To evaluate the impact and type of side-effects in patients treated with cetuximab and provide a description of the general measures and treatment.
MethodsRetrospective safety study. We included all patients that received cetuximab from January to December 2009. All information was obtained from the Pharmacy and Oncology Department's Access databases and reviewed the patient's medical history. All data was registered in an Excel workbook. Skin toxicity was graded by the current National Cancer Institute-Common Toxicity Criteria (NCI-CTC).
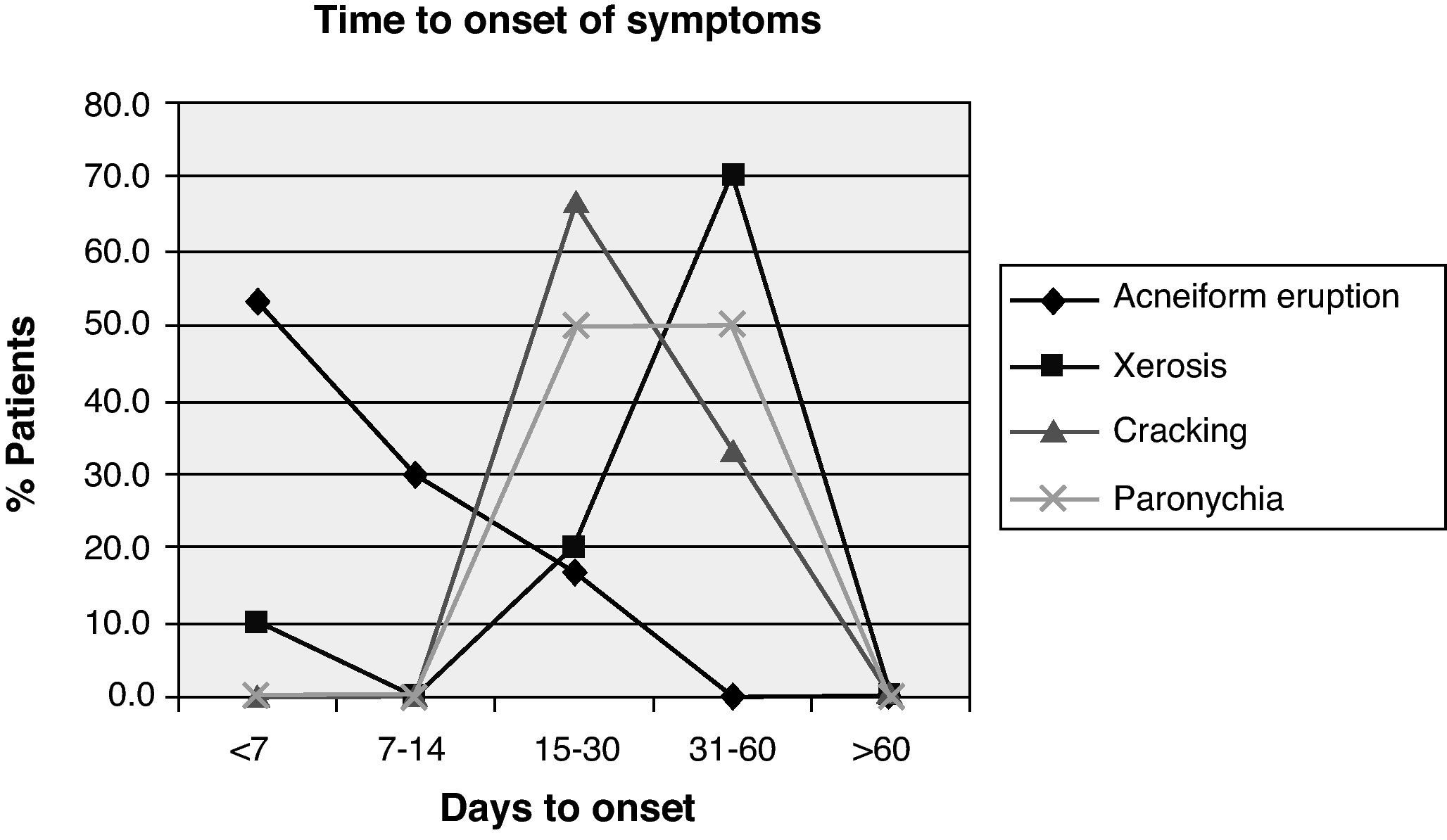
ResultsDuring the study period 43 patients received treatment with cetuximab. Acneiform eruption was present in 30 of the cases (69.8%): 14 patients with grade 1 (48.3%), 13 with grade 2 (44.8%) and 3 with grade 3 (10.3%). These adverse effects appeared in a median of seven (4–28) days. In a median of 40 (20–56) days, ten patients (23.3%) presented xerosis, and three (7%) suffered painful fissures in hands and feet after a median of 28 (21–35) days. Paronychia was present in two patients after a median of 42 (35–49) days. Finally, an alteration in hair growth was observed in two patients with overgrowth of facial hair and one patient with overgrowth of the eyelashes. Five patients presented important conjunctivitis. Three infusion reactions occurred.
