, Zara-goza: Reyes Abad Sazatornil. Hospital Universitari Germans Trias i Pujol, Badalona: Ángels Andreu Crespo. Hospital Universitario, Getafe: Marta Arteta Jiménez. Hospital Uni-versitario Ramón y Cajal, Madrid: Teresa Bermejo Vicedo. Fundación Oncológica, San Sebastián: Gerardo Cajaraville Ordoñana. Hospital Clínic, Barcelona: Carlos Codina Jané. Empresa Pública Hospital Costa del Sol, Marbella: Vicente Faus Felipe. Hospital Universitari i Politècnic La Fe, Valen- cia: Isabel Font Noguera. Hospital General Universitario Gregorio Marañón, Madrid: Ana Herranz Alonso. Hospital Clí-nico Universitario, Valladolid: Ana María López González. Hospital Universitario Fundación Alcorcón, Madrid: Mont-serrat Pérez Encinas. Hospital Universitario Virgen de la Arrixaca, Murcia: Amelia de La Rubia Nieto. Hospital Univer-sitario Virgen del Rocío, Sevilla: María Dolores Santos Rubio.
U. Baños Roldán (Hospital Universitario Virgen Macarena, Sevilla), C. Blanco Bermejo (Hospital Comarcal de la Axarquía, Torre del Mar, Málaga), J. Cabeza Barrera (Hospital Universitario San Cecilio, Granada), S. Corral Baena (Hospital San Juan de Dios del Aljarafe, Bormujos, Sevilla), V. Faus Felipe (Empresa Pública Hospital Costa del Sol, Marbella, Málaga), M.J. Fobelo Lozano (Hospital Universitario de Valme, Sevilla), A. Jiménez Morales (Hospital Virgen de las Nieves, Granada), M.D. López Malo de Molina (Hospital Universitario Reina Sofía, Córdoba), E. Molina Cuadrado (Hospital Torrecárdenas, Almería), A. Moreno Villar (Hospital San Juan de la Cruz, Úbeda, Jaén), V. Padilla Marín (Hospital de Antequera, Málaga), M.A. Rosado Souviron (Complejo Hospitalario Carlos Haya, Málaga), E. Sánchez Gómez (Hospital Juan Ramón Jiménez, Huelva), M.D. Santos Rubio (Hospital Universitario Virgen del Rocío, Sevilla), F. Verdejo Reche (Hospital de Poniente, El Ejido, Almería), A. Villa Rubio (Hospital SAS La Línea de la Concepción, Cádiz).
M.R. Abad Sazatornil (Hospital Universitario Miguel Servet, Zaragoza), M.A. Alcácera López (Hospital Clínico Universitario Lozano Blesa, Zaragoza), M.P. Amador Rodríguez (Hospital San Jorge, Huesca).
M.T. Iglesias García (Hospital Universitario Central de Asturias, Oviedo), A. Lozano Blázquez (Hospital de Cabueñes, Gijón), P. Terroba Alonso (Hospital San Agustín, Avilés).
A. Escrivá Torralva (Hospital Universitario Son Espases, Palma de Mallorca).
P. González Joyanes (Hospital Universitario Materno-Infantil de Canarias, Las Palmas de Gran Canaria), M.A. Ocaña Gómez (Complejo Hospital Universitario Ntra. Sra. de Candelaria, Santa Cruz de Tenerife), A.M. Quintana Holgado (Hospital General de La Palma), I. Rodríguez Pedrosa (Hospital Universitario de Canarias, La Laguna, Tenerife), M.A. Velaz Suárez (Hospital Universitario Insular de Gran Canaria, Las Palmas de Gran Canaria).
J. de la Puente Jiménez (Hospital Universitario Marqués de Valdecilla, Santander).
V.L. Areas del Águila (Hospital General de Ciudad Real), S. González Martínez (Hospital Universitario de Guadalajara), M. Martínez Camacho (Complejo Hospitalario de Toledo -Hospital Geriátrico Virgen del Valle, Toledo), C. Mora Herrera (Hospital Santa Bárbara, Puertollano, Ciudad Real), M. Pascual Martínez (Complejo Hospitalario Universitario de Albacete).
M.M. Andújar Arias (Complejo Asistencial de Ávila), M.I. Azcárate García (Complejo Hospitalario de Soria), T. Caro-Patón Carmona (Hospital Universitario Río Hortega, Valladolid), A.M. López González (Hospital Clínico Universitario de Valladolid), M.J. Otero López (Hospital Universitario de Salamanca), N. Revilla Cuesta (Hospital Santos Reyes, Aranda de Duero, Burgos), M. Rodríguez María (Hospital El Bierzo, Ponferrada, León).
