Hyperprolactinaemia is a significant side effect of antipsychotic medications and may cause sexual dysfunction. Although risperidone and paliperidone can induce prolactin elevation, previous studies suggest that switching from risperidone long-acting injectable (LAI) to paliperidone palmitate (PP) might reduce prolactin concentrations in early psychosis patients. We aimed to evaluate the effect of switching from risperidone to PP on sexual functionality and prolactin levels in patients with schizophrenia.
MethodsWe studied 38 patients with schizophrenia who were treated with risperidone (oral or R-LAI) monotherapy at stable doses for at least two months and had an indication to be switched to PP by their psychiatrists. Three assessments were completed: 1) baseline (preswitch), 2) 3 months post-switch, and 3) 6 months post-switch. Prolactin concentrations in plasma were determined. Sexual functioning was assessed with the Arizona Sexual Experience Scale (ASEX). Statistical analyses were conducted in 27 patients who had at least one follow-up visit. Longitudinal changes in prolactin levels and sexual function after switching from risperidone to PP were analysed with linear mixed models. Significance was set at p < 0.05.
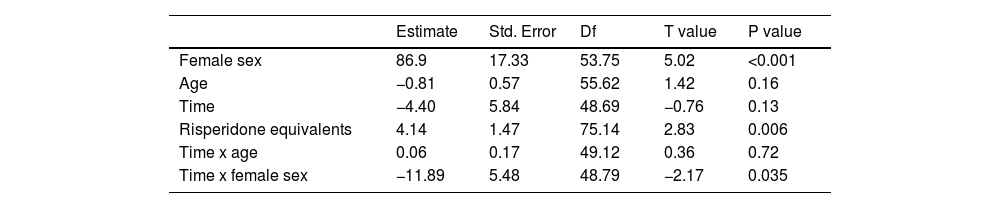
ResultsProlactin concentrations were reduced in women after the switch, with a significant time by sex effect (p = 0.035). Antipsychotic doses influenced prolactin levels (p = 0.006), such that higher antipsychotic doses were associated with higher prolactin concentrations. No significant differences were found in ASEX total scores at 6 months after the switch.
ConclusionsIn patients with schizophrenia, switching from risperidone to PP was associated with a reduction in prolactin concentrations in women but not men.
Lack of awareness of schizophrenia and poor adherence to treatment in patients suffering from the disease implies an increased risk of relapse and a worse long-term prognosis. Side effects are one of the leading causes of medication discontinuation. Hyperprolactinemia (HPRL) is a common finding in patients with schizophrenia, and in most cases, it is a side effect of antipsychotic medication.1 HPRL is related to the blockade of D2 receptors in the tuberoinfundibular pathway (since dopamine inhibits prolactin secretion in the pituitary).2,3 An incidence of HPRL of 42 to 89 % has been described among patients taking antipsychotic drugs.1,4-6 Many of the main clinical consequences of HPRL involve the reproductive system, including galactorrhoea, amenorrhea, and menstrual cycle irregularities in women and erectile and ejaculation dysfunction, alteration of spermatogenesis and reduction of muscle mass in men. HPRL also causes hypogonadism and decreased libido in both sexes.7,8 These effects can have a negative impact on the quality of life of patients, interfere with adherence to treatment9 and cause exacerbation of psychotic symptoms.10
Evidence suggests that long-acting injectable (LAI) antipsychotics facilitate compliance with treatment and are more effective in preventing relapse and reducing the risk of hospitalization.11-13 Risperidone and paliperidone and their LAI formulations (risperidone LAI and paliperidone palmitate, PP) are two second-generation antipsychotics that cause elevated prolactin levels.14 Although paliperidone, also called 9-hydroxyrisperidone, is the main active metabolite of risperidone, it exhibits pharmacokinetic and pharmacodynamic differences from risperidone that contribute to a better tolerability profile.15 PP can be administered monthly, compared to R-LAI, which is administered every two weeks. Previous research from our group also suggests that switching from R-LAI to PP in patients with a psychotic disorder at early stages of the disease is followed by a reduction in prolactin levels and an improvement in sexual functionality during a follow-up period of 3 months.16 Other research groups have also reported reduction in prolactin levels after switching from R-LAI to PP in male patients with schizophrenia.17
Our study aimed to replicate our previous study16 in a larger sample of patients with schizophrenia, extending the evaluation time to 6 months, as it is plausible that a longer follow-up duration might be necessary to detect clinical changes secondary to the reduction in HPRL. The main hypothesis of our study was that switching from risperidone to PP would improve sexual functionality and reduce prolactin levels in patients with schizophrenia. We included data from a larger prospective, observational, pragmatic study to assess functionality and cognition when switching from risperidone to PP.