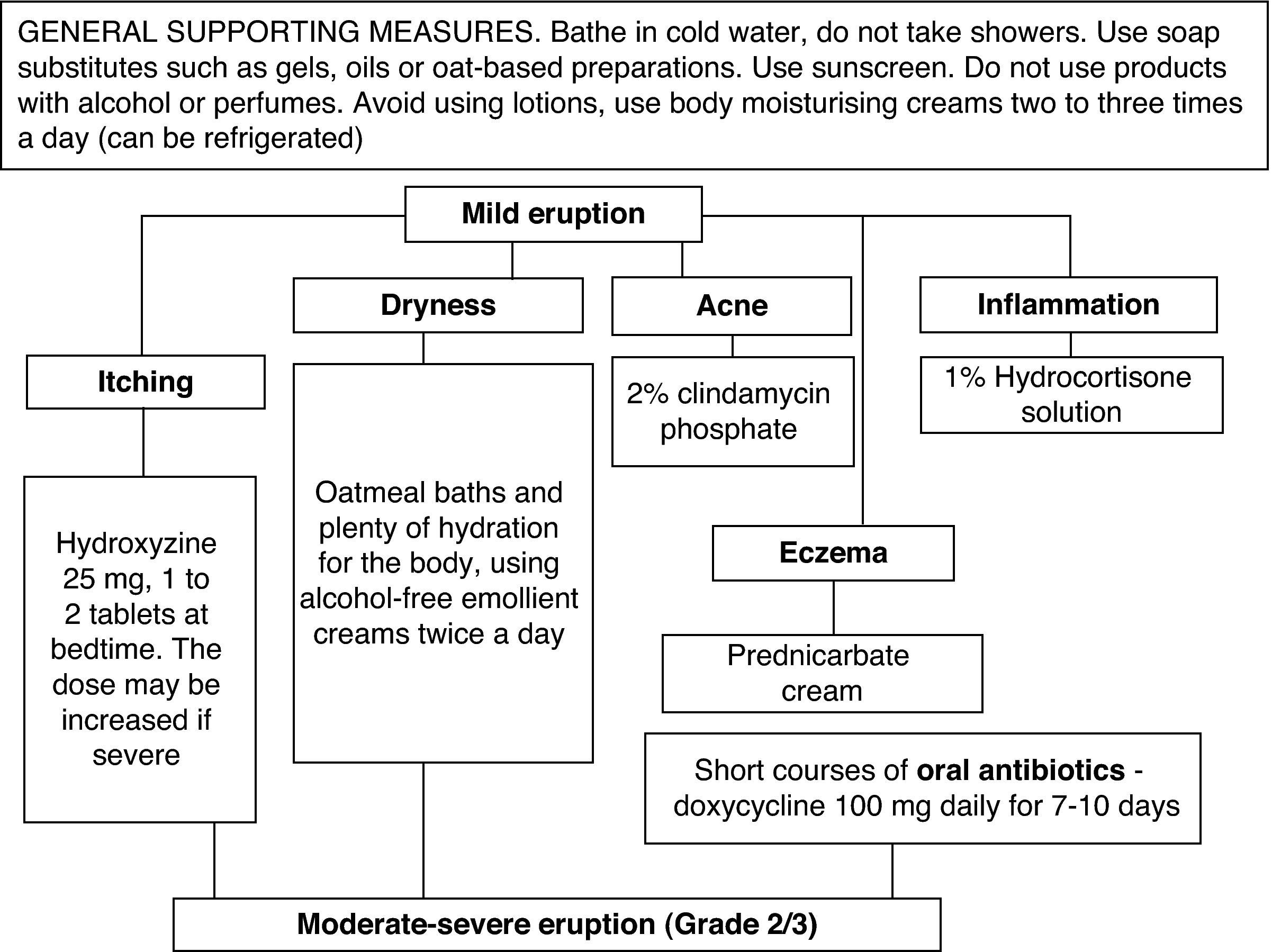
A grade-based treatment algorithm was used for all patients that presented cutaneous toxicity.
