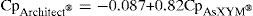To assess the technique employed by the autoanalyser Architect® i1000sr to determine digoxin in serum samples, compared with the assay developed for AsSYM® using microparticle enzyme immunoassay (Digoxin II).
MethodA prospective analysis of the samples from 100 requests to monitor patients being treated with digoxin. The samples were processed in AxSYM® and Architect®. The techniques were assessed using the linear regression coefficient, determination coefficient, mean absolute error, mean squared prediction error and the Bland-Altman method.
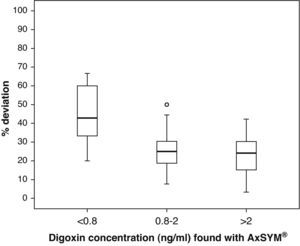
ResultsThe serum levels showed a correlation coefficient of 0.93. There was nearly a 40% difference for the concentrations between 0.8 and 2ng/ml and nearly 20% in the rest of the samples analysed.
ConclusionsThe Architect® system is precise; however, from a clinical monitoring point of view, it is unacceptably inaccurate when compared with the AxSYM®.
Evaluar en muestras séricas la técnica empleada por el autoanalizador Architect® i1000sr para la determinación de digoxina respecto al ensayo desarrollado para AxSYM® mediante enzimoinmunoanálisis de micropartículas (DigoxinII).
MétodoAnálisis prospectivo de las muestras procedentes de 100 solicitudes de monitorización de pacientes en tratamiento con digoxina. Las muestras fueron procesadas en AxSYM® y Architect®. Las técnicas se evaluaron mediante el coeficiente de regresión lineal, el coeficiente de determinación, el error absoluto medio, el error cuadrático medio de predicción y el método de Bland-Altman.
ResultadosLas concentraciones séricas mostraron un coeficiente de correlación de 0,93. La diferencia se aproxima al 40% para concentraciones entre 0,8 y 2 ng/ml y al 20% en el resto de muestras analizadas.
ConclusionesEl sistema Architect® es preciso pero inexacto, en una magnitud inaceptable desde el punto de vista de la monitorización clínica respecto al AxSYM®.
Pharmacokinetic monitoring of digoxin (DGX) is one of the most common types of monitoring in pharmacy departments. This cardiotonic agent's narrow therapeutic interval (0.8–2ng/ml), its high inter- and intra-individual variability, the strong correlation between its pharmacological activity and serum concentrations of DGX, the low specificity of the symptoms and signs of toxicity, and the availability of automated systems to measure drug levels all contribute to the fact that DGX monitoring is part of routine care for these patients.1
The presence of digoxin-like immunoreactive factors in biological matrices can cause false-positive results and affect the accuracy of monitoring tests. The cross-reactivity of digoxin-like factors may vary among different commercially available assays owing to different affinities for the antibodies being used. The presence of digoxin-like factors has been documented in newborns, pregnant women, patients with liver or kidney failure and in critical patients.2,3
DGX levels in serum or plasma have traditionally been measured using polarisation fluorescence immunoassay techniques like those developed for the AxSYM® or TDX® autoanalysers (Abbott Laboratories, Diagnostics Division, Abbott Park, IL 60064, United States).2 Recently, Abbott Laboratories developed 2 new Architect® system techniques for determining and measuring DGX levels. One is based on using chemiluminescent microparticles (Architect i1000SR®) and the other uses photometric assay methods (Architect c4000®).
The aim of this study was to use patient serum samples to evaluate the analytical technique used by Architect i1000SR® in determining digoxin levels and compare it to that of the AxSYM® test.
MethodProspective analysis of samples from 100 orders for serum level monitoring in patients treated with DGX. The samples were immediately processed in both analysers, AxSYM® and Architect i1000SR®, according to the analytical technique specifications of each.
The precision of both assays was evaluated by means of their respective tests recommended by the manufacturer (AxSYM®: Digoxin controls®, Abbot Laboratories; Architect i1000SR®: Abbot immunoassay-MMC, Liquid®, BIO-RAD). The precision test consisted of analysing the assays of 3 different, defined concentrations, 3 times a day during 4 days.