Materials and methodsParticipantsWe consecutively recruited 38 patients between 18 and 60 years old with a diagnosis of schizophrenia who were attending different facilities of the Department of Mental Health (mainly the Adult Mental Health Center and the Day Hospital) at Parc Taulí Hospital (Sabadell, Spain). The sample in this study overlaps with that of another study that explored functionality and cognitive changes when switching from risperidone to PP.18 The inclusion and exclusion criteria have been described previously.18 In brief, the inclusion criteria were a DSM-IV-TR diagnosis of schizophrenia, receiving risperidone treatment (oral or R-LAI) at stable doses during the previous two months, and having a clinical indication by the referring psychiatrist to switch from risperidone to PP. The exclusion criteria included unstable medical pathologies and severe neurological pathologies, electroconvulsive therapy, pregnancy or lactation (women), cognitive alterations, substance use (alcohol, cocaine or heroin dependence or abuse disorder with active use in the previous three months), antipsychotic polytherapy or intolerance/allergy to paliperidone.
Ethical approval was obtained from the local Ethics Committee, and all participants provided written informed consent.
This was an observational, open-label, longitudinal and pragmatic study based on routine clinical practice. The decision to switch from risperidone to PP was made by the psychiatrist according to clinical criteria. In the change from risperidone to PP, the recommendations of the product were followed. In patients switching from R-LAI to PP, the dose was doubled. The doses of PP and other psychotropic drugs could be modified during the study if the referring psychiatrist of each patient considered it appropriate.
Clinical assessment and prolactin measurementThe clinical evaluation was completed by a psychiatrist with the OPCRIT checklist v.4.0, and the diagnosis of schizophrenia was generated. Three assessments were completed: 1) baseline (preswitch), 2) 3 months post-switch, and 3) 6 months post-switch.
At the baseline visit, sociodemographic and clinical variables related to the course of schizophrenia were obtained in a semistructured interview. At all visits, substance use and current psychopharmacological treatment were recorded.
The following tests were administered by the same rater to assess psychopathological status at all visits: the Positive and Negative Syndrome Scale (PANSS)19 was used to assess positive, negative and general psychotic symptoms, and the Calgary Depression Scale for Schizophrenia20 was administered to rate depression symptoms. Sexual functioning was assessed with the Arizona Sexual Experience Scale (ASEX),21 a self-applied 5-item scale (scored from 1 to 6) that obtains a total score between 5 and 30, with high scores indicating sexual dysfunction. Previous studies defined a dichotomous variable for clinically significant sexual dysfunction as an ASEX total score >18, a score ≥5 on any single item or any three items with individual scores ≥4. The UKU Side Effect Rating Scale22 was used for side effect monitoring.
Hormone measurementsA fasting blood analysis was performed in the morning between 9 h and 10 h. Prolactin and sex hormone levels were determined in plasma. Plasma prolactin concentrations were measured by electrochemiluminescence immunoassays (Roche Diagnostics GmbH, Manheim, Germany). The sensitivities of the assays were 0.047 ng/ml for prolactin, 5 pg/ml for oestradiol, 0.030 ng/mL for progesterone and 0.025 ng/mL for testosterone. The intra-assay and interassay coefficients of variation (CVs) were below 6 %.