ConclusionsA considerable number of patients treated with cetuximab develop dermatological side-effects which left untreated could represent a threat to the efficacy of the therapy. Therefore effective management is mandatory, patient education and immediate treatment based on a grade-based algorithm to alleviate symptoms is necessary, so that patient compliance is guaranteed.
Evaluar la incidencia y el tipo de reacciones cutáneas secundarias al tratamiento con cetuximab y describir el protocolo terapéutico.
MétodoEstudio retrospectivo de seguridad. Se seleccionaron los pacientes que recibieron cetuximab desde enero a diciembre de 2009. Se obtuvo la información consultando bases de datos Access y posterior revisión de historias clínicas. Se registraron los datos en una hoja de cálculo Excel. Para la gradación de la gravedad de la erupción cutánea se siguió la clasificación NCI-CTCAE.
ResultadosRecibieron tratamiento 43 pacientes. Presentaron erupción acneiforme 30 (69,8%): 14, grado 1 (48,3%); 13, grado 2 (44,8%), y 3, grado 3 (10,3%). La mediana de días hasta aparición de síntomas fue de 7 (4-28). Presentaron xerosis, tras una mediana de 40 (20-56) días, 10 pacientes (23,3%) y 3 (7%) manifestaron fisuras en manos y pies tras 28 (21-35) días. En dos pacientes aparecieron trastornos ungueales (como paroniquia) tras 42 (35-49) días. En cuanto a alteraciones del crecimiento del pelo: hubo dos casos de hipertricosis y un paciente experimentó un gran sobrecrecimiento de las pestañas. Se presentaron conjuntivitis importantes en cinco casos. Tuvieron lugar tres reacciones infusionales.
Se aplicó a todos los pacientes un protocolo de tratamiento basado en la gravedad de la toxicidad cutánea.
ConclusiónEl perfil de seguridad de cetuximab concuerda con el descrito en los estudios publicados. Se trata de efectos adversos cutáneos de aparición muy frecuente y de importantes consecuencias relacionadas con la efectividad de la terapia. La información al paciente y detección y tratamiento precoz de la sintomatología según un protocolo consensuado pueden favorecer el buen cumplimiento y el éxito terapéutico.