Any potential digoxin-like factor interference was evaluated in samples from patients who were not taking DGX. Patients were placed in 2 groups: 7 patients with a high risk of having digoxin-like factors (those with kidney or liver failure or critical patients) and 9 low-risk patients (adults with normal liver and kidney function and unaffected vital functions).3 DGX concentrations greater than 0.3ng/ml were considered positive for the presence of digoxin-like factors.4
Concordance between the results obtained using both techniques was evaluated by means of the linear regression coefficient, the determination coefficient, the intra-class correlation coefficient, the mean absolute error (MAE), the mean squared prediction error (MSPE) and the Bland-Altman plot method.5 Values obtained using AxSYM® Digoxin II were considered as reference values. Values of P<.05 were considered statistically significant.
ResultsThe mean level measured by the AxSYM® system was 1.44ng/ml (95% CI: 1.27–1.61). Measured with Architect®, it was 1.09ng/ml (95% IC: 0.95–1.23). The inter-day variation coefficients (VC) for AxSYM® ranged between 5.6% and 8.4%; intra-day VCs were between 3.9% and 6.4%, and total VC was 7%. For Architect® system measurements, inter-day VCs ranged between 1.0% and 1.6% with intra-day coefficients between 0.8% and 1.5%. Total VC was 1.2%.