Statistical analysisThe calculation of the sample size of the original study has been described elsewhere.18
Of the 38 patients who started the study, 27 (71 %) had at least one follow-up visit. Therefore, the final sample for statistical analysis was composed of 27 patients. Longitudinal changes in prolactin levels and sexual function variables after switching from risperidone to PP were analysed with linear mixed models using R and the package Ime4.
The main analysis, which was hypothesis-driven, used prolactin concentrations as the main dependent variable. In this analysis, time (visit) was included as a fixed factor, along with other independent variables that were also considered fixed factors (age, sex, risperidone dose). We also tested the interaction between time (visit) and sex. Subjects were included in the model as random factors. Restricted maximum likelihood (REML) was used for fitting the model and estimating the effects of the variables included in the model. We conducted another analysis for sexual dysfunction considering ASEX scores as the main dependent variable.
ResultsTwenty-two (81.5 %) participants were men. The mean age was 35 years. The mean duration of the disease was 8.9 (2.1) months, and the age of onset of the disease was 26.1 years. Men were younger than women (33.1 ± 11.9 vs. 43.8 ± 11.6 years), although this clinically relevant difference was not statistically significant. There were no significant differences between men and women in antipsychotic doses at baseline (risperidone dose: 4.2 ± 2.4 in men vs. 5.3 ± 4.2, p = 0.454). The clinical and demographic characteristics of the sample are described in Table 1.
Baseline sociodemographic and clinical variables of 27 patients with schizophrenia who completed follow-up visits.
Abbreviations: R-LAI=risperidone long-acting injectable.
At 6 months after the switch from risperidone to PP, no significant changes in prolactin levels were detected. However, in women, prolactin concentrations were reduced (119.9 ± 65.5 to 83.0 ± 40.7 ng/ml; p < 0.001) with a significant time by sex interaction effect (p = 0.035) but no effect of age (p = 0.72) (Table 2). The sex by time interaction is depicted in Fig. 1. As shown in this figure, prolactin concentrations were relatively unchanged in men, whereas a reduction was observed in women. Antipsychotic doses (risperidone equivalents) influenced prolactin levels (p = 0.006), such that higher antipsychotic doses were associated with higher prolactin concentrations (Table 2).
No significant differences were found in the ASEX total scores at 6 months after the switch (Table 2). The prevalence of clinical sexual dysfunction was 51.9 % at baseline, 37.0 % at three months, and 36.4 % at 6 months. Longitudinal changes in clinical sexual dysfunction were not statistically significant (p = 0.125).
Antipsychotic doses measured as risperidone equivalents did not change during the study (Table 3). The participants did not present significant psychopathological changes in either positive psychotic symptoms or depressive symptoms.
Longitudinal changes in continuous variables.
Data are shown as mean (SD).
Abbreviations: PANSS= Positive and Negative Syndrome Scale; CDSS= Calgary Depression Scale for Schizophrenia; ASEX= Arizona Sexual Experience Scale.
Our study suggests that women diagnosed with schizophrenia under treatment with risperidone have higher prolactin levels than men, and throughout follow-up, these levels remain higher. The upper limit of normal prolactin levels is usually 30% higher for women than for men, so HPRL was defined as a serum prolactin level >410 mIU/L (∼19.3 ng/ml) for males and >510 mIU/L (∼24.1 ng/ml) for females.23 Prolactin concentrations in women change throughout the menstrual cycle, and between premenopausal and postmenopausal women, with lower prolactin values in postmenopause.24 In our sample, only one woman was younger than 40 years, which suggests that most women in our study were peri/postmenopausal. Our finding of higher mean serum prolactin levels in females is consistent with previous studies on subjects with established psychosis25 and subjects with first-episode psychosis.26 This result is expected given that females are primed to respond to prolactin during physiological states such as pregnancy and breast feeding.23 The reduction in prolactin concentration after switching to PP that is observed more clearly in women is in accordance with other studies that have suggested sex differences in the relationship between risperidone metabolism and plasma prolactin levels in psychiatric patients.26 In the study by Suzuki et al,27 a positive correlation was found between 9-OH-risperidone and prolactin concentrations in women but not in men, suggesting that risperidone and paliperidone metabolism might be influenced by sex.