Cetuximab is a chimeric IgG1 monoclonal antibody which specifically targets the extracellular domain of the epidermal growth factor receptor (EGFR), which is a transmembrane glycoprotein of the tyrosine kinase family that is expressed in a number of human tissues controlling cell multiplication and proliferation.1,2 However, the EGFR is overexpressed or upregulated in a wide variety of solid tumours, including intestinal cancers (65%–75%), and this is related with increased metastasis, decreased survival and a poor prognosis.3 Cetuximab is indicated by the European Medicines Agency (EMEA) for the treatment of metastatic colorectal carcinoma resistant to conventional chemotherapy, which expresses EGFR with wild-type KRAS gene4 and locally advanced squamous cell carcinoma of the head and neck, in combination with radiation or platinum therapy in metastatic or recurrent disease.1
This family of EGFR inhibitors does not include many of the serious adverse effects posed by conventional chemotherapy in its toxicity profile. However, these compounds, in particular cetuximab, are associated with a variety of highly prevalent adverse dermatological effects. This receptor is also expressed in the basal layer of the epidermis, stimulating its growth, inhibiting differentiation and accelerating wound healing.5–10 The epidermal cell blockade by the antibody leads to side effects such as acneiform eruptions, changes in the scalp and hair growth, nail and eye disorders, xerosis and pruritus. Skin effects are frequent and have serious consequences associated with the psychosocial and physical health of the patient. Over 80% of patients present with acneiform eruption, with a third requiring some sort of intervention5 and up to 15% being severe. This effect appears in the first 3 weeks of treatment and is dose-dependent.1,5–10 Other common adverse reactions are diarrhoea, hypomagnesaemia11,12 and increased levels of liver enzymes. More severe, albeit infrequent, reactions are hypersensitivity reactions13–15 and pulmonary toxicity.16
The aim of this study was to assess the incidence and type of skin reactions secondary to treatment with cetuximab and describe the treatment protocol applied.
MethodsThis was a retrospective safety study from January to December 2009 in a general hospital of 592 beds. All patients who received cetuximab as a second or third line treatment for wild-type KRAS metastatic colorectal cancer during that period of time were selected. All patients underwent screening for KRAS mutational status in the hospital using a validated method. Cetuximab was administered by intravenous infusion once a week. The first dose was 400mg/m2 and subsequent weekly doses were 250mg cetuximabperm2 of body surface.
Information was obtained from a pharmacy department database (Microsoft Office Access 2003) containing anti-cancer drug dispensing records; an oncology department database (Pigeo, Microsoft Access 2003), containing information on treatment administered, day hospital patient visits, outpatient oncology information; and a medical record review. Information about demographics, grade, type and location of skin reactions, treatment received, other skin adverse effects, infusion reactions and time from the administration of cetuximab to the onset of adverse effect symptoms/signs was entered onto an Excel spreadsheet.
Skin reaction severity was graded according to the NCI-CTCAE classification,17 as follows: Grade 1: macular or papular eruption or erythema that affects less than 10% of the body surface area (SA), with no associated symptoms and no therapeutic intervention required; Grade 2: macular or papular eruption or erythema with pruritus or other associated symptoms that affect 10%–30% of the SA, having a specific, significant psychosocial impact and requiring therapeutic intervention; Grade 3: papules or pustules that affect more than 30% of the SA, which may or may not be associated with symptoms such as pruritus and associated with local superinfection (indicating the use of oral antibiotics), with the formation of lesions or ulcerations, having a significant impact on the patient's daily life; and Grade 4: exfoliative or ulcerative dermatitis: papules/pustules affecting any part of the SA, which may be associated with symptoms such as pruritus, with associated superinfection requiring IV antibiotic treatment, and having possible fatal consequences.
The quantitative variables studied were expressed as median (minimum–maximum) and categorical variables as frequency and percentage.
The study was approved by the hospital's clinical research ethics committee, with informed consent from patients not being considered necessary, as it was a retrospective study.
ResultsBetween January and December 2009, 43 patients (34 men and 9 women) with a median age of 66.2 years (34–82) were treated with cetuximab. An acneiform eruption was seen in 30 (69.8%) of them, mainly on the face, nose, perilabial area, neck, back and trunk. The median to the onset of the first signs/symptoms was 7 days (4–28), i.e. always within the first 4 weeks. Of these 30 patients, 14 had a grade 1 eruption (48.3%), 13 had a grade 2 eruption (44.8%) and 3 patients had a grade 3 eruption (10.3%).
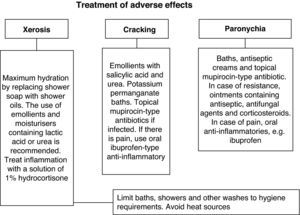
All patients received the same treatment according to the hospital protocol (Fig. 1). For a mild rash, a topical treatment consisting mainly of abundant hydration was prescribed, with an antihistamine for pruritus. For acne, the use of a 2% clindamycin phosphate was suggested or a 1% prednicarbate cream or hydrocortisone solution if there was inflammation. Grade 2 and 3 eruptions had to be treated with short courses of oral antibiotics, while severe cases (Grades 3 or 4) were referred to the dermatology consultation department if standard therapy failed to control the symptoms.