DGX serum concentrations above 0.3ng/ml were not measured in either of the 2 groups (high-risk, n=7; low risk, n=9) in which the presence of digoxin-like factors was checked.
Concordance analysis for DGX serum levels measured with the AxSYM® and Architect® systems reveals a positive and statistically significant correlation coefficient (r2=0.93; P<.001) according to the equation shown below:
The 95% confidence interval for the ordinate in the origin was from −0.007 to −0.166 (P=.03) and the slope from 0.77 to 0.86 (P<.001). The intra-class correlation coefficient was 0.92.
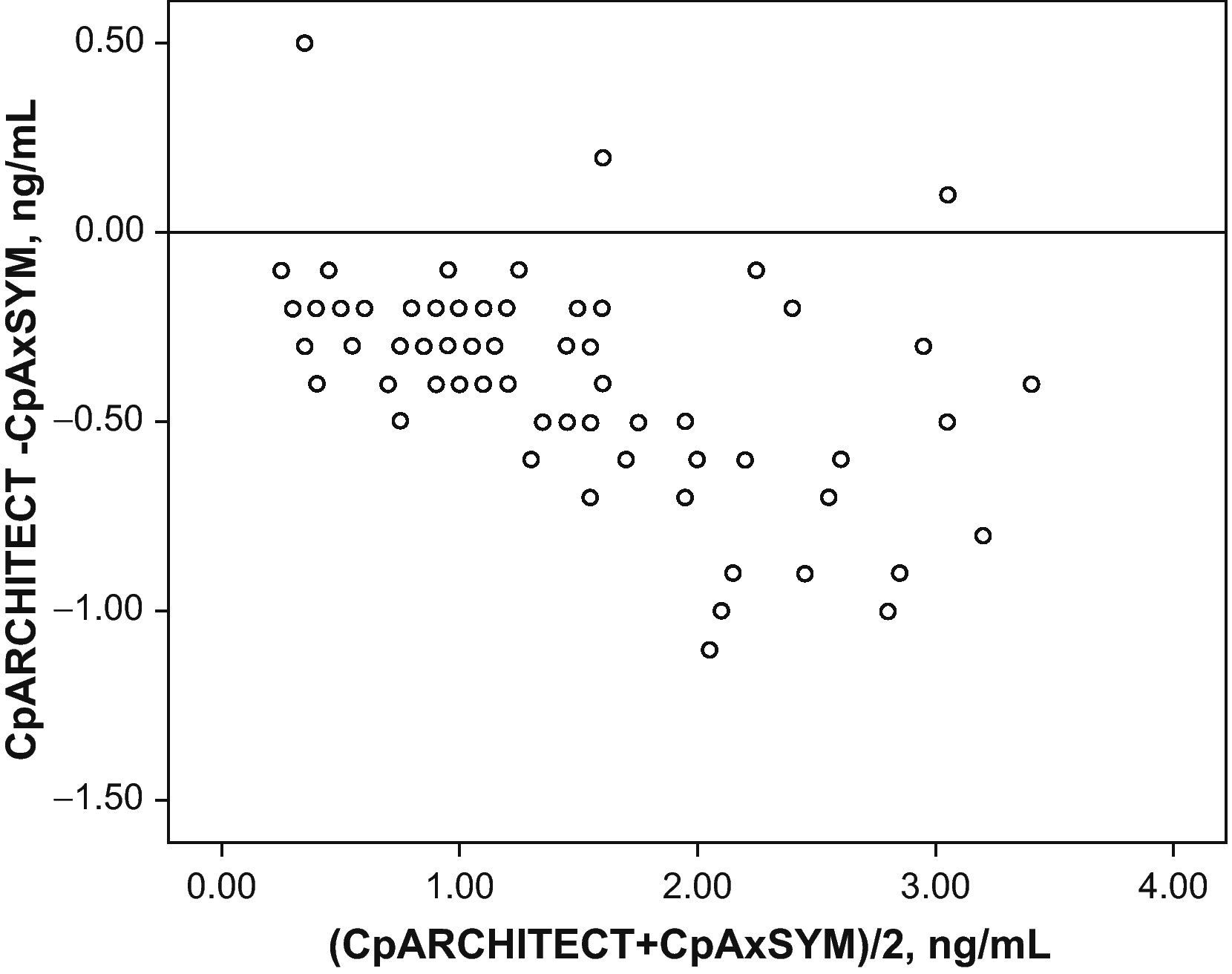
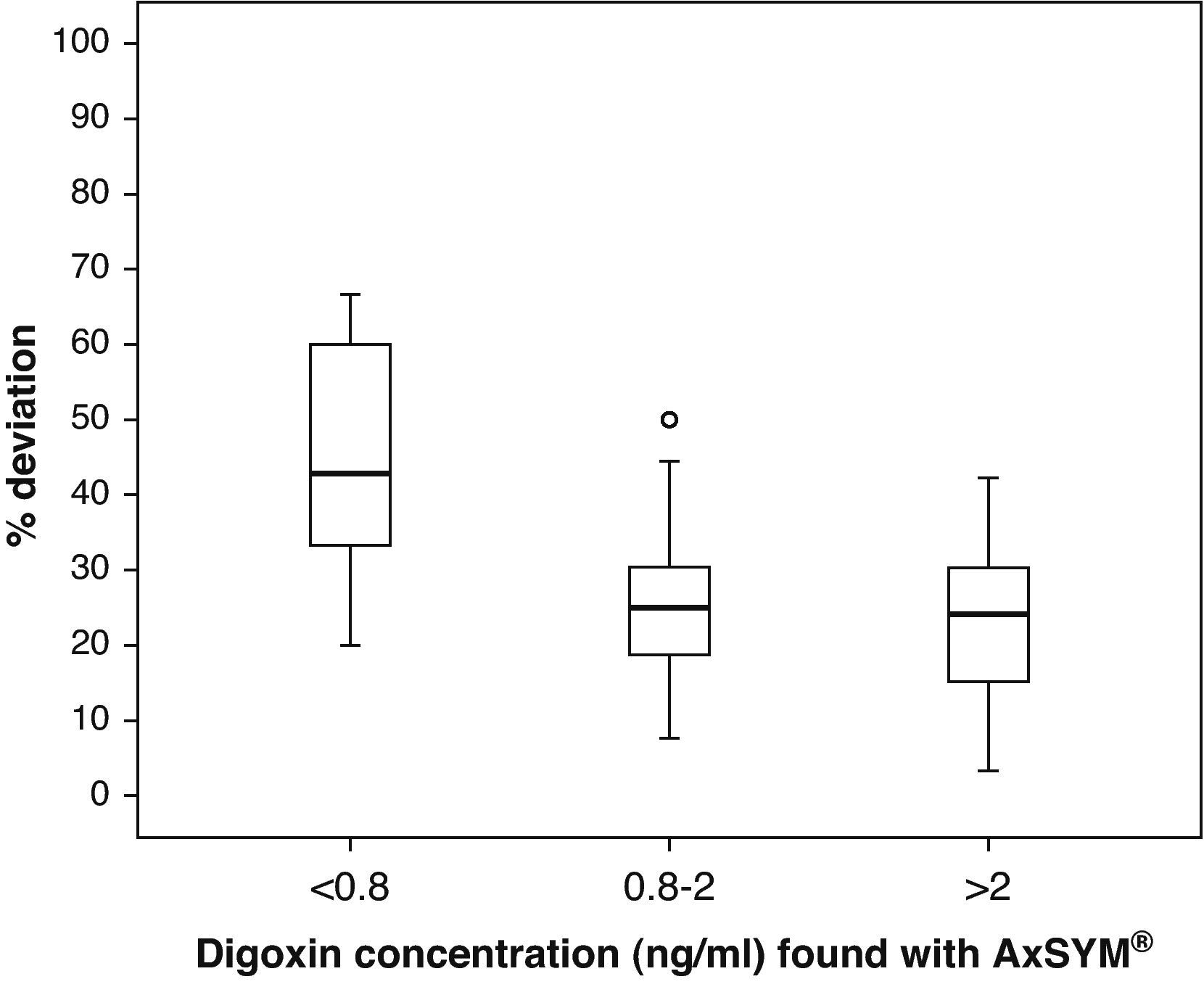
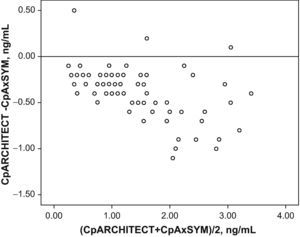
Fig. 1 shows the correlation between the difference in the analysed levels (CpArchitect®–CpAsXYM®) with respect to their mean value [(CpArchitect®+CpAsXYM®)/2]. Fig. 2 shows the deviation between the Architect® and the AxSYM® measurements as a percentage, according to the levels analysed by means of the AxSYM® system. The MAE was 0.37±0.22 and the MSPE 0.18±0.24.