Our study has several clinical implications. The results suggest that switching from R to PP in women with schizophrenia and HPRL could be an option before considering other strategies that have been recommended for improving antipsychotic-induced HPRL, such as adding aripiprazole.28 In a previous study by our group conducted in early psychotic patients,16 prolactin levels were reduced after switching from R-LAI to PP, although we did not assess sex differences in that study. Our current study replicates the findings by Montalvo et al16 in a sample of chronic patients with schizophrenia, which suggests that switching from R to PP might be useful in a wider population (both early psychosis and chronic schizophrenia). Moreover, other studies have also reported a reduction in prolactin concentrations after switching from risperidone to paliperidone in elderly individuals.29
Previous studies have postulated that the difference in the pharmacokinetics of risperidone and paliperidone palmitate could explain the reduction in prolactin levels after the switch. Since we did not determine the levels of 9-hydroxyrisperidone before and after the switch, we do not know if the changes in prolactin levels could be explained by changes in 9-hydroxyrisperidone levels due to the different metabolisms of risperidone-LAI and PP. Madaan et al30 suggested that HPRL in patients treated with risperidone-LAI or PP is driven by 9-OH-risperidone and the higher occupancy of D2 at the pituitary level compared with the striatum. As already mentioned, Suzuki et al27 reported sex differences in the relationship between 9-OH-risperidone and prolactin concentrations. In our study, there were no sex differences in risperidone doses, and mixed linear regression analyses were adjusted for risperidone equivalents, which suggests that sex differences are independent of antipsychotic dosing.
Another potential explanation for the observed sex differences could be the existence of differences in treatment adherence between men and women at baseline. As poor treatment adherence is more common in male than in female psychiatric patients,31-34 if male participants had poorer adherence to oral risperidone at baseline, the reduction in prolactin concentrations could also be smaller than that in compliant participants.
In relation to sexual dysfunction, we did not observe improvements in ASEX total scores after the switch. Although a slight reduction in clinically significant sexual dysfunction was observed, this difference was not statistically significant. This result is consistent with a previous study by our group16 that did not report improvements in sexual dysfunction after the switch from R-LAI to PP. In this study, we included patients with chronic schizophrenia who had a longer duration of illness and more negative symptoms than the participants in the study by Montalvo et al,16 two variables that could contribute to a poorer response in sexual dysfunction, as negative symptoms are known risk factors for this condition.35
The main limitation of our study is that it was not randomized, allowing for the possibility of selection bias. The number of women was also low, which limits the possibility of exploring many clinical predictors of prolactin changes in each sex or conducting sex-stratified analyses for exploring longitudinal changes in categorical outcomes (e.g., clinically significant sexual dysfunction). The variability in prolactin concentrations throughout the menstrual cycle might be deemed a limitation, given our inclusion of both premenopausal and postmenopausal women. However, considering that 4 out of 5 women were over 40 years old, we anticipate fewer fluctuations in prolactin concentrations compared to if they were younger. Study strengths include the inclusion of participants recruited in real-world clinical practice under pragmatic conditions, as well as the follow-up period of 6 months, which extends the evidence of other previous studies that have used shorter follow-up periods.18
ConclusionsSwitching from risperidone to PP might reduce prolactin concentrations in women with schizophrenia without changes in sexual dysfunction. Therefore, the strategy of switching from risperidone to PP in women with schizophrenia and HPRL could be a therapeutic option for improving prolactin concentrations before considering other prolactin-reducing therapeutic strategies (e.g., aripiprazole addition, switching to aripiprazole or cabergoline treatment).
Ethics statementThe study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
All patients were informed about the study and signed a written informed consent form before participation in the study. Ethical approval was obtained by the Ethics Committee of Parc Taulí Hospital.
This study was financially supported by Janssen. Our institution was the promoter of the project (I3PT – PI: Javier Labad). Janssen did not participate in the design of the study or the analyses of the data obtained during the study.