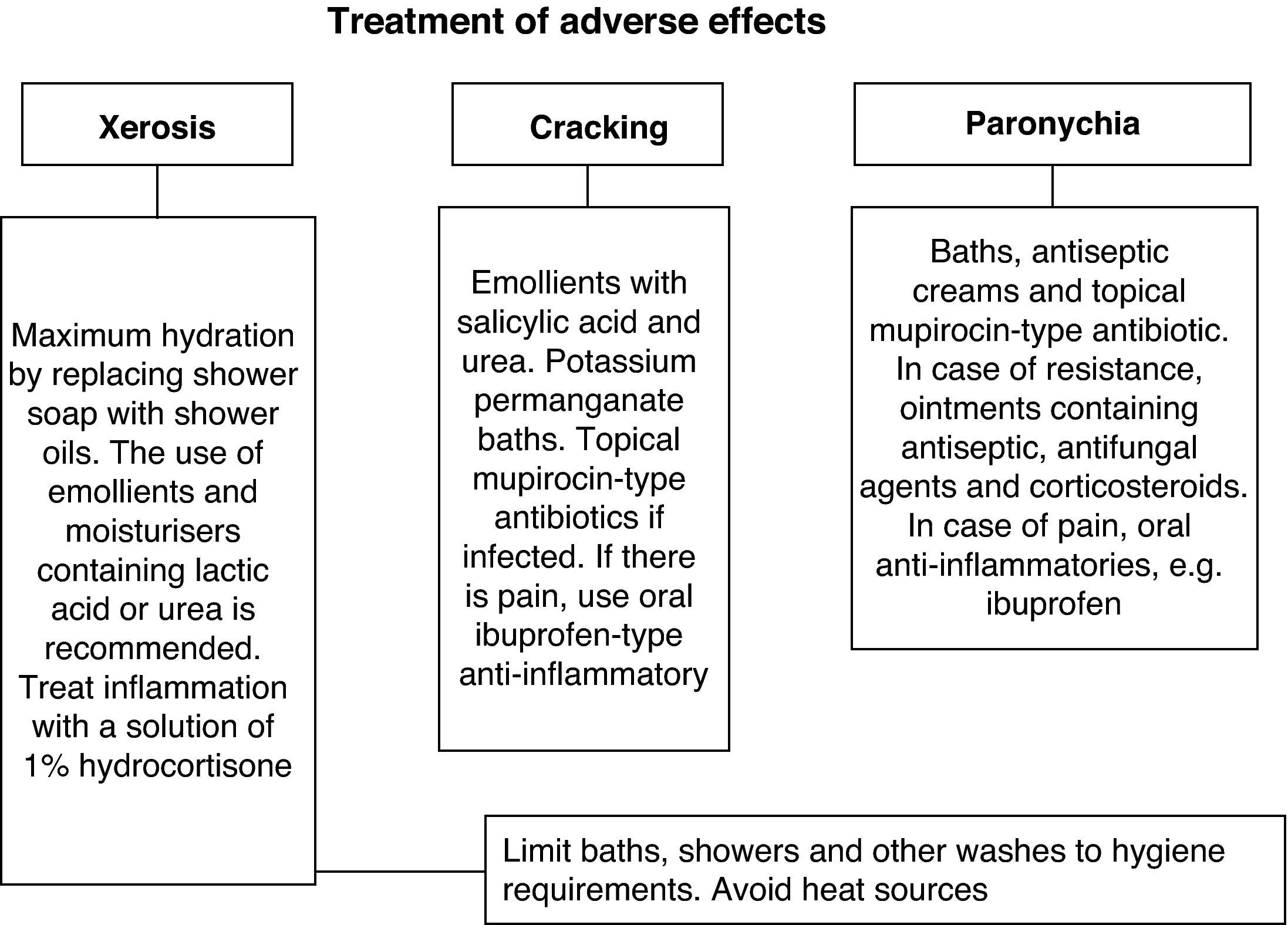
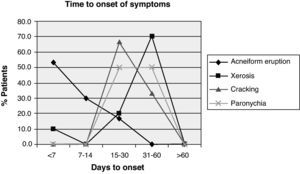
Other, less frequent, presentations were as follows: xerosis in 10 patients (23.3%) with a median onset of 40 days (20–56); cracks in the hands and feet in 3 patients (7%), which appeared after a median 28 days (21–35); and nail disorders in 2 patients after a mean of 42 days (35–49), see Fig. 2. The treatments significantly alleviated the symptoms (Fig. 3).