Box diagram of the percentage of deviation in the digoxin measurement performed with the Architect® system compared to the AxSYM® system, considering the concentrations measured by the latter. In the first group (<0.8ng/ml), the result found for a sample with a 500% deviation is not shown so as not to distort the graph.
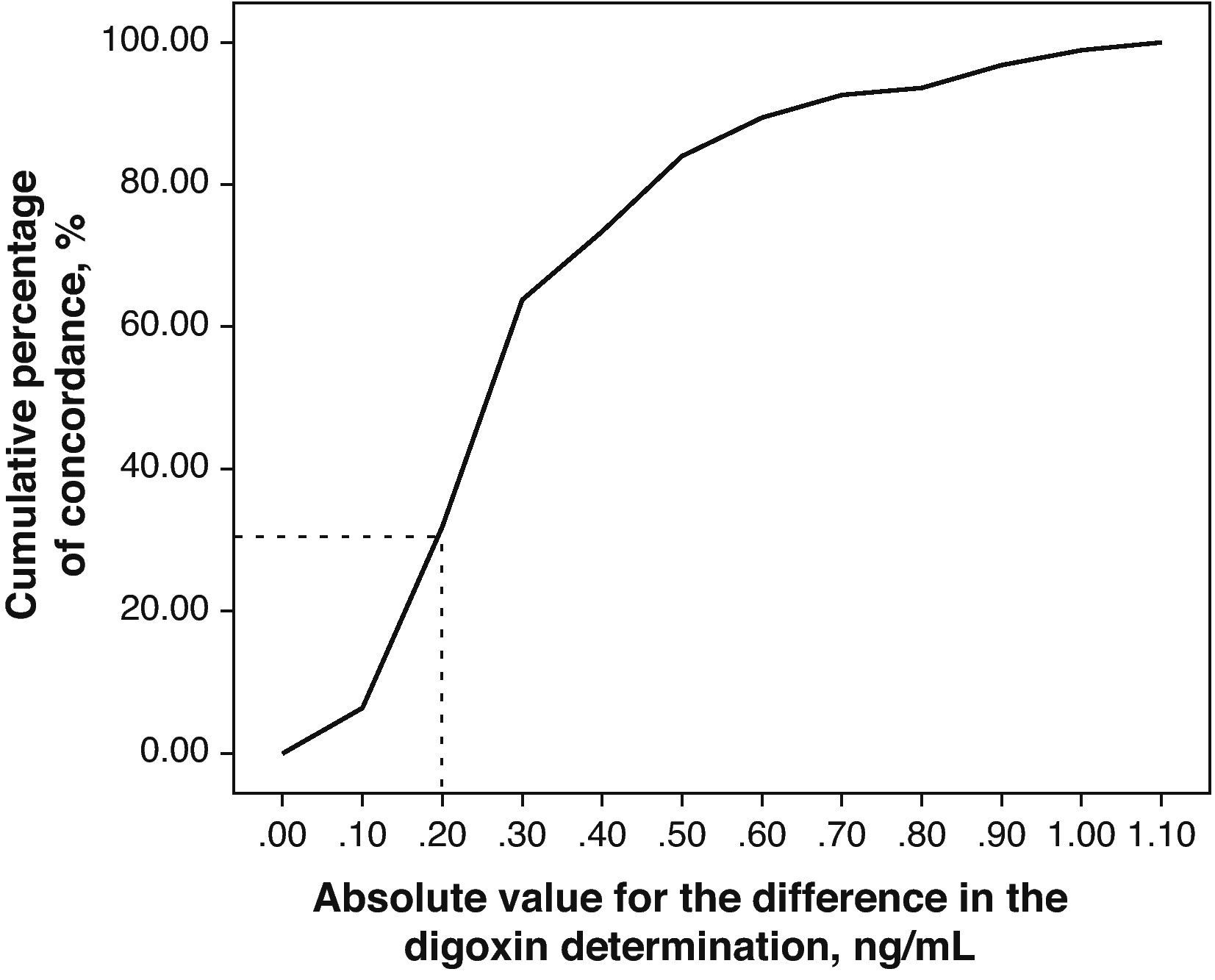
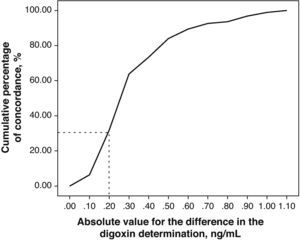
The relevance of these differences can be evaluated by representing the absolute value of the differences existing between the DGX concentration measurements for both techniques vs the cumulative percentage of concordance (Fig. 3).
DiscussionBoth tests showed good levels of precision with VCs below 10% for the entire range of assayed concentrations. However, Architect i1000SR® showed less variability than AxSYM®, with a total VC of 1.2% vs 7%.
Our study found no cases of interference caused by digoxin-like factors. The presence of these factors has been documented extensively and different authors have detected cross-reactivity with DGX through the use of various analytical techniques. However, discrepancies between these techniques are considerable.6–8 Azzazy et al.4 compared susceptibility to possible interference from digoxin-like factors among AxSYM® and 6 other techniques using 233 samples of different origins. Samples from patients with liver failure or from the umbilical cord were those that most often showed cross-reactivity. Nevertheless, AxSYM® was the least susceptible system, and interference only appeared in 10% of patients with kidney failure, 36% of patients with liver failure, and 34% of samples from the umbilical cord. Suppression of acid pre-treatment, which was used in previous DGX assays, has reduced interference frequency.3,4
We did not find cross-reactivity with digoxin-like factors in our study and it can be attributed to its small sample size. Such interference could also be present in the Architect® assay, and therefore, undertaking a larger study is absolutely necessary. A future study must use a number of serum samples from patients with kidney or liver failure to evaluate the absence of interference by digoxin-like factors.