A. Andreu Crespo (Hospital Universitari Germans Trias i Pujol, Badalona, Barcelona), T. Arranz Castella (Consorci Sanitari de L’Alt Penedés, Villafranca del Penedés, Barcelona), T. Boada Port (Clínica Corachán, Barcelona), C. Codina Jané (Hospital Clínic i Provincial de Barcelona), E. Fernández de Gamarra Martínez (Hospital de la Santa Creu i Sant Pau, Barcelona), M.Q. Gorgas Torner (Corporació Sanitària Parc Taulí, Sabadell, Barcelona), P. Jolonch Santasusagna (Hospital Santa Tecla, Tarragona), M. Juan Aguilar (Hospital Verge de la Cinta, Tortosa, Tarragona), J.J. Machí Ribes (Hospital de Viladecans, Barcelona), J.M. Martínez Cutillas (Hospital Universitari Vall d’Hebrón, Barcelona), L. Pastó Cardona (Hospital Universitari de Bellvitge, L’Hospitalet de Llobregat, Barcelona), A. Pérez Plasencia (Hospital Universitari Dr Josep Trueta, Girona), P. Salvador Collado (Hospital Universitari Sant Joan de Reus, Reus, Tarragona), J.A. Schoenenberger Arnaiz (Hospital Universitari Arnau de Vilanova, Lleida), O. Urbina Bengoa (Parc de Salut Mar, Barcelona), A. Vilaseca Tomàs (Althaia Xarxa Assistencial de Manresa, Barcelona).
M.J. Estepa Alonso (Complejo Hospitalario Universitario de Badajoz -Hospital Infanta Cristina, Badajoz).
S. Albiñana Pérez (Hospital Arquitecto Marcide, El Ferrol, La Coruña), S. González Piñeiro (Complejo Hospitalario Universitario A Coruña, La Coruña).
M.J. Alfaro Alfaro (Fundación Hospital Calahorra, La Rioja).
M. Arteta Jiménez (Hospital Universitario de Getafe, Madrid), G. Baldominos Utrilla (Hospital Universitario Príncipe de Asturias, Alcalá de Henares, Madrid), T. Bermejo Vicedo (Hospital Universitario Ramón y Cajal, Madrid), C. Calderón Acedo (Hospital Universitario de Móstoles, Madrid), A. Herranz Alonso (Hospital General Universitario Gregorio Marañón, Madrid), M.A. Lucena Campillo (Hospital Severo Ochoa, Leganés, Madrid), M. Manso Manrique (Hospital Universitario Puerta de Hierro, Madrid), M. Moro Agud (Hospital Universitario La Paz, Madrid), M. Pérez Encinas (Hospital Universitario Fundación Alcorcón, Madrid), O. Serrano Garrote (Hospital 12 de Octubre, Madrid).
M.T. Antequera Lardón (Hospital Reina Sofía, Murcia), M.C. Mira Sirvent (Hospital Santa María del Rosell, Cartagena, Murcia), A. de la Rubia Nieto (Hospital Universitario Virgen de la Arrixaca, Murcia).
C. Lacasa Arregui (Clínica Universitaria de Navarra, Pamplona), F. Marcotegui Ros (Complejo Hospitalario de Navarra-Hospital Virgen del Camino, Pamplona), V. Napal Lecumberri (Complejo Hospitalario de Navarra- Hospital de Navarra, Pamplona).
M. Álvarez Lavín (Hospital de Basurto, Bilbao), G. Cajaraville Ordoñana (Fundación Oncológica, San Sebastián), A. Campino Villegas (Hospital de Cruces, Barakaldo, Vizcaya), N. Mauleón Echeverría (Hospital Donostia, San Sebastián), J. Peral Aguirregoitia (Hospital de Galdakao, Vizcaya), A. Quintana Basterra (Hospital Txagorritxu, Vitoria).
M.T. Aznar Saliente (Hospital Universitari Sant Joan d’Alacant, Alicante), M.D. Bellés Medall (Hospital La Plana, Vila-real, Castellón), M. Climente Martí (Hospital Universitario Dr. Peset, Valencia), I. Font Noguera (Hospital Universitari i Politècnic La Fe, Valencia), J.D. Rosique Robles (Hospital de Sagunto, Valencia), M.T. Torrecilla Junyent (Hospital Clínico Universitario, Valencia), J.M. Ventura Cerdá (Hospital General de Castellón).
The components of the group are listed in Appendix A.
Please cite this article as: Otero López MJ, et al. Elaboración de una relación de nombres de medicamentos similares en los que se recomienda utilizar letras mayúsculas resaltadas. Farm Hosp. 2011;35:225–35.