Other manifestations were also seen, such as changes in hair growth: 2 patients had hypertrichosis and 1 patient experienced a large overgrowth of the eyelashes; while 5 patients had significant conjunctivitis, which led to delaying the treatment in 2 cases.
There were 3 infusion reactions (6.9%): 2 of them were serious and involved changing the treatment to panitumumab. This is a fully human IgG2 monoclonal antibody that binds with high affinity and specificity to the ligand-binding domain of human epidermal growth factor receptor (EGFR).
Cetuximab treatment was discontinued temporarily or permanently in 9 patients (20.9%) due to adverse skin or infusion reactions. In 7 cases (16.3%), the treatment was stopped and resumed at a lower dose after recovery. This was due to significant skin toxicity in 5 cases, conjunctival toxicity in 2 cases and moderate/severe infusion reaction in 1 case. Treatment was stopped permanently in 2 cases (4.6%) due to severe infusion reactions.
DiscussionThe number of clinical applications for the anti-EGFR family is increasing, and its toxic effects are therefore increasingly more important. Treatment with cetuximab involves adverse skin effects in more than 80% of patients. The majority of skin reactions appears during the first 3 weeks of therapy and generally resolves without sequelae over time after discontinuation of treatment, provided that the recommended dose adjustments are observed. However, these skin reactions have a significant impact on the physical and psychological health of the patient, due to the unpleasant appearance of the lesions and corresponding discomfort caused, despite their reversible nature and lack of high risk involved for the patient. As a result, adherence to the treatment and of course quality of life may be adversely affected. Proper management of the toxicity, therefore, helps to maximise the benefit and minimise the negative effects of the treatment, improving the patient's quality of life.5,9
Since Busam et al.18 first described skin toxicity secondary to treatment with anti-EGFR agents, numerous studies19–23 have described the appearance of anecdotal cases. In 2003, Walon et al.20 proposed the inclusion of cetuximab in the list of drugs that may induce acneiform eruption after 2 cases. Later reviews5–9 evaluated the incidence and type of reactions occurring in patients under treatment, and related them to a timeline and the efficacy of the treatment. Reviewing the literature, it can be seen that this skin rash has been called many things: acneiform rash, maculopapular rash, acne-like rash, folliculitis, etc. Lacouture and co-workers7 believed the term ‘papulopustular eruption’ to provide the most accurate description. However, terms such as acneiform reaction,6 eruption or rash are commonly used to describe this adverse reaction in our study, due to its distribution on the body surface.
In the group of patients we studied, 69.8% had acneiform eruption mainly on the face, nose, perilabial area, neck, back and trunk. In the first week, sensitivity disorders, erythema and oedema were seen; a papulopustular eruption was seen in the 2nd week and scabs formed in the 3rd week. The percentage of reactions recorded confirms the high incidence of skin toxicity found in the literature.5–7,10 The same did not appear with cracking, which occurred in 7% of our patients compared to 100% of those in the Busam et al.18 study, or 60% as observed by Roé et al.10 Only 5% of our patients had nail lesions, compared with 40% identified by Busam et al.18 and 30% by Roé et al.10 This may be due to an under-reporting of non-skin rash events in the medical records.
The time to the onset of symptoms is similar to those reported by other groups.10 In most cases, the intensity of skin reactions was reduced after repeated doses of the drug and the effects were reversible after discontinuation. However, some cases needed the dose to be reduced, postponed or even suspended early, due to intolerable toxicities. Li et al.9 recorded up to 10% of treatment interruptions. In our series, 16.3% of treatments were interrupted at some point in the therapy and 4.6% were permanently stopped.