The correlation between the laboratory results from both techniques is excellent (r2=0.93). However, the significantly different slope in one case (b=0.82; CI 95%: 0.77–0.86; P>.001) suggests a systematic deviation in the values obtained with Architect® from those obtained with AxSYM. Values quantified using the Architect® system are lower than those from the AxSYM® system, and this discrepancy increases as values increase (Fig. 3). There was a difference of nearly 40% for the concentrations between 0.8ng/ml and 2ng/ml, and of nearly 20% in the other samples analysed. Furthermore, 50% of the 21 samples shown by AxSYM® to contain potentially toxic concentrations (>2ng/ml) were within the therapeutic range, according to the Architect® analysis; and 15% of the samples shown by AxSYM to be within the therapeutic range appeared as sub-therapeutic values according to the Architect® analysis. If we accept a maximum difference, of 0.2ng/ml for example, between DGX serum levels measured using both techniques in order to make them equivalent, they would only be concordant in approximately 35% of the measurements that were made (Fig. 3).
Results from the present study demonstrate the need of evaluating accuracy and precision for all analytical techniques incorporated into pharmacokinetic monitoring of drugs used in clinical practice.
To conclude, if we accept that the assay to determine serum DGX levels by means of the AxSYM® system is the standard of reference, the serum DGX assay performed using Architect® would be precise but inexact to such an extent that it is unacceptable for use in clinical monitoring.
Conflict of InterestThe authors have no conflict of interest to declare.
Please cite this article as: Albert Vicent E, et al. Comparación del ensayo de digoxina con el sistema Architect®i1000 sr respecto al sistema AxSYM®. Farm Hosp. 2011;35:256–9.