However, our study had a low rate of infusion reactions reported. Lee et al.16 analysed the impact of these infusion reactions in time and caregiver personnel costs, due to the frequency of occurrence, and identified these reactions in 32% of patients. Our study recorded only 3 (6.9%) serious infusion reactions during treatment; however, mild or moderate reactions were not recorded.
The treatment protocol used in our hospital was similar to that found in the literature.5–10 However, there is no standard treatment established from randomised trials, so treatment was based on clinical practice. In 2005, Segaert et al. developed a treatment procedure for skin toxicity and Lacouture et al.8 did the same in 2007. In 2008, the results of a panel of experts were published, which contained recommendations for the treatment of skin eruption caused by receiving erlonitib,23 and in 2009 the Canadian clinical practice guidelines for managing skin eruptions secondary to anti-EGFR therapy5 were published. For a mild rash, treatment options include topical corticosteroids of low to medium strength, 2% clindamycin gel and 1% hydrocortisone solution.5,8 Some authors advise against the use of steroids,9,24 as they are a known cause of acneiform eruption. Pimecrolimus has been used successfully in isolated cases; however, a recent prospective, uncontrolled study showed no clinical benefit for it.25 Other topical options used are benzoyl peroxide, fusidic acid, erythromycin, metronidazole,6,7 with little improvement. Retinoids are also used7; however, the possible exacerbation of xerosis must be assessed, and some groups do not support their use as they do not consider the eruptions to be acne.9,26 Finally, oral tetracyclines are a good option.19
Another possibility studied is the search for preventive strategies: the controlled study STEPP27 compared the use of treatment when therapy was started or once toxicity was displayed. There was a 50% reduction in the incidence of adverse moderate/severe (Grade 2 or higher) in those receiving preventive treatment.5
There are several treatment strategies currently being studied in clinical trials. After conducting a randomised, double-blind, placebo-controlled study, Scope et al. suggested that prophylactic treatment with oral minocycline once a day reduced the occurrence of moderate/severe cases, whereas the topical application of tazarotene had no clinical benefit for any of these patients.28 Subsequently, a randomised, placebo-controlled, double-blind study was published, which concluded that oral tetracycline treatment twice daily did not prevent the eruption from developing, but reduced the occurrence of severe cases.29 Other treatment options for prevention or treatment9 are currently being studied, based on the mechanism of occurrence of skin toxicity, such as the topical application of menadione twice daily.
Randomised studies are needed to support prophylactic treatment in these patients and to establish a standard evidence-based treatment.
The main limitation of this study is its retrospective nature, which in our case introduced information bias due to incomplete filling of medical records, especially for adverse events considered to be less well-known or serious. However, information on the skin toxicity of cetuximab in clinical practice was provided.
In conclusion, this cetuximab safety profile study shows that skin type adverse effects are common and present a predictable pattern of occurrence, as described in other studies. Knowing the high incidence of skin toxicity secondary to treatment with cetuximab and the symptom onset profile helps us predict these cutaneous manifestations, monitor them and treat them early.
The consensus from a number of sources points to the importance of active care by a multidisciplinary team in treating these adverse effects, due to the significant consequences of compliance and, therefore, the efficacy of the therapy. It is very important to monitor these patients to prevent dose reduction or treatment interruption. An appropriate treatment programme based on the grade of the exanthema should be established, which limits the incidence of severe adverse effects and improves tolerability. Patients must be well informed through good pharmaceutical care, with prompt recognition of symptoms and treatment of injuries as quickly as possible, which will encourage greater compliance and, ultimately, an improved quality of life for the patient.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Rodríguez-Murphy E, et al. Toxicidad cutánea asociada a cetuximab en cáncer colorrectal metastásico. Farm Hosp. 2011;35(3):114–120.
This paper has been presented in electronic poster format at the 55 Congreso de la SEFH, held in Madrid in 2010, and is pending acceptance.